- *Corresponding Author:
- KR. V. Rajan
Department of Pharmacology, Andhra University College of Pharmaceutical Sciences, Andhra Pradesh, 530003, India
E-mail: kvinayrajan@gmail.com
| Date of Received | 26 October 2023 |
| Date of Revision | 22 April 2024 |
| Date of Acceptance | 18 August 2024 |
| Indian J Pharm Sci 2024;86(4):1456-1468 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Impatiens balsamina, commonly known as balsam rose, is widely grown in India and China as an ornamental plant. The flowers of this plant have been reported as a rich source of polyphenols, especially flavonols. In China and some parts of India, this plant has been traditionally utilized for various clinical conditions, both wholly and partially. As this plant is a rich source of flavonols, the current study was carried out to investigate its antihyperlipidemic activity. The methanolic extract of Impatiens balsamina flowers was tested against dexamethasone-induced hyperlipidemia in male Wistar rats. 10 mg/kg body weight of dexamethasone was used to induce hyperlipidemia in rats combined with the co-treatment by utilizing the methanolic extract of Impatiens balsamina flowers of 200 mg/kg and 400 mg/kg orally for 8 d. Lipid parameters, glucose and oxidative stress markers were analyzed in serum and liver tissues, respectively. Dexamethasone treatment caused sustained elevation of serum lipid parameters, glucose levels and oxidative stress markers. Co-treatment with the methanolic extract of Impatiens balsamina significantly reduced the elevated serum lipid profile, glucose levels and oxidative stress markers in dose-dependent manner. Pronounced antihyperlipidemic activity of methanolic extract of Impatiens balsamina flowers was observed at the dose of 400 mg/kg. Histopathological studies of rats’ liver also reflected the same. The present study provides strong evidence for the antihyperlipidemic activity of methanolic extract of Impatiens balsamina flowers without any observed side effects at the studied dosage.
Keywords
Dexamethasone, hyperlipidemia, flavonols, Impatiens balsamina, kaempferol, polyphenols, hepatic steatosis
The occurrence of hyperlipidemia is currently increasing at a remarkable rate throughout the world and there is a close connection between hyperlipidemia and Cardiovascular Disease (CVD) which has been well documented[1]. Hyperlipidemia is characterized by an elevated Triglyceride (TG) levels and Free Fatty Acid (FFA) content with decreased High Density Lipoprotein- Cholesterol (HDL-C) and normal or slightly elevated Low Density Lipoprotein-Cholesterol (LDL-C) levels in blood circulation[2]. Lipid metabolism is very essential to maintain blood lipid levels and CVD is caused by dysfunction of lipid metabolism[3]. Cholesterol that we take through diet is absorbed into the intestine and is transported in the form of chylomicrons to the liver as it is the predominant organ in maintaining the lipid homeostasis[4]. Cholesterol absorption pathway has been recognized as an important pharmacological intervention for hyperlipidemia due to its close relationship with lipid metabolism. The intestinal absorption of cholesterol can therefore be reduced to prevent hyperlipidemia[5]. Apart from this to treat hyperlipidemia, lifestyle modifications such as low-fat diet intake, weight loss, exercise and pharmacotherapy play major role. Despite being 1st line drugs, statins do not universally correct metabolic abnormalities and are associated with several side effects particularly myopathy, if taken for a long period. The use of other synthetic hypolipidemic drugs may lead to hyperuricemia, diarrhea, nausea, myositis, gastric, irritation, flushing, dry skin and abnormal liver function[6]. Hence, there is a need to develop natural hypolipidemic drugs for preventing and treating hyperlipidemia[7].
Nutraceuticals have been used long back, but recent scientific and clinical evidences began supporting their potential therapeutic action[8]. Hippocrates stated, let food be thy medicine and medicine be thy food, describing the significance of nutraceuticals as substances that can be considered foods or part of food, attributing to therapeutic purpose including disease prevention and treatment[9]. International Lipid Expert Panel (ILEP) has also approved these therapies[10]. Studies on food consumption trends and ecological studies provide early evidence on the role of diet on CVD[11]. Due to their pathophysiological role, nutraceuticals may block the intestinal cholesterol absorption, inhibit liver cholesterol synthesis and enhance LDL-C excretion. It is possible that these agents may be of considerable help to patients with low-to-moderate hyperlipidemia, as well as in those with statin related side effects[12].
