- *Corresponding Author:
- C. Chellaram
Department of Applied Biotechnology, Sur College of Applied Sciences, Sur-411, Sultanate of Oman
E-mail: chellaramc.sur@cas.edu.om
| Date of Received | 30 July 2021 |
| Date of Revision | 10 May 2022 |
| Date of Acceptance | 20 March 2023 |
| Indian J Pharm Sci 2023;85(2):376-387 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Salacia oblonga (Celastraceae), a traditional ayurvedic herbal plant commonly called as ponkoranti. The antidiabetic and Histopathological studies of aqueous stem extracts of Salacia oblonga carried in Streptozotocin-nicotinamide induced diabetic rats which were orally treated with glibenclamide and Salacia oblonga stem extracts at lower (250 mg/kg) and higher (500 mg/kg) concentrations. The normal rats were administrated for the oral glucose tolerance test at 0, 30, 60 and 120 min after glucose load. Fasting blood glucose levels were measured by glucose oxidase strips and glucometer. The protective effect was evaluated by studying the effect of antioxidant enzymes, lipid peroxidation and Histopathological changes after the treatment period. Treatment done with 500 mg/kg of stem extract produced more effect with improved protein (1.18±1.45 mg), superoxide dismutase (229.15±1.52 U/mg protein), Catalase (19.51±1.35 U/min/mg protein), Glutathione (1.54±.29 U/min/mg protein), Glutathione peroxidase (1.74±1.56 U/min/mg) and reduced lipid peroxidation (1.76±2.31 μm/min/mg). From the study, it was found that the stem extracts potentially increase the antioxidants and reduced the lipid peroxidation. The rats which were treated with Salacia oblonga and glibenclamide significantly reduced the glucose level (p<0.001). Histopathological alterations were restored after the treatment with stem extracts of Salacia oblonga at lower and higher concentrations. The significant effect on the vital organs such as liver and pancreas demonstrates the herbal usage of potential endangered plant. The spectral analysis (gas chromatography-mass spectrometry) explored the potential natural chemical constituents which were responsible for the antioxidant and antidiabetic activity. Further preclinical and clinical trials are required to confirm the potential of Salacia oblonga stem extract.
Keywords
Salacia oblonga, antidiabetic, streptozotocin, glibenclamide, pancreas
Diabetes mellitus is a metabolic disorder initiated by excess glucose limit in blood. The inadequate secretion of insulin is the major intension in human body[1]. Alpha amylase and alpha glucosidase are the two important enzymes responsible for diabetes[2]. The long-term exposure of increased blood glucose leads to failure of kidney, hypertension, reduce of vision, obesity, tissue damage and lipid disorder[3]. Diabetes arise when our body is inefficacious to take enough insulin effectively or when the pancreas does not generate sufficient insulin[4]. The symptoms are thirsty, abnormal urination, frequent hunger, loss of body weight, headache, long duration for wound healing, skin irritation, loss of vision and tiredness. It causes due to lack of body movement, unhealthy diets, raised blood cholesterol, obesity and glucose[5,6].
Herbal plants play an interesting role in pharmaceutical field and are widely used all over the world for healing purpose. They have rich in therapeutic values and are widely used by pharmaceutical companies. It is possible to extract different chemical constituents from plants, which are active against insects, microorganisms and diseases. The secondary metabolites such as phenols, alkaloids, terpenoids, flavonoids, tannins and saponins are naturally synthesized by the plants through metabolic actions[7]. The medicinal activity of plants can give health, financial and social benefits which are considered as the back bone of traditional medicine. The antidiabetic compounds play an important role in glucose metabolism and reduce the blood sugar level and complications related to diabetes[8,9].
Salacia oblonga (S. oblonga) is a woody strangling climber associated with the family of Celastraceae. Its leaves are oblong, blunt at apex, narrow at base and green color. Flowers are greenish yellow and orange red fruit. It is frequently called as Saptrangi, Ekanayaka and Ponkoranti due to its golden color root bark. The root and stem extractions of S. oblonga used to cure diabetes and obesity[10,11]. It is available in the biosphere reserve of tropical Africa and the southern regions of India and Sri Lanka. In long term analysis, S. oblonga plays an important role in the traditional Ayurveda medicine[12]. The extract of S. oblonga has the similar activity of standard drug acarbose, which is a therapeutic agent of type 2 diabetes[13]. It is also used for curing gonorrhea, asthma, itchiness, joint pain, obesity, thirst and fever. It may reduce the cardiovascular risk in kidney patients[14,15]. The nutrients such as iron, calcium, phosphorous and fiber contents available in S. oblonga can be utilized as liver tonic[16].
In olden period, the root extract of S. oblonga had been used as traditional ayurvedic medicine to cure madhumeha[17]. It is due to the inhibiting action of alpha amylase and alpha glucosidase enzymes. In the present investigation, the stem extract of S. oblonga explores the antidiabetic action and the effect of antioxidant enzymes on diabetic rats. Histopathological alterations of pancreatic tissues of diabetic rats and their effects on higher (500 mg/kg) and lower (250 mg/kg) concentrations of S. oblonga stem extracts uphold the potential action of herb.
Materials and Methods
Chemicals:
Streptozotocin, glibenclamide (Himedia; Mumbai), 2-2-Diphenyl-1-picryl Hydrazyl, glucometer (Accuchek Roche Diagnostics, USA). Semi-autoanalyzer (Photometer 5010, V5+, Germany) with enzymatic kits procured from Primal Healthcare Limited, Lab Diagnostic Division, Mumbai, India. All other chemicals and reagents used are analytical grade.
Collection of plant materials and extract preparation:
Healthy plants of S. oblonga were collected from Western Ghats (15°22'40.5" N, 75°07'41.0" E, Altitude 700 m) Hubli, Dharwad district, Karnataka state, South India. The plant was authenticated by Dr.Vijayakumar, Associate professor, Hindu College, Nagercoil-629002. The stems were separated and washed thoroughly to remove all impurities like soil and dust particles. Then it was cut down into small pieces and dried in shadow for 5 w. The dried stems were powdered by ball mill and maintained at 10°.
Preparation of stem extracts:
About 30 g of dried sample of S. oblonga stem powder was extracted with 300 ml of five different solvents (ethanol (75 %), acetone, water, chloroform and petroleum ether) for one minute using an Ultra Turax mixer (13 000 rpm). It was soaked overnight at room temperature. Then the samples were filtered through Whatman No.1 filter paper in a Buchner funnel, the filtrate was evaporated under vacuum in a rotary evaporator at 40° and then dissolved in respective solvents. Phytochemical studies revealed the maximum percentage of alkaloids, terpenoids, flavonoids, tannins, saponins and other phytochemicals in aqueous stem extract which was used for experimental studies.
