- Corresponding Author:
- M. C. Sidhu
Department of Botany, Panjab University, Chandigarh-160 014, India
E-mail: mcsidhu@gmail.com
| Date of Submission | 09 August 2013 |
| Date of Revision | 21 June 2014 |
| Date of Acceptance | 25 June 2014 |
| Indian J Pharm Sci 2014; 76(4): 323-331 |
Abstract
The petroleum ether leaf extract of Ficus krishnae has been evaluated for the management of diabetes in alloxan induced diabetic rats. Phytochemical screening of the leaf extract for various chemical compounds has also been carried out. Leaf extract was administered continuously for 21 days orally at a dose of 200 mg/kg. Along with this, the blood glucose level was monitored at regular intervals to understand the activity of the extract. The leaf extract has decreased the blood glucose level of diabetic rats which was comparable to an antidiabetic standard drug, glibenclamide, given at a dose of 2.5 mg/kg. It has been observed that the leaves of Ficus krishnae possess antidiabetic activity and it reduces the blood glucose level significantly. The phytochemical screening of leaf has revealed the presence of carbohydrates, flavonoids, tannins, glycosides, terpenoids, steroids, alkaloids, gums and mucilage, phlobatannins, reducing sugars and phenolic compounds. The Fourier Transform Infrared analysis of glibenclamide and leaf powder has displayed some common absorption spectra. This shows that leaf powder has a molecule which is close to glibenclamide. Wavelength Dispersive X-Ray Fluorescence spectroscopy have shown the presence of cellulose, Ca, Si, K, Cl, Mg, P, S, Al, Fe, Na, Sr, Pd, Zn, Mn, Cr, Mo, Br, Ni, Rb and Zr. It is assumed that these elements alongwith other chemical compounds of the plant species may play a role in the management of diabetes. The Raman Specta of both glibenclamide and leaf powder has also shown some similarities. The results obtained during the present investigation have revealed the antidiabetic activity of Ficus krishnae leaves. The phytochemical screening has indicated the various chemical constituents likely to be responsible for this activity. The Fourier Transform Infrared, Wavelength Dispersive X-Ray Fluorescence and Raman Specta of the leaf powder suggested that there is some glibenclamide like molecule or its derivatives which is imparting antidiabetic activity.
Keywords
Diabetes, Ficus krishnae, glibenclamide, phytochemicals, FTIR, WD-XRF, Raman spectroscopy
The plants have been used to treat different health related troubles in many countries of the world since time immemorial [1]. It has also been reported that the continuous use of synthetic drugs has increased the health related problems because of their side effects [2]. Due to this, plant based medicines are becoming popular throughout the world. Ficus is one of the largest genus with 800 species occurring throughout the world, ranked twenty-first in angiosperms [3,4]. One hundred and fifteen species are distributed all over India and nearly 43 species in Meghalaya alone [5].
Ficus krishnae L. is also known as Krishna fig or Krishna?s butter cup in English and Makkhan Katori in Hindi. It is mainly found in India, tropical Africa and Sri Lanka [6]. It is 10 m in height, fast growing tree with spreading branches and aerial roots. Leaves are simple, dorsiventral with acute apex, entire margin and reticulate venation. Upper surface of the leaf is dark green while lower surface is light green in colour. The unique feature of the tree is that the leaves have a pocket like fold at the base. It has been used in number of folklore medicines. Various plant parts are used to treat ulcers, vomiting, fever, inflammations and leprosy. The plant is also used as as aphrodisiac, as a tonic, in piles and gonorrhoea. Stem bark and leaves are useful in diabetes. The aerial roots are styptic, useful in syphilis, biliousness, dysentery, inflammation of liver [7,8]. This shows the medicinal importance of this species. Based on this, it has been selected for present investigation.
Different phytochemicals present in plants are like steroids, terpenoids, carotenoids, flavonoids, alkaloids, tannins, glycosides [9]. Phytochemicals are chemical compounds that occur abundantly in different plant communities. The term phytochemicals refers to a wide variety of compounds, produced in different parts of the plant species, affecting human health but are not essential nutrients. The researchers have estimated that there are approximately 10,000 different phytochemicals having potential to manage different health related problems [10,11]. Different chemical constituents can be detected by performing various preliminary tests. Further, Fourier Transform Infrared (FTIR) spectroscopy analysis providing details of various functional groups present in the sample, can confirm the preliminary detected phytochemicals. The Wavelength Dispersive X-Ray Fluorescence (WD-XRF) study can revealed the presence of different macro and micro elements present in material under investigation. By doing so, one can find out the number of various chemical compounds present in the sample. After this, these compounds can be separated out through different techniques and the effect of each component can be accessed.
The leaves of Ficus krishnae have been used to study its antidiabetic activity in male albino wistar rats. The plant material was tested for the presence of various chemical compounds so as to find out the actual component responsible for this activity. The phytochemical related observations have been supported by FTIR spectroscopy. The material has also been studied for the presence of micro and macro elements using WD-XRF spectroscopy. Further studies can be conducted to compare the chemical properties of standard antidiabetic drug with the compounds present in the leaf powder of the plant so that it can be recommended for its commercial use by the pharmaceutical industry if it is at par or superior than the available standard medicines.