Impatiens balsamina flowers often grown as ornamental plants are indigenous to India and are mostly utilized in China for its various traditional medicinal properties. Flowers of this plant are rich source of polyphenols especially flavonols such as kaempferol, quercetin, rutin and depsides[13,14]. It was well documented that polyphenols play a vital role in protective effect against CVD due to its potent antioxidative effect. Therefore, the Methanolic Extract of Impatiens balsamina Flowers (MEIbF) were undertaken to evaluate its antihyperlipidemic activity against Dexamethasone (DEX)-induced hyperlipidemia in male Wistar rats.
Materials and Methods
Drugs and chemicals:
DEX sodium phosphate was obtained as a generous sample from Mythri Pharmaceuticals, Pvt. Ltd. (Hyderabad, India). All the other chemicals used in the study were of analytical grade while the plant extract, MEIbF was purchased from Vital Herbs (India).
Qualitative phytochemical screening of MEIbF was carried out where the extract was screened for the presence of active phytochemicals by following standard methods[15] (Table 1).
| Phytochemical screening methods | Phytochemicals in MEIbF |
|---|---|
| Carbohydrates | |
| Molisch’s test Benedict’s test |
Positive (+ve) +ve |
| Flavonoids | |
| Shinoda test Alkaline reagent test |
+ve +ve |
| Alkaloids | |
| Hager’s test | +ve |
| Saponins | |
| Foam test | Negative (-ve) |
| Glycosides | |
| Liebermann’s test Salkowski’s test |
+ve +ve |
| Proteins | |
| Biurets test Ninhydrin test |
-ve -ve |
| Triterpenoids | |
| Horizon test | -ve |
| Phenols and tannins | |
| Ferric chloride test | +ve |
Table 1: Qualitative Phytochemical Screening
Carbohydrates screening:
Molisch’s test: 3 ml of MEIbF was mixed with alcoholic Alpha (α)-naphthol and concentrated Sulfuric (H2SO4) acid. The formation of a violet ring at the interphase denoted the presence of carbohydrates.
Benedict’s test: Small amount of MEIbF was boiled with Benedict’s reagent where the appearance of reddish-brown precipitate indicated the presence of carbohydrates.
Flavonoids screening:
Shinoda test: MEIbF was mixed with magnesium ribbon fragments and concentrated Hydrochloric (HCl) acid. The development of a scarlet-pink color was considered to be as an indicator for the presence of flavonoids.
Alkaline reagent test: MEIbF was mixed with Sodium hydroxide (NaOH). The formation of an intense yellow color, later turning to colorless upon addition of diluted acid was considered as an indicator for the presence of flavonoids.
Saponins screening:
MEIbF was shaken vigorously with distilled water where the presence or formation of stable foam was considered as an indicator of the presence of saponins.
Glycosides screening:
Liebermann’s test: MEIbF was mixed with chloroform, acetic acid and H2SO4. Color change to violet-red or blue-green was considered as an indicator of the presence of the steroidal nucleus, i.e., the glycone portion of the glycoside.
Salkowski’s test: MEIbF was mixed with chloroform and H2SO4. The appearance of a reddish-brown color was utilized as an indicator of the presence of the steroidal ring, i.e., the glycone portion of the glycoside.MEIbF was mixed with chloroform and H2SO4. The appearance of a reddish-brown color was utilized as an indicator of the presence of the steroidal ring, i.e., the glycone portion of the glycoside.
Proteins screening:
Biurets test: MEIbF was mixed with biuret reagent and formation of a violet or red color revealed the presence of proteins.
Ninhydrin test: MEIbF was boiled with ninhydrin solution and appearance of violet color was utilized as an indicator for the presence of amino acids and proteins.
Triterpenoids screening:
Horizon test: MEIbF was mixed with trichloroacetic acid and formation of red precipitate indicated the presence of triterpenoids.
Alkaloids screening:
Hager’s test: MEIbF was mixed with Hager’s reagent and formation of yellow precipitate revealed the presence of alkaloids.
Phenols and tannins screening:
Ferric chloride test: MEIbF was mixed with Ferric chloride (FeCl3) solution. A blue-green or black coloration was indicated the presence of phenols and tannins.
Experimental animals:
For the present study, adult male albino Wistar rats weighing between (170-210) g were purchased from VAB Biosciences (Hyderabad, India). Prior to the commencement of the study, all the animals were acclimatized for 7 d at room temperature of 22° of relative humidity, 55 %-65 % with 12:12 h light dark cycle in polypropylene cages. The animals had free access to standard rat pellets diet (purchased from VAB Biosciences, Hyderabad, India) and water ad libitum.