Experimental animals:
Adult male albino rats from the KMCH College of Pharmacy, Coimbatore-641048 with the approval of ethical committee were used for the present study. The animals were housed in clean polypropylene cages and maintained with well-ventilated temperature in a controlled animal house with a constant 12 h light/ dark schedule. The animals were fed with standard rat pelleted diet and have provided clean drinking water with ad libitum.
Evaluation of antihyperglycemic activity of S. oblonga:
The antihyperglycemic activity of stem extracts of S. oblonga was committed with the induction of diabetes followed by the oral glucose tolerance test on normal rats.
Experimental induction of diabetes:
The animals were divided into five groups of six animals each. They were kept overnight fasting and the initial fasting blood glucose level checked from the tip of rat tail vein.
Group I: Control (only normal saline); Group II: Streptozotocin (60 mg/kg) (IP)+Nicotinamide 120 mg/kg (p.o); Group III: Streptozotocin (60 mg/ kg)+Nicotinamide 120 mg/kg (p.o) rats treated with Glibenclamide 20 mg/kg (p.o); Group IV: Streptozotocin (60 mg/kg)+Nicotinamide 120 mg/ kg (p.o) rats treated with S. oblonga (250 mg/kg); Group V: Streptozotocin (60 mg/kg)+Nicotinamide 120 mg/kg (p.o) rats treated with S. oblonga (500 mg/kg).
Streptozotocin was dissolved in citrate buffer (pH 4.5) and nicotinamide was dissolved in normal saline. Non-insulin dependent diabetes mellitus was induced in overnight fasted rats by a single intraperitoneal injection of 60 mg/kg streptozotocin, 15 min after the i.p administration of 120 mg/kg of nicotinamide. Hyperglycemia was confirmed by the increased level of blood glucose, and was determined at 72 h. The animals used for the study have blood glucose concentration more than 250 mg/dl[18].
The vehicle (saline), standard glibenclamide and the stem extracts were administered to the respective group animals for 28 d. The fasting blood glucose levels of animals were estimated on 1st, 7th, 14th, 21st and 28th d from the tip of rat tail vein.
Oral glucose tolerance test in normal rats:
Normal rats were subjected for oral glucose tolerance test by the standard procedure[19]. It was carried out with two different concentrations of S. oblonga stem extracts and standard drug glibenclamide. Albino Wistar rats of either sex weighing 150-200 g were divided into 5 groups consisting of 6 rats in each group.
Group I: Distilled water; Group II: Only Glucose (2 g/kg p.o); Group III: Glibenclamide (2 mg/ kg p.o)+Glucose (2 g/kg p.o); Group IV: Extract of S. oblonga (250 mg/kg p.o)+Glucose (2 g/kg p.o); Group V: Extract of S. oblonga (500 mg/kg p.o)+Glucose (2 g/kg p.o)
Distilled water, glibenclamide (2 mg/kg) and S. oblonga stem extracts (250 and 500 mg/kg p.o.) were administered to the respective groups of rats. Glucose (2 g/kg) was fed 30 min after pretreatment with distilled water, glibenclamide and stem extracts. The effect of S. oblonga stem extracts at lower and higher concentrations was measured by testing the glucose level. The blood glucose levels were measured at the interval of 0, 30, 60, 120 and 240 min after glucose load by using blood glucose test strips and glucometer (Accu-chek Advantage II; Roche, Germany).
Determination of antioxidant enzymes and Lipid Peroxidation (LPO):
Antioxidant enzymes such as Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), reduced Glutathione (GSH) and LPO were determined in the liver tissues of all the tested rats.
Preparation of tissue homogenate:
After the treatment with aqueous stem extracts of S. oblonga with both concentrations, the rats were sacrificed their liver which was secluded. Then it was washed with normal saline and stored for 12 h, for in vivo antioxidant studies. The separated liver was homogenized with motor driven Teflon coated homogenizer with 0.1 M Tris-HCl buffer (pH 7.4) to get 10 % homogenate. The homogenate was centrifuged at 10 000 rpm for 10 min at 5°. The supernatant was used for in vivo antioxidant studies.
Determination of proteins:
Total protein content was determined by Lowry et al.[20]. About 0.1 ml of liver homogenate was mixed with 0.9 ml of water and 4.5 ml of alkaline copper sulphate reagent. The mixture was kept at room temperature for 10 min. About 0.5 ml of Folin’s reagent was added with the mixture. After 20 min, blue colour developed which was measured at 640 nm in a double beam Ultra Violet-Visible (UVVIS) spectrophotometer (UV 1700, Szhimadzhu). The amount of protein present in the sample was expressed as mg/dl.
Determination of SOD:
SOD was measured by the standard method with slight alterations[21]. About 0.5 ml of liver homogenate was diluted with 0.5 ml of distilled water. To this mixture, 0.25 ml of chilled ethanol and 0.15 ml of chloroform were added. The mixture was shaken thoroughly and centrifuged at 2000 rpm. The supernatant solution was separated. About 0.5 ml filtrate was taken separately and 1.5 ml of carbonate buffer (pH 10.2) was added. The reaction was started by the addition of 0.4 ml epinephrine. The change in optical density per minute was measured at 480 nm.
Determination of CAT:
The CAT activity was assayed by the standard method[22]. About 0.1 ml of liver homogenate was taken with 1.0 ml of phosphate buffer (pH 7.0) and hydrogen peroxide (0.2 M). The reaction was arrested by the addition of 0.2 ml dichromate acetic acid reagent. Standard hydrogen peroxide in the range of 4 to 20 μl were taken and treated similarly. The tubes were heated in a boiling water bath for 10 min. The green color developed was read at 570 nm in a Double beam UV-VIS spectrophotometer. CAT activity was expressed as U/mg.
Determination of GPX:
GPX was measured according to the standard procedure[23]. About 0.2 ml each of ethylene diaminetetraacetic acid (0.8 mM), sodium azide (10 mM), reduced GSH (4 mM), hydrogen peroxide (2.5 mM) and 0.4 ml of sodium phosphate buffer (0.32 M) was added with 0.1 ml of liver homogenate. All the ingredients were mixed well and the mixer was incubated at 37° for 10 min. The reaction was arrested by the addition of 0.5 ml of Trichloro acetic acid and the tubes were centrifuged. About 0.5 ml of supernatant was separated and 3 ml of sodium hydrogen phosphate and 1 ml of Ellman's reagent were added. The mixture was kept at room temperature and the color developed was read at 412 nm immediately in a double beam UV-VIS spectrophotometer. GPX activity was expressed as μg/mg.