Materials and Methods
Male Albino Wistar rats (250-300 g) were used in the experiment. They were obtained from the Central Animal House of Panjab University, Chandigarh. The experimental work was approved by the Institutional Animal Ethics Committee (IAEC) of Panjab University, Chandigarh. Animals were kept in clean and well-aerated cages in the animal house. The animals had free access to feed and drinking water.
Collection of plant material
The leaves of Ficus krishnae L. were collected from the Botanical garden of Panjab University, Chandigarh during 2011-2012. Leaves were washed properly with tap water and then with distilled water. Leaves were shade dried and powdered using electric grinder. The powder was then used for extraction process. Herbarium of plant specimen has been deposited in the Herbarium of Botany Department (PAN No.- 20233), Panjab University, Chandigarh.
Preparation of leaf extract
The fresh leaves of Ficus krishnae L. were washed thoroughly first with tap water and then in distilled water. Leaves were shade dried at room temperature for almost a week or till they were completely dried. The dried leaves thus obtained were powdered using an electronic grinder. One hundred and twenty grams of powder was extracted with 1 l of petroleum ether as the solvent. Extraction was carried out in Soxhlet apparatus at a temperature ranging between 60-80°. The extract was then concentrated in rotary evaporator at 80° which yield a semi-solid mass. This mass was then dried in an oven at 40° and later used in the experiment.
Induction of diabetes in test animals
The diabetes was induced in fasted rats by intraperitoneal injection of alloxan monohydrate. In nut shell, during fasting period, the rats were allowed to free access of water. After 18 h of fasting, a freshly prepared single dose of alloxan (120 mg/kg) dissolved in 0.4 ml of saline was given to each one of them. After 1 h, the rats were provided with feed and drinking water ad libitum. The blood glucose level was checked after 48 h of the injection of alloxan monohydrate. Only those animals with blood glucose level beyond 200 mg/dl of blood were used in the experiment.
Experimental design
A total of 20 rats were taken (15 diabetic+5 normal) for the experiment. They were divided into four groups. Group-I having normal control, untreated rats, fed with normal diet, Group-II contain diabetic control rats, also fed with normal diet, Group-III composed of diabetic rats were given standard drug, glibenclamide, orally at a dose of 2.5 mg/kg, dissolved in 2% v/v Tween 80 solution and Group-IV diabetic rats were given petroleum ether extract of Ficus krishnae leaves orally at a dose of 200 mg/kg, dissolved in 2 ml/mg olive oil.
Treatment with respect to group III and IV was conducted for 21 days. In our pilot studies, it was observed that the vehicles (i.e. olive oil and Tween 80) did not show any significant change in blood glucose levels as compared to the normal or the control groups. Therefore, special control groups fed only with vehicle were not maintained in any of the experiments.
Testing of blood glucose level
The blood glucose level of each animal of all four groups was monitored on 0, 7, 14 and 21 days. Blood samples were collected from the retro-orbital vein in test-tubes under mild anaesthesia. All these samples were centrifuged at 1500-2000 rpm for 10 min which separate serum from the whole blood. Serum was pipetted out which was further used to estimate the blood glucose levels with a spectrophotometric method.
Statistical analysis
Data were represented as mean±standard error mean (n=5). Data was analysed by one way analysis of variance (ANOVA) followed by post hoc test P values less than 0.05 were considered as significant.
Phytochemical evaluation
Different chemical compounds like alkaloids, carbohydrates, flavonoids, glycosides, gums and mucilage, phenols, phlobatannins, reducing sugars, saponins, steroids, tannins and terpenoids have been determined from the leaf extract of the plant under investigation using different tests [9,12-18].
Fourier transform infrared (FTIR) analysis
FTIR analysis was done in Perkin Elmer Spectrum 400 FTIR/FTFIR spectrometer. Leaf powder was used for the detection of various functional groups. This test was conducted at Sophisticated Analytical Instrumentation Facility (SAIF), Central Instrumentation Laboratory (CIL) and University Centre for Instrumentation and Microelectronics (UCIM), Panjab University, Chandigarh.
Raman spectroscopy
Glibenclamide tablet, petroleum ether leaf extract and raw leaf powder of Ficus krishnae and pure petroleum ether were used without further purification. Raman spectra were recorded on a JY Horiba iHR550 spectrograph, equipped with gratings of 1800 lines/mm and peltier cooled CCD detector. Measurements were carried out in 90o geometry with the incident light linearly polarized and scattered light was detected unpolarized. The excitation line at 488·0 nm was provided by air cooled Ar+ laser. Power of incident light was 40 mW. Twenty scans, each of 15 sec duration, in the wavenumber region 1800- 100 cm-1 were added together to increase signal to noise ratio. Due to fluorescent, we have removed the fluorescence background by fitting it with a non-linear curve and subtracting the fitted background from the recorded Raman spectra.
Wave dispersive X-Ray fluorescence (WD-XRF) analysis
WD-XRF analysis was done in WD-XRF Tiger S8 for the detection of various macro and micro elements present in leaf powder of Ficus krishnae. This test was performed at SAIF, CIL and UCIM, Panjab University, Chandigarh.
Results and Discussion
In the present study, leaf extract of Ficus krishnae was prepared in petroleum ether. The extract thus obtained was used for its phytochemical analysis and finally for its antidiabetic activity.