Determination of reduced GSH:
Reduced GSH was estimated by Ellman’s procedure[24]. About 250 μl of tissue homogenate was taken in a 2 ml Eppendroff tube. Then one ml of 5 % Trichloro acetic acid was added and the solution was centrifuged at 3000 rpm for 10 min at room temperature. The supernatant was separated and 250 μl was taken with 1.5 ml of phosphate buffer (0.2 M) and mixed well. About 250 μl of Ellman’s reagent (0.6 mM) (DTNB solution) was added to the above mixture and the absorbance was measured at 412 nm within 10 min. A standard graph was plotted using GSH reduced solution (1 mg/ml) and GSH content present in the tissue homogenates was calculated by interpolation. Amount of GSH was expressed as μg/ mg protein.
Determination of LPO:
LPO was estimated by the standard method[25]. 1 ml of liver homogenate was mixed with 0.2 ml of sodium dodecyl sulfate (4 % w/v), 1.5 ml 20 % acetic acid in 0.27 M hydrochloric acid (pH 3.5) and 15 ml of 0.8 % thiobarbituric acid (pH 7.4). The mixture was heated in a hot water bath at 85° for 1 h. Then it was cooled and centrifuged for 10 min. The intensity of the pink colour developed was read against a reagent blank at 532 nm.
Histological assessment:
Histopathology is the microscopical study of tissues for the demonstration of pathological alterations. It involves the five different stages such as collection of morbid tissues from necropsy, fixation, preparation of sections, staining and microscopical examination.
Collection and fixation of materials:
Thin pieces of 3 to 5 mm, thickness of pancreatic tissues were collected and gross morbid changes were shown along with normal tissue. The pancreatic tissues were kept in fixative for 24-48 h at room temperature. 10 % of formalin solution was used as fixative. The fixation serves to harden the tissues by coagulating the cell protein, to prevent destruction of tissues, to preserve the structure and to prevent shrinkage.
Haematoxylin and eosin method of staining:
Deparaffine the section, by xylol 5 to 10 min and xylol was removed by absolute alcohol. Then, clean the section in tap water and stained with Haematoxylin for 3-4 min and again cleaned under tap water. The sections were allowed in tap water for few minutes and counter stained with 0.5 % eosin until section appears light pink and then washed with tap water. Then it was blotted and dehydrated in alcohol and cleared with xylol. The section was mounted on a Canada balsam or DPX Mountant and kept the slide dry and remove air bubbles for Histopathological evaluation under photomicroscope (×40).
Spectral analysis of S. oblonga stem extracts:
The natural chemical constituents in S. oblonga stem extract were analysed by using Gas Chromatography– Mass Spectrometry (GC-MS)-5975C Agilent system composed of an auto sampler and a gas chromatograph interfaced with a mass spectrometer. The highest percentage of antioxidant fraction obtained from the aqueous stem extract by Column Chromatography was used for the GC-MS analysis. The important conditions are, column Elite-1 fused silica capillary column (300.25 mm ID×1 EM df, composed of 100 % dimethyl poly siloxane), operating in electron impact mode at 70 eV. A constant flow of 1.51 ml/min and an injection volume 1 μl of helium (99.999 %) gas was used as carrier gas (split ratio 10:1). The temperature of injector and ion source were maintained at 240° and 200° respectively. The oven temperature was programmed from 70° (isothermal for 2 min) with an increase of 10° per min to 300° per min, ending with a 9 min isothermal at 300°. Mass spectra were taken at 70 eV, with a scan range 40-1000 m/z. Solvent cut time was 5 min, MS start time and end time being 5 min and 35 min respectively.
Statistical analysis:
The results of statistical analysis were expressed as mean±Standard Error of Mean (SEM) in the Graph pad 5.1 versions. Statistical significance test for comparison was done by one way ANOVA followed by Dunnett’s Test (n=6); Mean values were tested for the significant difference between the normal control group with diabetic control group (***p<0.001) whereas the treated groups were compared with non treated diabetic control group (***p<0.001, **p<0.01 and *p<0.05).
Results and Discussion
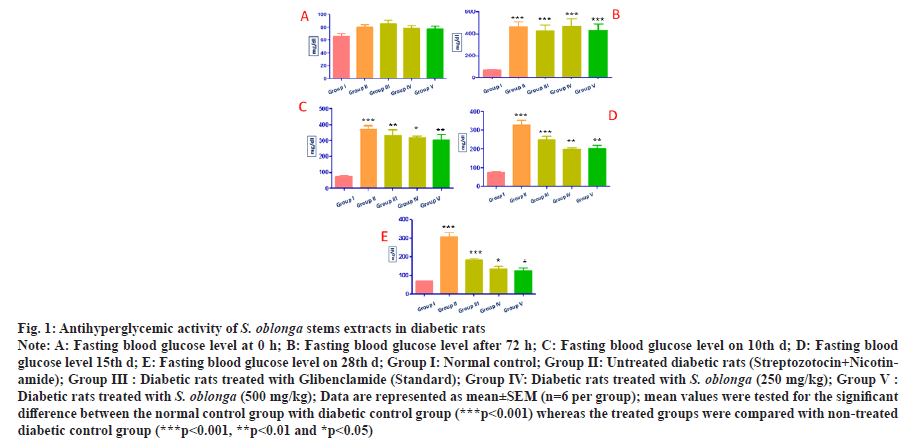
The anti-diabetic action of S. oblonga stem extracts was experimentally confirmed by in vivo study. The effect of oral administration of S. oblonga stem extracts in diabetic rats was determined by measuring the fasting blood glucose level before and after the administration of Streptozotocin and Nicotinamide. The initial blood sugar level was measured for all the five groups of animals. Fasting blood sugar level on 3rd, 10th, 15th and 28th d also measured. There was no significant difference of blood sugar level for the normal control rats identified throughout the experiment. The blood glucose levels of Type-2 diabetic induced control rats were increased from the initial level 80±4.131 mg/dl to 463.33±47.164 mg/ dl on the next interval of the experiment. Diabetic control rats showed significantly increased level (***p˂0.001) of blood glucose compared with normal control rats. The stem extracts of S. oblonga at the concentration of 250 mg/kg p.o. reduced the blood glucose level from 466±67.26 mg/dl to 133.33±14.29 mg/dl and the same extract at higher concentration (500 mg/kg p.o.) potentially reduced the blood glucose level from 430±57.677 mg/dl to 126.67±12.56 mg/dl. These results explored the stem extracts of S. oblonga significantly cut the blood glucose level almost similar to the standard drug glibenclamide, which reduce the blood glucose level from 426.66±52.578 mg/dl to 181.67±8.33 mg/dl on 28th d. There was a significant reduction of blood glucose level observed in treated diabetic groups with glibenclamide (***p˂0.001) and S. oblonga stem extracts 250 mg/kg p.o. and 500 mg/kg p.o (*p˂0.05) when compared with the non treated diabetic group. The variation of blood glucose levels at each interval was represented in the figure (fig. 1).
Fig. 1: Antihyperglycemic activity of S. oblonga stems extracts in diabetic rats
Note: A: Fasting blood glucose level at 0 h; B: Fasting blood glucose level after 72 h; C: Fasting blood glucose level on 10th d; D: Fasting blood
glucose level 15th d; E: Fasting blood glucose level on 28th d; Group I: Normal control; Group II: Untreated diabetic rats (Streptozotocin+Nicotinamide);
Group III : Diabetic rats treated with Glibenclamide (Standard); Group IV: Diabetic rats treated with S. oblonga (250 mg/kg); Group V :
Diabetic rats treated with S. oblonga (500 mg/kg); Data are represented as mean±SEM (n=6 per group); mean values were tested for the significant
difference between the normal control group with diabetic control group (***p<0.001) whereas the treated groups were compared with non-treated
diabetic control group (***p˂0.001, **p˂0.01 and *p˂0.05)
The hyperglycemia appears due to the excess production of glucose from hepatic and it leads to serious disorder[26]. Streptozotocin and nicotinamide induced Type-2 diabetic rats produced high blood glucose level. Generally, Nicotinamide is administered to protect the insulin secreting cells against streptozotocin. Thus the beta cells are not completely damaged and it is used to induce Type 2 diabetes. In the present study, there was a significant reduction of glucose level at the concentration of 500 mg/kg p.o. of stem extract of S. oblonga than 250 mg/kg p.o. extract. Similar studies carried out on the root extracts of S. oblonga, leaf and root extract of Andrographis paniculata, Tinospora cordifolia and Trigonella foenum-graecum corroborate the glucose reduction potential of herbal plants[27-28]. The phytochemicals such as phenols, flavonoids, terpenoids, tannins, alkaloids and saponins are responsible for antidiabetic action[29].
The ability of body to take glucose can be estimated by glucose tolerance test on normal rats. In oral glucose tolerance test, the blood samples were analyzed at 0, 60, 120,180 and 240 min intervals. Initially, the concentration of blood glucose level was seen normal and slight change among the different groups. The blood glucose level was rapidly increased after glucose loading at the 1st h interval. The glucose levels of treated groups were not significant at the 1st and 2nd h interval. The treated groups with glibenclamide and S. oblonga stem extracts (250 mg/ kg p.o. and 500 mg/kg p.o.) were showed significant reduction of glucose level (***p˂0.001) from the 4th to 5th h interval when compared with control rats. The changes in the blood glucose level of treated groups and diabetic control were shown in the Table 1.
| Glucose Level | Control | Only Glucose | Glucose+STD | Glucose+Ext L.D | Glucose+Ext H.D |
|---|---|---|---|---|---|
| Initial | 55±17.99 | 52±16.85 | 47.83±15.82 | 57.83±18.9 | 60±19.81 |
| 1st h | 78.33±4.94 | 273±122.7ns | 240±108.4ns | 198.3±94.88ns | 191±86.08ns |
| 2nd h | 75±3.16 | 253.3±113.4ns | 186.7±83.89ns | 165±74.73ns | 145.7±65.52ns |
| 3rd h | 83.33±5.72 | 238.3±17.4*** | 215±2.88*** | 226.7±4.41*** | 218.3±6.009*** |
| 4th h | 81.67±3.33 | 199.3±6.546*** | 171.7±6.91*** | 183.3±3.801*** | 173.3±4.59*** |
| 5th h | 79.83±4.61 | 84.17±37.96 | 48.17±21.76 | 57±25.53 | 53±23.77 |
Note: The data expressed as mean±standard error of mean; statistical significance test for comparison was done by one way ANOVA followed by Dunnett’s Test (n=6); mean values were tested for the significant difference between the normal control group with diabetic control group (***p<0.001) whereas the treated groups were compared with non-treated diabetic control group (***p<0.001); ns: non-significant
Table 1: Effects of S. Oblonga Stem Extracts in Normal Rats by Oral Glucose Tolerance Test
Generally, oral glucose tolerance test measures the body’s ability to use glucose. It is used to diagnose the pre-diabetes and diabetes mellitus. The present study supports the active potential of aqueous stem extract of S. oblonga at higher and lower concentrations. Similar studies carried out in the ethanol extract of Hedyotis leschenaultiana and Cynoglossum zeylanicum has been proven as a potential drug for diabetic complications[30,31]. The oral glucose tolerance test conducted on the extract of Phyllanthus acidus shown the nontoxic nature of plant at lower concentration[32]. The hypoglycemic effect of plant extracts were due to the presence of flavonoids, terpenoids, saponins and other secondary metabolites[33]. The antihyperglycemic effect of stem extract of S. oblonga was compared with the standard Glibenclamide which confirmed the potentiality of herbal extract.
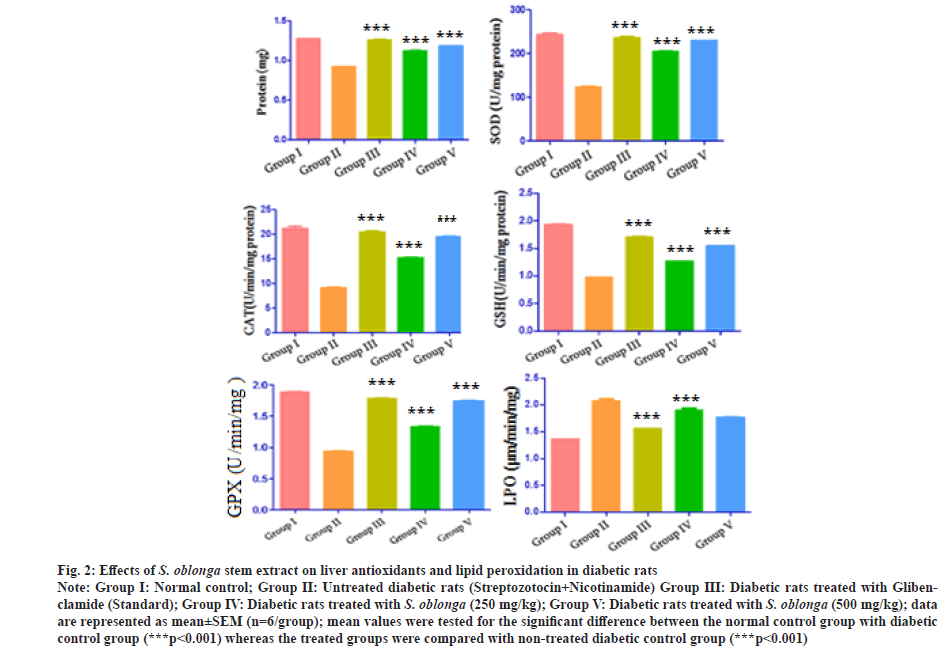
After the treatment of 28 d, the liver antioxidants such as protein, SOD, CAT, reduced GSH, GPX and LPO were measured. The diabetic control rats showed reduction in the protein, SOD, CAT, GSH and GPX which inferred the continuous oxidative stress. The diabetic rats treated with the standard glibenclamide and S. oblonga stem extracts (250 mg/kg p.o. and 500 mg/kg p.o.) significantly increased the level of antioxidant enzymes (***p˂0.001) when compared with the diabetic group of rats which explored the reduction of reactive oxygen species. Treatment done with 500 mg/kg of stem extract produced improved protein (1.18±1.45 mg), SOD (229.15±1.52 U/mg protein), CAT (19.51±1.35 U/min/mg protein), GSH (1.54±.29 U/min/mg protein) and GPX (1.74±1.56 U/min/mg). The present study indicates the stem extract of S. oblonga could be a potent source for antioxidants.