The antidiabetic activity of the petroleum ether leaf extract of the Ficus krishnae was studied in alloxaninduced diabetic male albino wistar rats. Diabetes was induced in 15 rats and they were divided into three groups i.e. group II, III and IV. The group I acted as normal control, which did not receive any special treatment. Group II served as diabetic control while III and IV groups of diabetic rats treated with antidiabetic drug, glibenclamide and leaf extract of Ficus krishnae, respectively. The leaf extract was given orally to diabetic rats of group IV at the dose of 200 mg/kg continuously for 21 days. Treatment of petroleum ether leaf extract showed a significant reduction in the blood glucose level as compared to group II. The antidiabetic drug, glibenclamide was also given orally to group III diabetic rats at the dose of 2.5 mg/kg up to 21 days. This group acted as positive control. The variation in blood glucose level in normal and experimental rats on 0, 7, 14 and 21 days of treatment has also been recorded. In normal control, blood glucose level reading was 110±5.98, 106±7.98, 93.1±7.61 and 87.8±8.08 on 0, 7, 14 and 21 days, respectively. In diabetic control, initial blood glucose level reading was 566±64.7. The blood glucose level was decreased to 537±31.9, 451±43.8 and 310±12.6 on 7, 14 and 21 days, respectively. The glibenclamide showed blood glucose levels of 552±34.3, 252±38.7, 229±63.9 and 98.3±20.3 at dose of 2.5 mg/kg whereas leaf extract showed blood glucose levels of 575±59.6, 386±71.1, 223±61·6 and 98.5±20.3 at dose of 200 mg/kg at 0, 7th, 14th and 21 days, respectively (Table 1). The leaf extract of Ficus krishnae showed similar reduction in blood glucose level to that of antidiabetic drug after 21 days. Hence, present study showed a good antidiabetic response of leaf extract against the experimental animals.
| Groups* | Initial | 7th day | 14th day | 21st day |
|---|---|---|---|---|
| Normal controla1 | 110±5·98 | 106±7·98 | 93·1±7·61 | 87·8±8·08 |
| Diabetic controla2 | 566±64·7a | 537±31·9a | 451±43·8a | 310±12·6a |
| Diabetic control+ antidiabetic drug |
552±34·3a | 252±38·7a,b | 229±63·9b | 98·3±20·3b |
| Diabetic control+ plant extract |
575±59·6a | 386±71·1a,b | 223±61·6b | 98·5±20·3b |
*Blood glucose is expressed as mg/dl and each value is a mean±standard error of the mean of n=5; aP=0·05 compared to normal control and bP=0·05 compared to diabetic control
Table 1: Blood Glucose Levels
Ficus krishnae is used in traditional plant remedies for diabetes but detailed experiments have not been carried out for its antidiabetic activity. Another research group had studied the antidiabetic activity of leaf extract of Ficus krishnae in alloxan-induced diabetic rats. The experiment was conducted for 14 days. They treated diabetic rats with leaf extract at the dose of 200 and 400 mg/kg. Leaf extract at a dose of 400 mg/kg (77±3.28) was more effective in reducing the blood glucose level than a dose of 200 mg/kg (99±2.45). The effect of 400 mg/kg dose was equivalent to the standard drug, glibenclamide given at a dose of 2·5 mg/kg (70.17±2.45) for the same time [19]. We have conducted the experiment for 21 days and there was a significant reduction in blood glucose level with a dose of 200 mg/kg. This reduction was similar to an antidiabetic drug, glibenclamide given for same period. This shows that petroleum ether leaf extract of Ficus krishnae is antidiabetic in nature.
Antidiabetic activity of leaf and fruit extracts of Ficus religiosa had also been studied earlier [20]. The administration of fruit extract (100 and 250 mg/kg) decreased the blood sugar level but maximum effect was observed at 250 mg/kg. But presently studied Ficus krishnae leaf extract showed similar activity at a dose of 200 mg/kg. This indicates that Ficus krishnae leaf extract is more effective than fruit extract of Ficus religiosa.
The blood glucose level of groups given leaf extract and an antidiabetic drug, glibenclamide were determined separately to compare the effect of both. The leaf extract has antidiabetic activity comparable to standard drug. Hence it is assumed that leaf extract contains some chemical compound having glibenclamide like activity. Ficus nervosa ethanolic leaf was tested for its antidiabetic activity against alloxan-induced diabetic rats and found to be less effective as compared to glibenclamide drug [21]. These findings are different from our observations which may be due to difference in the chemical properties of two Ficus species.
Phytochemical analysis of petroleum ether extract of leaves of Ficus krishnae has revealed the presence of carbohydrates, flavonoids, glycosides, phenolic compounds, gums and mucilage, tannins and terpenoids. It is assumed that any of these chemical compounds individually or in combination with others may be responsible for antidiabetic activity of this species. Whereas, the aqueous and ethanol extracts of the same sample contains alkaloids, carbohydrates, flavonoids, glycosides, gums and mucilage, phenolic compounds, phlobotannins, reducing sugars, steroids, tannins and terpenoids. The variation in phytochemicals obtained from three extracts may be due to difference in the nature of the solvents (Table 2). The flavonoids obtained from the ethanolic extract of Geniosporum prostratum were found to be responsible for antidiabetic activity [22]. The antidiabetic activity and phytochemical screening of roots of Cayratia trifolia was conducted and flavonoids were said to be responsible for this activity [23]. Flavonoids have also been reported during present investigation in the leaves of Ficus krishnae. This shows that the antidiabetic activity of Ficus krishnae leaf extract is likely to be because of flavonoids.
| Phytochemicals | Extracts | ||
|---|---|---|---|
| Petroleum ether | Aqueous | Ethanol | |
| Alkaloids | - | - | + |
| Carbohydrates | + | + | + |
| Flavonoids | + | + | + |
| Glycosides | + | + | + |
| Gums and Mucilage | traces± | + | traces± |
| Phenolic compounds | + | + | + |
| Phlobotannins | - | + | + |
| Reducing sugars | - | + | + |
| Saponins | - | - | - |
| Steroids | - | - | + |
| Tannins | + | + | + |
| Terpenoids | + | + | + |
Table 2: Phytochemical Screening of Leaf In Different Solvents
The water and 80% methanol leaf extracts of Ficus krishnae contained various phytoconstituents like alkaloids, cardiac glycosides, flavonoids, tannins and carbohydrates [24]. But during present investigation steroids, terpenoids, reducing sugars, phlobotannins, gums and mucilage and phenolic compounds have been found additionally. This may be due to the difference in organic solvent used for extraction. The phytochemical study of leaf and bark (mixture) extract of Ficus infectoria in petroleum ether, chloroform, ethyl acetate, methanol and ethanol:water revealed the presence of carbohydrates, glycosides, alkaloids, proteins, amino acids, phytosterols, tannins and flavonoids [25]. Some of these phytochemicals are common with Ficus krishnae.
The phytochemical screening of Ficus carica leaf extracts in petroleum ether, ethyl acetate, ethanol and aqueous solvents showed the presence of carbohydrates, coumarins, steroids and sterols, flavonoids, fixed oils, proteins and amino acids, glycosides and triterpenoids [26]. Some of these compounds are similar to Ficus krishnae. The presence of some common chemical compounds in different species of Ficus indicates their closeness at the biochemical level. Hence any of these common phytochemical can be obtained from the species which is present in abundance. By doing so, the need of plant species for various medical formulations can be met without harming the floristic diversity. This will ultimately helps in the conservation of plant diversity.
We investigated the antidiabetic potential of petroleum ether extract of Ficus krishnae leaf considering the reviewed literature. However, we also investigated the presence of different phytochemicals in comparatively more polar solvents like aqueous and ethanol. It would be of interest in our future studies to investigate their antidiabetic potentials since there have been some differences in the constituents.
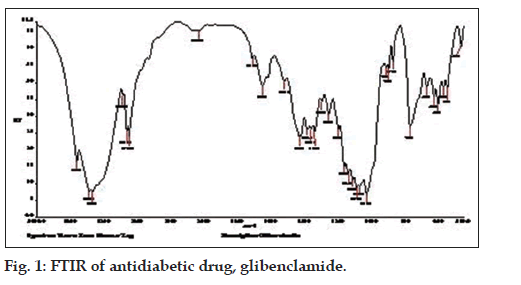
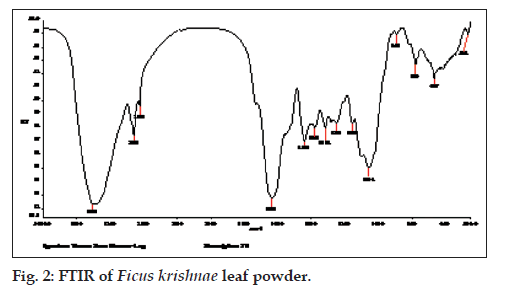
The Fourier Transform Infrared spectroscopy of an antidiabetic drug, glibenclamide and leaf powder of the Ficus krishnae has been carried out to find out the various functional groups. The FTIR absorption spectra of glibenclamide and leaf powder have been presented in figs. 1 and 2, respectively. The functionalities present in these respective samples have been compared with the values reported in the literature [27] (Table 3). The details of the various functionalities of the leaf powder are also listed (Table 4).