LPO leads cell damage, which was measured for all the group of rats. There was an increase of LPO (2.08±1.10 μm/min/mg of protein) for the streptozotocin and nicotinamide induced diabetic rats. The LPO tendency was also measured for the treated rats with glibenclamide and stem extracts (250 mg/kg p.o. and 500 mg/kg p.o.) 1.56±1.06, 1.90±1.26 and 1.76±2.31 and μm/min/mg of protein respectively. The treated groups showed significant reduction (***p˂0.001) of LPO when compared with diabetic control rats. The effect of oral administration of S. oblonga on liver antioxidants of streptozotocin and nicotinamide induced diabetic rats were graphically represented in the fig. 2. From the study, it was found that the action of stem extracts significantly increase the antioxidants and reduce the LPO.
Fig. 2: Effects of S. oblonga stem extract on liver antioxidants and lipid peroxidation in diabetic rats
Note: Group I: Normal control; Group II: Untreated diabetic rats (Streptozotocin+Nicotinamide) Group III: Diabetic rats treated with Glibenclamide
(Standard); Group IV: Diabetic rats treated with S. oblonga (250 mg/kg); Group V: Diabetic rats treated with S. oblonga (500 mg/kg); data
are represented as mean±SEM (n=6/group); mean values were tested for the significant difference between the normal control group with diabetic
control group (***p<0.001) whereas the treated groups were compared with non-treated diabetic control group (***p˂0.001)
The clinical studies and experiments carried out on diabetes suggested that it was linked with oxidative stress, strain, reactive oxygen species, hydrogen peroxide and hydroxyl radicals[34]. Free radicals play an important role for the damage of beta cells.
Streptozotocin induced diabetes can produce reactive species leads the damage of important organs in our body like kidney, liver and eyes[35]. A variation in defense system may alter the antioxidant enzymes such as SOD, CAT, GSH, and GPX. The enzymes such as SOD, GSH and CAT play vital role for the elimination of reactive oxygen species. They are considered as first line defense antioxidants[36]. The hydrogen peroxide produced by SOD is removed as water by the action of other enzymes GSH, GPX and CAT. Thus our body is preserved from the toxic nature of oxygen radical. In the present study, the liver antioxidants such as SOD, CAT, GSH and GPX were reduced in diabetic control rats than normal rats. It specified the inactiveness of defense system. Many investigators proved the diminishing nature of antioxidants on diabetic rats[37]. Similar results were reported on the studies carried out on the root extracts of S. oblonga and Moringa oleifera leaves. The decreased antioxidant enzymes were improved by the treatment of root extracts and leaf extracts[38,39]. It supports that the stem extract of S. oblonga be a potent source for the increase of antioxidant enzymes at lower and higher concentrations.
LPO is the most important one for chronic diabetes. It may induce inflammation and many diabetic complications[40]. The anti-LPO study conducted on the root extract of S. oblonga effectively reduces the LPO[41]. In the present study, LPO severely increased for the diabetic rats. Then it was decreased by the treatment of stem extracts of S. oblonga and the results were compared to the standard. Similar studies carried out on Morin effectively reduce the LPO on diabetic rats[42].
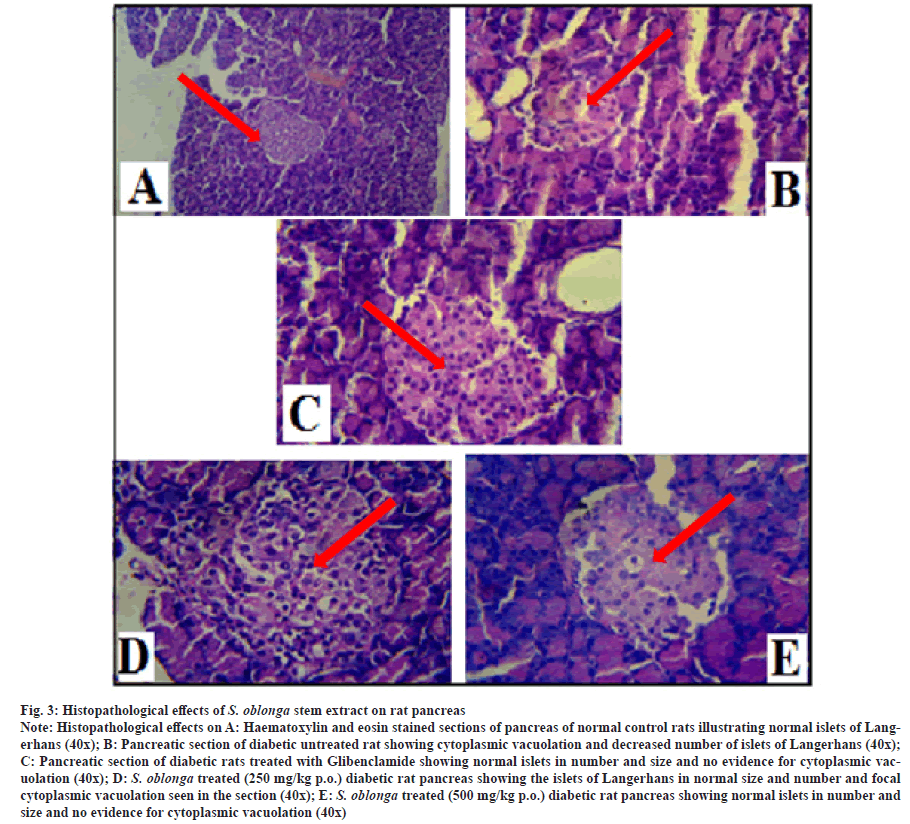
The Histopathological studies were carried out on the pancreas of normal, diabetic and treated groups (glibenclamide and S. oblonga stem extracts (250 mg/kg p.o. and 500 mg/kg p.o.) of experimental rats. The histological effects of stem extracts on rat pancreas were shown by the arrow mark in the photomicrograph (x40) of fig. 3. The section of normal rat pancreas was shown normal acini and the section from islets also normal in number and size. There is no evidence of inflammation or cytoplasmic vacuolation seen in the section studied. The photomicrograph of the pancreatic section of untreated diabetic rats was shown normal acini but the islets were shown cytoplasmic vacuolation and decreased in number. The pancreatic section of diabetic rats treated with glibenclamide was shown normal acini and the section from islets shown normal in number and size. There was no evidence of cytoplasmic vacuolation seen in the section studied. Comparison of normal and diabetic groups clearly shows the destruction of islet cells in diabetic rats as they were irregularly shaped and atrophic.