| IR frequencies-(Glibenclamide) corresponds to |
IR frequencies (leaf powder) |
Literature IR frequencies range for the respective functionality |
|
|---|---|---|---|
| 2931 cm-1, | C-H stretching | 2921 cm-1, | 2960-2850 cm-1 |
| 2900 cm-1 | 2851 cm-1 | ||
| 1653 cm-1 | C=O stretching (amides) | 1635 cm-1 | 1650-1620 cm-1 |
| 1523 cm-1 | N-H deformation | - | 1550-1510 cm-1 |
| 1430 cm-1 | C-H deformation in CH2 | 1441 cm-1 | 1450-1425 cm-1 |
| 1363 cm-1, | Asymmetric SO2 | - | 1370-1330 cm-1 |
| 1342 cm-1 | stretching | ||
| 1259 cm-1, | C-O stretching | 1251 cm-1, | 1270-1200 cm-1, |
| 1201 cm-1, | (C=C-O-C) | 1056 cm-1 | 1070-1020 cm-1 |
| 1074 cm-1 | |||
| 1165 cm-1 | Symmetric SO2 stretching | 1158 cm-1 | 1180-1150 cm-1 |
| 900 cm-1 | S-N stretching | 893 cm-1 | 910-900 cm-1 |
| 774 cm-1 | C-Cl stretching | 667 cm-1 | 800-600 cm-1 |
IR: Infrared
Table 3: Comparative Ir Frequencies of Glibenclmide and Leaf Powder
| Mode of vibration (functionality) | Range | IR Freq. |
|---|---|---|
| N-H stretch in amines | 3500-3300 cm-1 | 3411 cm-1 |
| Alkanes sp3 C-H stretch | 3000-2840 cm-1 | 2921 cm-1 |
| I) Alkanes sp3 C-H Stretch | one at | 2851 cm-1 |
| II) Stretch, aldehyde | 2860-2800 cm-1 | |
| hydrogen (-CHO) consists of a pair | other at | |
| of weak bonds | 2760-2700 cm-1 | |
| I) C=C stretch | 1660-1600 cm-1 | 1635cm-1 |
| II) C=O stretch in amides | 1680-1630 cm-1 | |
| III) N-H bond in 1° amines | 1640-1560 cm-1 | |
| -CH3 bond in alkanes | 1450 cm-1 | 1441 cm-1 |
| I) C-F stretch | 1400-1000 cm-1 | 1384 cm-1 |
| II) Nitro group has a strong absorption | 1390-1300 cm-1 | |
| I) C-N stretch in amines | 1350-1000 cm-1 | 1318 cm-1 |
| II) Asymmetric S=O stretch in | 1350-1140 cm-1 | |
| Sulphones, Sulphonyl chlorides, | ||
| Sulphates, Sulphonamides | ||
| I) C=O bending in ketones appears | 1300-1100 cm-1 | 1251 cm-1 |
| as a medium intensity peak | ||
| II) Phenyl alkyl ethers give two | 1250-1040 cm-1 | |
| strong bonds | ||
| III) C-O stretch in alcohols, ethers, | 1300-1000 cm-1 | |
| esters, carboxylic acids, anhydrides | ||
| IV) C-N stretch in amines | 1350-1000 cm-1 | |
| V) Aryl fluorides absorb | 1250 and | |
| 1100 cm-1 | ||
| I) C-N stretch in amines | 1350-1000 cm-1 | 1158 cm-1, |
| II) C-O stretch in alcohols, ethers, | 1300-1000 cm-1 | 1056 cm-1 |
| esters | ||
| III) S=O symmetric stretch, strong | 1150 cm-1 | |
| I) Alkenes out of plane bonds | 1000-650 cm-1 | 893 cm-1 |
| II) Aromatics out of plane | 900-690 cm-1 | |
| I) Alkenes out of plane bonds | 1000-650 cm-1 | 780 cm-1 |
| II) Aromatics out of plane bond | 900-690 cm-1 | |
| III) C-Cl stretch in aliphatic chlorides | 785-540 cm-1 | |
| I) Alkenes out of plane bonds | 1000-650 cm-1 | 667 cm-1 |
| II) C-Cl stretch in aliphatic chlorides | 785-540 cm-1 | |
| C-X for bromides/iodide | <667 cm-1 | 466 cm-1 |
IR Freq: Infrared frequencies
Table 4: Fourier Transform Infrared Analysis of Leaf Powder
The FTIR analysis of leaf powder of Ficus krishnae have revealed the presence of different peaks pertaining to various functional groups. The peaks at 2921 cm-1 and 2851 cm-1, 1635 cm-1, 1441 cm-1, 1251 cm-1 and 1056 cm-1, 1158 cm-1, 893 cm-1 and 667 cm-1 pertaining to C-H stretching, C=O stretching (amides), C-H deformation in CH2, C-O stretching (C=C-O-C), symmetric SO2 stretching, S-N stretching and C-Cl stretching, respectively. These functionalities are very much present in the structure of an antidiabetic drug, glibenclamide. Recently, another research group has studied the FTIR peaks for pure glibenclamide drug. Their findings for glibenclimide were 3354.32 cm-1, 1652.52 cm-1, 1628.12 cm-1, 1161·19 cm-1, 1035·31 cm-1, 1591·34 cm-1, 2974·54 cm-1, 2920·14 cm-1, 1332·86 cm-1 and 666·68 cm-1 pertaining to NH stretching, C=O stretching, NH bending, CN stretching (aliphatic), C=C (aromatic), -CH3 stretching, C-H stretching (aliphatic), S=O stretching and C-H bending, respectively which are close to our findings for leaf powder of Ficus krishnae [28]. This shows that Ficus krishnae leaf powder contains some chemical compound which is close to glibenclamide molecule or its derivatives.
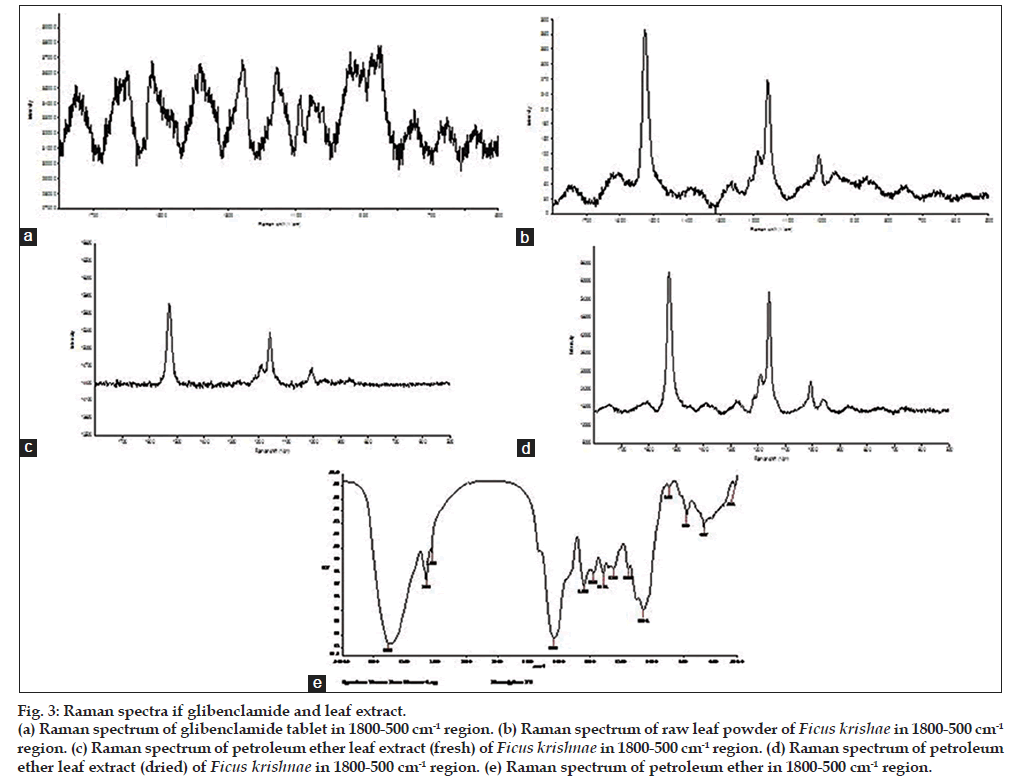
Observed spectra of glibenclamide tablet, petroleum ether leaf extract, raw leaf powder of Ficus krishnae and pure petroleum ether are shown in fig. 3a-e. Positions of all the observed bands along with their assignments are listed in Table 5. Raman band at 1745 cm-1 in the spectrum of glibenclamide tablet (fig. 3a) can be assigned to the amide carbonyl moiety. In this vibrational mode, in-plane N-H bending of amide group takes place. C=O stretching of carbonyl groups are responsible for the observed Raman bands at 1600 cm-1 and 1619 cm-1. Raman bands at 1384 cm-1 and 1157 cm-1 can be assigned to the sulphonyl stretching vibrations. Bands at 1053 cm-1 and 1087 cm-1 arise due to C-O stretching vibrations. C-H bending vibration gives rise to a shoulder at 1355 cm-1. C=C stretching mode appears at 1472 cm-1. Band at 653 cm-1 can be assigned to the stretching vibration of C-Cl group. We observe a number of bands at 753 cm-1, 859 cm-1, 874 cm-1, 903 cm-1, 923 cm-1, 939 cm-1 and 1023 cm-1 in the =C-H bending region [29].
Figure 3: Raman spectra if glibenclamide and leaf extract.
(a) Raman spectrum of glibenclamide tablet in 1800-500 cm-1 region. (b) Raman spectrum of raw leaf powder of Ficus krishae in 1800-500 cm-1
region. (c) Raman spectrum of petroleum ether leaf extract (fresh) of Ficus krishnae in 1800-500 cm-1 region. (d) Raman spectrum of petroleum
ether leaf extract (dried) of Ficus krishnae in 1800-500 cm-1 region. (e) Raman spectrum of petroleum ether in 1800-500 cm-1 region.
| Raman shift (cm-1) | Assignment | ||||
|---|---|---|---|---|---|
| Glibenclamide tablet |
Raw powder |
Extract | Petroleum ether |
||
| Fresh | Dried | ||||
| 1745 | 1744 | 1746 | N-H bending | ||
| 1619 | 1624 | C=O stretching | |||
| 1600 | 1605 | 1612 | C=O stretching | ||
| 1525 | 1525 | 1528 | 1525 | C=C stretching | |
| 1472 | C=C stretching | ||||
| 1456 | |||||
| 1384 | 1378 | 1382 | Sulphonyl stretching | ||
| 1355 | C-H bending | ||||
| 1341 | |||||
| 1305 | |||||
| 1271 | 1277 | ||||
| 1257 | C-H bending | ||||
| 1214 | 1215 | 1213 | |||
| 1188 | 1192 | 1190 | |||
| 1174 | |||||
| 1157 | 1158 | 1159 | 1159 | Sulphonyl stretching | |
| 1145 | |||||
| 1127 | C-O stretching | ||||
| 1087 | 1081 | C-H bending | |||
| 1068 | |||||
| 1053 | C-O stretching | ||||
| 1040 | |||||
| 1023 | 1018 | C-H bending | |||
| 1007 | 1006 | 1006 | 1006 | ||
| 958 | 957 | 958 | |||
| 939 | C-H bending | ||||
| 923 | C-H bending | ||||
| 903 | C-H bending | ||||
| 891 | |||||
| 874 | 867 | 867 | 865 | 868 | C-H bending |
| 859 | C-H bending | ||||
| 814 | |||||
| 800 | |||||
| 785 | |||||
| 753 | 751 | 750 | 748 | C-H bending | |
| 733 | 733 | ||||
| 711 | |||||
| 653 | 654 | C-Cl stretching | |||
| 610 | |||||
| 566 | 564 | ||||
Table 5: Raman Bands Obtained For Different Materials
Various Raman bands of raw powder and petroleum ether extract of leaf powder of Ficus krishnae match well with each other and some of the bands of tablet.