Fig. 3:Histopathological effects of S. oblonga stem extract on rat pancreas
Note: Histopathological effects on A: Haematoxylin and eosin stained sections of pancreas of normal control rats illustrating normal islets of Langerhans
(40x); B: Pancreatic section of diabetic untreated rat showing cytoplasmic vacuolation and decreased number of islets of Langerhans (40x);
C: Pancreatic section of diabetic rats treated with Glibenclamide showing normal islets in number and size and no evidence for cytoplasmic vacuolation
(40x); D: S. oblonga treated (250 mg/kg p.o.) diabetic rat pancreas showing the islets of Langerhans in normal size and number and focal
cytoplasmic vacuolation seen in the section (40x); E: S. oblonga treated (500 mg/kg p.o.) diabetic rat pancreas showing normal islets in number and
size and no evidence for cytoplasmic vacuolation (40x)
Haematoxylin and eosin sections of the diabetic rat pancreas treated with aqueous stem extracts of S. oblonga at 250 mg/kg p.o. and 500 mg/kg p.o. concentrations were shown normal acini and the section from islets shown normal in number and size but focal cytoplasmic vacuolation seen in the section treated with lower concentration of stem extract. The histological effect of diabetic rats treated with 500 mg/kg p.o. extracts shown no evidence of inflammation or cytoplasmic vacuolation. This finding is in agreement with the previous study [43].
The Histopathological studies carried out on the pancreatic tissues of diabetic rats to confirm the antihyperglycemic action of S. oblonga stem extracts. In this study the Histopathological examination of sections of the pancreas from the diabetic control group of rats showed massive pathological changes as compared to the normal structure observed in the normal group. The photomicrograph of beta cells in the pancreatic islets of the normal rats was looks like patches. Destruction of beta cells reflects the cytotoxity of streptozotocin. Microscopic examination of pancreatic sections of the untreated diabetic group revealed a breakdown of anatomical features including necrotic changes, beta cell degranulation and severe vacuolation. From the previous study, cluster of inflammation cells could also be seen among the cells[44]. The diabetic rats treated with 500 mg/kg p.o of aqueous stem extract of S. oblonga shows maximum number in the regeneration of beta-cells than 250 mg/kg p.o. Similar histological studies were carried out on the pancreas of diabetic rats by using the root extract of S. oblonga, reduced diabetes and the tissues were normal after treatment[45].
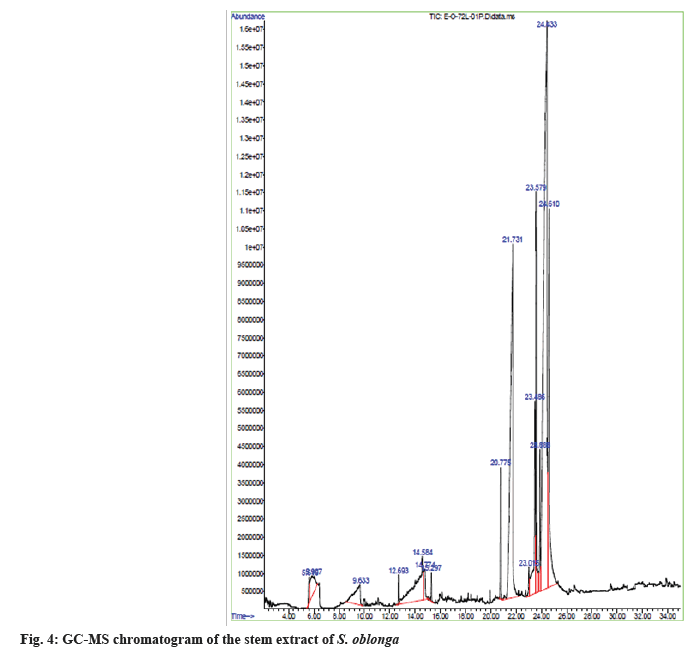
The natural phytoconstituents superintend the pharmaceutical characteristics of stem extract of S. oblonga. They are exposed by comparing the spectral data obtained on the GC-MS with the data base of National Institute Standard and Technology (NIST) having more than 62 000 patterns. The name of the natural chemical constituents and their structure were identified with the help of mass spectrum obtained. The GC-MS chromatogram of the stem extract of S. oblonga was represented in the fig. 4. There are fifteen bioactive compounds were identified and was represented in the Table 2 with their name, structure and molecular weight.