For example bands at 1745 cm-1, 1619 cm-1, 1600 cm-1, 1525 cm-1, 1384 cm-1 and 1157 cm-1 in tablet spectrum are also present in the spectra of raw powder and petroleum ether extract of leaf powder suggesting thereby the presence of glibenclamide like molecule in the leaf of Ficus krishnae. One may argue that the spectra of extract show bands due to petroleum ether which can match with the spectrum of glibenclamide. In order to rule out the possibility of the above matching bands arising out of petroleum ether, we have also recorded Raman spectra of petroleum ether (fig. 3e). It can be concluded that only the band at 867 cm-1 of extract matches with the petroleum ether band at 868 cm-1. Therefore, on the basis of matching of Raman bands of glibenclamide and leaf powder/extract, we may infer the presence of glibenclamide like molecules or its derivative in leaf of Ficus krishnae.
The WD-XRF is one of the best techniques for simultaneous analysis of the major and minor elements in the sample. The leaf powder of Ficus krishnae has been analysed using the WD-XRF technique. The WD-XRF analysis has revealed the presence of Na, Mg, Al, Si, Cl, K, P, S, Ca Mn, Fe, Ni, Cu, Zn, Br, Rb, Sr, Mo and Zr elements (Table 6). For determining the concentrations of the elements, the matrix of the sample was taken to be that of cellulose (C6H10O5). Nineteen elements, viz., K, S, Cl, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Br, Rb, Sr and Pb were found in the leaves and roots of Catharanthus roseus, Solenostemon monostachyus, Phyllanthus amarus and Boerhavia diffusa using Energy Dispersive XRF (ED-XRF) technique [30]. Out of these, the Cr, V, Zn and Mn elements are known to possess antidiabetic activity. Two elements, Zn and Mn, are also observed in presently studied leaf powder of Ficus krishnae. Similarily Mg, Cr, Mn, Fe, Ni, Cu, Zn, Sr, Mo and Pb were observed in five antidiabetic medicinal plants, viz., Bidens pilosa, Erythrina abyssinica, Aspilia pluriseta, Strychnos henningii and Catta edulis through ED-XRF and Atomic Absorption Spectroscopy (AAS) techniques [31]. The Mg, Fe and Zn elements are also present in Ficus krishnae leaf powder. This strengthens the claim of this species as antidiabetic.
| Element | Concentration? (ppm) |
|---|---|
| 11Na | 140 |
| 12Mg | 4150 |
| 13Al | 350 |
| 14Si | 24280 |
| 15P | 1380 |
| 16S | 1290 |
| 17Cl | 6910 |
| 18K | 11500 |
| 20Ca | 37430 |
| 25Mn | 20 |
| 26Fe | 180 |
| 28Ni | 10 |
| 29Cu | 15 |
| 30Zn | 22 |
| 35Br | 11 |
| 37Rb | 6 |
| 38Sr | 130 |
| 40Zr | 4 |
| 42Mo | 12 |
?The error in concentration values of the major elements is~5% and that of the minor elements it increases up to 20% ppm
Table 6: Levels of Elements Determined In Ficus Krishnae Using Wd-Xrf
Some elements play significant role in the management of diabetes. Zn is one of such element which is an extremely important part of insulin [32]. Zn is known to assist in the regulation of insulin level in the blood [33]. Mg plays an important role in the release of insulin and maintenance of the pancreatic beta cells [34]. Mn is an essential micronutrient for plants and animals and its deficiency may cause diabetes, nervous instability, convulsions [35]. This shows that Zn, Mg and Mn play a role in the management of diabetes. Presently studied plant Ficus krishnae material possesses these three elements. Therefore, we conclude that these elements along with other chemical compounds of the plant powder are likely to be responsible for antidiabetic activity of this species.
Acknowledgements
The authors are thankful to Professor Ahluwalia, Chairperson, Department of Botany, Panjab University, Chandigarh for providing necessary facilities during this inveatigation. We are also grateful to Professor Ashwani Koul, Department of Biophysics, Professor Saini and Professor Devinder Mehta, Department of Physics, Dr. Aman Bhalla, Department of Chemistry, Panjab University, Chandigarh for their valuable guidance and scientific suggestions.
References
- Hoareau L, Dasilva EJ. Medicinal plants: A re-emerging health aid. Elec J Biotech 1999;2:56-70.
- Ghule ST, Patil DK. Development of medicinal plants. Kisan World 2001;28:33-4.
- Frodin DG. History and concept of big plant genera. Taxon 2004;53:753-76.
- Adebayo EA, Ishola OR, Taiwo OS, Majolagbe ON, Adekeye BT. Evaluations of the methanol extract of Ficus exasperata stem bark, leaf and root for phytochemical analysis and antimicrobial activities. Afr J Plant Sci 2009;3:283-7.
- Chaudhary LB, Sudhakar JV, Kumar A, Bajpai O, Tiwari R, Murthy GV. Synopsis of the genus Ficus L. (Moraceae) in India. Taiwania 2012;7:193-216.