| S No | RT | Name of the chemical component | Molecular formula | Molecular weight (g/mol) | Peak area % | Structure |
|---|---|---|---|---|---|---|
| 1 | 5.618 | 1-silacylo-2,4-hexadiene | C5H6Si | 94 | 0.54 |  |
| 2 | 5.91 | sulfuric acid, dimethyl ester | C2H6O4S | 126 | 2.07 | 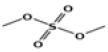 |
| 3 | 9.636 | glycerine | C3H8O3 | 92 | 2.75 | 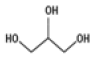 |
| 4 | 12.7 | 3H-pyrazol-3-one, 1,2-dihydro-1,2,5-trimethyl- | C6H10N2O | 126 | 0.25 | 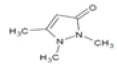 |
| 5 | 14.58 | 1,2,3,4-butanetetrol, [S-(R*,R*)]- | C4H10O4 | 122 | 6.52 | 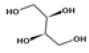 |
| 6 | 14.78 | 1,2-ethanediol, 1-(2-phenyl-1,3,2-dioxaborolan-4-yl)-, [S-(R*,R*)]- | C10H13BO4 | 208 | 1.29 | 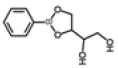 |
| 7 | 15.3 | benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | C15H22 | 202 | 0.35 | 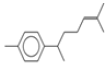 |
| 8 | 20.78 | hexadecanoic acid, methyl ester | C17H34O2 | 270 | 1.55 |  |
| 9 | 21.73 | n-hexadecanoic acid | C16H32O2 | 256 | 19.65 |  |
| 10 | 23.01 | heptadecanoic acid | C17H34O2 | 270 | 0.49 |  |
| 11 | 23.46 | 10,13-octadecadienoic acid, methyl ester | C19H34O2 | 294 | 4.33 | 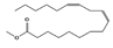 |
| 12 | 23.58 | 9-octadecenoic acid (Z)-, methyl ester, | C19H36O2 | 296 | 5.47 | 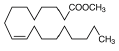 |
| 13 | 23.89 | methyl stearate | C19H38O2 | 298 | 1.64 | 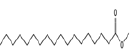 |
| 14 | 24.44 | 6-octadecenoic acid | C18H34O2 | 282 | 42.09 | 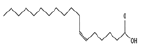 |
| 15 | 24.61 | octadecanoic acid | C18H36O2 | 284 | 11.01 | 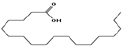 |
Note: RT: Retention Time
Table 2: Chemical Compounds Identified using GC-MS Analysis
Among the natural compounds obtained, hexadecanoic acid methyl ester has proven as antidiabetic compound[46], 9-octadecenoic acid methyl ester can inhibit alpha glucosidase enzyme, 10,13-octadecadienoic acid methyl ester is an hepatoprotective agent which can protect liver from oxidative stress and free radicals. The antioxidant, n-hexadecanoic acid can protect our body from tissue damage[48] and the heptadecanoic acid can prevent pre-diabetes from human[49]. Similar studies were done by researchers on herbal extractions, explored the pharmaceutical biological activities[50].
In conclusion, the natural chemical constituents present in the stem extracts of S. oblonga were responsible for the potential pharmaceutical properties. The aqueous stem extract beneficially reduce the glucose level, LPO and increase the level of liver antioxidants. The Histopathological effects on the pancreas of diabetic rats by the action of stem extracts proved the antidiabetic potential of herbal plant S. oblonga. However more studies are entailed to isolate and characterize the active antidiabetic compounds from the aqueous stem extracts of S. oblonga.
Ethical statement:
All animal procedures were performed after the approval from the Institutional Animal Ethics Committee, KMCH College of Pharmacy, Coimbatore (Reg.No: 685/PO/ Re/S/2002/CPCSEA) and by the suggestions for the perfect care and utilization of laboratory animals.
Acknowledgements:
The authors would like to express gratitude for the KMCH college of Pharmacy, Coimbatore to provide lab facilities and the Chairman and Principal of Veltech Multitech Engineering College to give support to complete the research work successfully.
Conflict of interest:
The authors disclosed no conflict of interest.
References
- Naowaboot J, Nanna U, Chularojmontri L, Tingpej P, Pannangpetch P. Effect of Thunbergia laurifolia water extracts on hepatic insulin resistance in high-fat diet-induced obese mice. Asian Pac J Trop Biomed 2021;11(3):97.
- Priya G, Gopalakrishnan M, Sekar T. Evaluation of antidiabetic potential of selected species of Salacia Leaf Extract. Int J Adv Pharm 2016;5:107-5.
- Medagama AB. Salacia reticulata (Kothala himbutu) revisited; A missed opportunity to treat diabetes and obesity? Nut J 2015;14:21.
[Crossref] [Google Scholar] [PubMed]
- Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity-A review of ten years of herbal medicine research (1990-2000). Dubai Diab Endocrinol J 2006;14(1):1-25.
- Kumar S, Kumar V, Prakash O. Antidiabetic and antilipidemic effects of Cassia siameal leaves extract in streptozotocin induced diabetic rats. Asian Pac J Trop Med 2010;3:871-3.
- Mamun-or-Rashid AN, Hossain MS, Hassan N, Dash BK, Sapon MA, Sen MK. A review on medicinal plants with antidiabetic activity. J Pharmacogn Phytochem 2014;3(4):149-59.
- Jadid N, Arraniry BA, Hidayati D, Purwani KI, Wikanta W, Hartanti SR, et al. Proximate composition, nutritional values and phytochemical screening of Piper retrofractum vahl. fruits. Asian Pac J Trop Biomed 2018;8(1):37-43.
- Deepak KG. Nageswara Rao Reddy N, Surekha C. Role of Antidiabetic Compounds on Glucose Metabolism–A special focus on medicinal plant: Salacia sps. Med Chem 2014;4:373-81.
- Tran N, Pham B, Le L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020;9(9):252.
- Li Y, Huang TH, Yamahara J. Salacia root, a unique Ayurvedic medicine, meets multiple targets in diabetes and obesity. Life Sci 2008;82(21-22):1045-9.
[Crossref] [Google Scholar] [PubMed]
- Takke AK, Pol SV, Bhoje RB. A review on saptrangi (Salacia oblonga Wall): A medicinal herb. Asian J Pharm Technol Innov 2016;4:33-9.
- Williams JA, Choe YS, Noss MJ, Baumgartner CJ, Mustad VA. Extract of Salacia oblonga lowers acute glycemia in patients with type 2 diabetes. Am J Clin Nutr 2007;86(1):124-30.
- Kushwaha PS, Singh AK, Keshari AK, Maity S, Saha S. An updated review on the phytochemistry, pharmacology, and clinical trials of Salacia oblonga. Pharmacogn Rev 2016;10(20):109.
[Crossref] [Google Scholar] [PubMed]
- Surekha C. Salacia as an ayurvedic medicine with multiple targets in diabetes and obesity. Ann Phytomed 2015;4:46-53.
- Singh RG, Rathore SS, Wani IA, Agrawal A, Dubey GP. Effects of Salacia oblonga on cardiovascular risk factors in chronic kidney disease patients: A prospective study. Saudi J Kidney Dis Transpl 2015;26(1):61.
- Deepak KG, Suneetha G, Surekha C. A simple and effective method for vegetative propagation of an endangered medicinal plant Salacia oblonga Wall. J Nat Med 2016;70:115-9.
[Crossref] [Google Scholar] [PubMed]
- Bagnazari M, Saidi M, Chandregowda MM, Prakash HS, Nagaraja G. Phyto-constituents, pharmacological properties and biotechnological approaches for conservation of the anti-diabetic functional food medicinal plant Salacia: A review note. Appl Food Biotechnol 2017;4(1):1-0.
- Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin-nicotinamide induced type 2 diabetes mellitus. J Appl Biomed 2008;6(1):31-7.
- Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J Ethnopharmacol 2006;107(3):374-9.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
[Google Scholar] [PubMed]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984;21:130-2.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47(2):389-94.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W. Selenium: Biochemical role as a component of glutathione peroxidase. J Sci 1973;179(4073):588-90.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophy 1959;82(1):70-7.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM: A balanced overview. Diabetes Care 1992;15(3):318-68.
- Huang TH, Yang Q, Harada M, Uberai J, Radford J, Li GQ, et al. Salacia oblonga root improves cardiac lipid metabolism in Zucker diabetic fatty rats: Modulation of cardiac PPAR-α-mediated transcription of fatty acid metabolic genes. Toxicol Appl Pharmacol 2006;210(1-2):78-85.
- Zhang XF, Tan BK. Antihyperglycaemic and anti‐oxidant properties of Andrographis paniculata in normal and diabetic rats. Clin Exp Pharmacol Physiol 2000;27(5‐6):358-63.
- Asghari AA, Mokhtari-Zaer A, Niazmand S, Mc Entee K, Mahmoudabady M. Anti-diabetic properties and bioactive compounds of Teucrium polium L. Asian Pac J Trop Biomed 2020;10(10):433.
- Sornalakshmi V, Tresina Soris P, Paulpriya K, Packia Lincy M, Mohan VR. Oral glucose tolerance test (OGTT) in normal control and glucose induced hyperglycemic rats with Hedyotis leschenaultiana DC. Int J Toxicol Pharmacol Res 2016;8:59-62.
- Anitha M, Sakthidevi G, Muthukumarasamy S, Mohan VR. Effect of Cynoglossum zeylanicum (Vehl ex Hornem) Thunb. ex Lehm on oral glucose tolerance in rats. J Appl Pharm Sci 2012;2(11):75-8.
- Chaimum-aom N, Chomko S, Talubmook C. Toxicology and oral glucose tolerance test (OGTT) of Thai medicinal plant used for diabetes controls, Phyllanthus acidus L. (Euphorbiaceae). Pharmacogn J 2017;9(1).
- El-Demerdash FM, Yousef MI, Abou El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol 2005;43(1):57-63.
[Crossref] [Google Scholar] [PubMed]
- Arulselvan P, Subramanian SP. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra-structural changes of pancreatic β-cells in experimental diabetes in rats. Chem Biol Interact 2007;165(2):155-64.
[Crossref] [Google Scholar] [PubMed]
- Yazdanparast R, Ardestani A. In vitro antioxidant and free radical scavenging activity of Cyperus rotundus. J Med Food 2007;10(4):667-74.
[Crossref] [Google Scholar] [PubMed]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 2018;54(4):287-93.
- Singh V, Singh SP, Singh M, Kumar A. Evaluation of antioxidant, hypoglycemic and hypolipidemic effects of the phytoconstituents of Cinnamomum tamala in rats. Indian J Pharm Sci 2018;80(1):161-72.
- Krishnakumar K, Augusti KT, Vijaymmal PL. Hypoglycaemic and anti-oxidant activity of Salacia oblongaWall. Extract in Streptezotocin-induced diabetic rats. Indian J Physiol Pharmacol 1999;43(4):510-4.
[Google Scholar] [PubMed]
- Bamagous GA, Al Ghamdi SS, Ibrahim IA, Mahfoz AM, Afify MA, Alsugoor MH, et al. Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators. Asian Pac J Trop Biomed 2018;8(6):320.
- Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, et al. Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. North Am J Med Sci 2013;5(3):213.
[Crossref] [Google Scholar] [PubMed]
- Krishnakumar K, Augusti KT, Vijayammal PL. Anti-peroxidative and hypoglycaemic activity of Salacia oblonga extract in diabetic rats. Pharm Biol 2000;38(2):101-5.
[Crossref] [Google Scholar] [PubMed]
- Rehman K, Rashid U, Jabeen K, Akash MS. Morin attenuates L-arginine induced acute pancreatitis in rats by down regulating myeloperoxidase and lipid peroxidation. Asian Pac J Trop Biomed 2021;11(4):148-54.
- Al-Janabi OS, Amer MS, Khayri MH. Effects of the extracts of olive and Morus alba leaves on experimentally STZ induced diabetes in male rats. Int J Sci Res 2013;4(3):1526-32.
- Margaret AO, Stephen AO, Meshack IO, Sunday AA, Abidemi O. Histological studies of pancreatic β-cells of streptozotocin induced diabetic Wistar rats treated with methanolic extract of Sphenocentrum jollyanum. J Pharm Innov 2013;2(2);8-12.
- Deepak KG, Challa S, Suhasin G, Nagesewara Rao Reddy N, Elansary HO, El-Ansary DO. Antidiabetic and antilipidemic activity of root extracts of Salacia oblonga against streptozotocin-induced diabetes in Wistar rats. Processes 2020;8(3):301-12.
- Ahmad Z, Zamhuri KF, Yaacob A, Siong CH, Selvarajah M, Ismail A, et al. In vitro anti-diabetic activities and chemical analysis of polypeptide-k and oil isolated from seeds of Momordica charantia (bitter gourd). Molecules 2012;17(8):9631-40.
- Melappa G, Channabasava R, Chandrappa CP, Sadananda TS. In vitro antidiabetic activity of three fractions of methanol extracts of Loranthus micranthus, identification of phytoconstituents by GC-MS and possible mechanism identified by GEMDOCK method. Asian J Biomed Pharm Sci 2014;4(34):34.
- Mazumder K, Nabila A, Aktar A, Farahnaky A. Bioactive variability and in vitro and in vivo antioxidant activity of unprocessed and processed flour of nine cultivars of Australian lupin species: A comprehensive substantiation. Antioxidants 2020;9(4):282-304.
- Mitri J, Tomah S, Furtado J, Tasabehji MW, Hamdy O. Plasma free fatty acids and metabolic effect in type 2 diabetes, an ancillary study from a randomized clinical trial. Nutrients 2021;13(4):1145-53.
[Crossref] [Google Scholar] [PubMed]
- Agada R, Thagriki D, Lydia DE, Khusro A, Alkahtani J, Al Shaqha MM, et al. Antioxidant and anti-diabetic activities of bioactive fractions of Carica papaya seeds extract. J King Saud Univ Sci 2021;33(2):101342.