- Biswas K. Observations on the systematic position of Ficus krishnae. Curr Sci 1934;3:424-7.
- Kirtikar KR, Basu BD. Indian medicinal plants. Vol. 3. Dehradun India: International Book Distributors; 2005.
- Chetty MK, Sivaji K, Rao TK. Flowering plants of chittoor district. 2nd ed. Tirupati: Students Offset Printers; 2008.
- Ajayi IA, Ajibade O, Oderinde RA. Preliminary phytochemical analysis of some plant seeds. Res J Chem Sci 2011;1:58-62.
- Ganatra SH, Durge SP, Patil SU. Preliminary phytochemicals investigation and TLC analysis of Ficus racemosa leaves. J Chem Pharma Res 2012;4:2380-4.
- Dhandapani R, Sabna B. Phytochemical constituents of some medicinal plants. Anc Sci Life 2008;37:1-7.
- Evans WC. Trease and Evans Pharmacognosy. 15th ed. London: W. B. Sounders and Company; 2002.
- Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. London (NY): Chapman and Hall Std; 1998.
- Idu M, Igeleke CL. Antimicrobial activity and Phytochemistry of Khaya senegalensis roots. Intern J Ayur Herb Med 2012;2:415-22.
- Kokate CK. Practical Pharmacognosy. Delhi, India: Vallabh Prakashan; 1994.
- Sofowora A. Medicinal plants and Traditional medicine in Africa. Ibadan: Spectrum Books Ltd; 1993.
- Trease GE, Evans WC. Pharmacognosy. 12th ed. Eastbourne, UK: Balliere Tindall; 1983.
- Trease GE, Evans WC. Pharmacognosy. 13th ed. London: ELBS; 1989.
- Lakshmi SM, Kumar AS, Srikanth S, Vidyultha KT, Jyothi G, Choudari DM, et al. Antidiabetic and antihyperlipidaemic activity of Ficus krishnae L. in alloxan induced diabetic rats. Int J Preclin Pharma Res 2010;1:14-8.
- Choudhary S, Pathak AK, Khare S, Kushwah S. Evaluation of antidiabetic activity of leaves and fruits of Ficus religiosa Linn. Int J Pharm Life Sci 2011;2:1325-7.
- Devi MR, Subramanian NS, Anbazhagan S. Antidiabetic activity of Ficus nervosa leaf in alloxan induced diabetic rats. Int J Pharm Chem Biol Sci 2012;2:196-201.
- Upadhayay A, Girhepunje K, Pal R, Thirumoorthy N. Phytochemical analysis and antidiabetic activity of ethanolic extract of Geniosporum prostratum aerial parts on STZ-induced diabetic rats. World J Pharm Res 2012;1:353-70.
- Batra S, Batra N, Nagori BP. Preliminary phytochemical studies and evaluation of antidiabetic activity of roots of Cayratia trifolia (L.) Domin in alloxan induced diabetic albino rats. J Appl Pharm Sci 2013;3:97-100.
- Joshi MK, Gorwadiya HC, Pandya DJ. Phytopharmacognostical study on ?Makkan katori?: Ficus krishnae. Int J Biomed Res 2012;3:427-30.
- Kumar A, Jha KK, Kumar D, Agrawal A, Gupta A. Preliminary phytochemical analysis of leaf and bark (mixture) extract of Ficus infectoria plant. Pharm Innov 2012;1:83-9.
- Kalaskar MG, Shah DR, Raja NM, Surana SJ, Gond NY. Pharmacognostic and phytochemical investigation of Ficus carica Linn. Ethnobot Leaf 2010;14:599-609.
- Pavia DL, Lampman GM, Kriz GS. Introduction to spectroscopy. 3rd ed. India: Thomson Business Information India Private Limited; 2006.
- Dash SK, Khan AS, Das SR, Padhan A, Rout D, Behera BC. Formulations and in vitro evaluation of sustained released glibenclamide microspheres. Int J Pharm Sci Res 2012;3:1433-43.
- Rehder S, Sakmann A, Rades T, Leopold CS. Thermal degradation of amorphous glibenclamide. Eur J Pharm Biopharm 2012;80:203-8.
- Djama AA, Goffri MC, Koua AA, Ofosu FG, Aboh IJ. Heavy metal analysis of some antidiabetic medicinal plants in Cote d?Ivoire. Curr Res J Biol Sci 2012;4:633-7.
- Piero NM, Joan NM, Cromwell KM, Maina D, Joseph NJ, Eliudm NN, et al. Trace elements content of selected Kenyan antidiabetic medicinal plants. Int J Curr Pharm Res 2012;4:39-42.
- Kinlaw WB, Levine AS, Morley JE, Silvis SE. Mcclain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med 1983;75:273-7.
- Zargar AH, Shah NA, Masoodi SR, Laway BA, Dar FA, Khan AR, et al. Copper, Zinc, Magnesium levels in non- insulin dependent diabetes mellitus. Postgraduate Med J 1998;74:665-8.
- Durlach J, Altura BM. Magnesium, diabetes and carbohydrate metabolism. Magnesium 1983;2:173-336. 35. Berman E. Toxic metals and their analysis. London: Heyden and Sons Limited; 1980.