This study was carried out with the approval of the Institutional Animal Ethics Committee (IAEC-19/ AU-Pharm/2022-23) Andhra University College of Pharmaceutical Sciences, Andhra University, Andhra Pradesh, India.
Induction of hyperlipidemia:
Prior to the start of the experiment rats were kept fasting for 18 h with water ad libitum. The rats were subcutaneously injected with 10 mg/kg body weight of DEX for 8 d consecutively[16].
Experimental groups:
In the current study rats were randomly allocated into 4 groups (n=6), Group I, II, III and IV. The total treatment course lasted for 8 d to induce hyperlipidemia. Rats in group I (Normal/vehicle control) received 0.3 % sodium carboxymethyl cellulose orally and Water for Injection (WFI) subcutaneously. Group II (Disease/DEX control) rats received 0.3 % sodium carboxymethyl cellulose orally and DEX (10 mg/kg body weight) subcutaneously. Similarly, group III (MEIbF 200 mg/kg) was treated with 200 mg/kg of MEIbF orally along with 10 mg/kg body weight of DEX subcutaneously to induce hyperlipidemia while group IV (MEIbF 400 mg/kg) rats received combination of 400 mg/kg of MEIbF orally with 10 mg/kg body weight of DEX subcutaneously.
Experimental procedure:
Due to aqueous insolubility, MEIbF was suspended in a vehicle containing 0.3 % sodium carboxymethyl cellulose. Dose of MEIbF (200 mg/kg and 400 mg/kg) was selected based on the reported acute toxicity study and previous research conducted on other clinical conditions[17]. The study period was spanned for 8 d consecutively. Upon completion of 8th d study period, rats were kept for overnight fasting, checked for body weights and blood samples (2 ml) were collected via retro-orbital plexus using a capillary tube for serum lipid profile estimation using commercial diagnostic kits. After blood collection, animals were humanely euthanized by cervical dislocation method under diethyl ether anaesthesia in an airtight closed container.
Evaluation of relative liver weight:
Following the euthanasia of the rats, their livers were removed and rinsed in ice-cold saline to remove residual blood. Subsequently, the livers were gently dried between layers of filter paper and individually weighed using an analytical balance. The relative liver weight was calculated using the prescribed formula[18].
Relative liver weight=organ weight/body weight×100
Biochemical analysis:
For the evaluation of lipid profile, fasting blood samples were obtained from rats via retro-orbital plexus sampling technique. The obtained blood samples were centrifuged at 3000 rpm for 15 min using centrifuge for serum separation. The resulting clear supernatant serum samples were transferred into clean, sterilized tubes and used for subsequent lipid profile estimation using semi autoAnalyzer.
Lipid profile included the evaluation of the Total Cholesterol (TC), Triglycerides (TG), HDL in blood using commercial excel diagnostic kits. LDL and VLDL levels were evaluated using Friedewald equation[19,20].
Atherogenic index=log(TG/HDL)
Evaluation of oxidative stress markers: Liver tissue homogenate preparation was carried out where the liver tissues were isolated and perfused with ice-cold saline. 1 g of liver tissue was weighed and 10 % w/v homogenate was prepared in 0.1 M cold phosphate buffer with 7.4 pH. Then the homogenate was subjected to centrifugation at 12 000 rpm for 15 min at 4°. Thus resulting supernatant was then collected and stored at -20° for subsequent estimation of the endogenous antioxidant parameters such as protein content, lipid peroxidation, Superoxide Dismutase (SOD), Glutathione (GSH) and Catalase (CAT).
Total protein content: The total protein content in the liver was determined using the Lowry method against standard Bovine Serum Albumin (BSA)[21].
Lipid peroxidation: To measure the extent of lipid peroxidation in tissue supernatant, Malondialdehyde (MDA) content was estimated using Thiobarbituric Acid Reacting Substances (TBARS) assay, as per Tirkey et al.[22] method.
SOD: It was estimated according to the method followed by Misra et al.[23].
GSH: To estimate the reduced GSH in tissue supernatant, Ellman’s method was employed[24].
Estimation of CAT: Tissue CAT activity was estimated using Claiborne[25] method.
Histopathology:
For histopathological analysis, liver tissues of rats from all experimental groups were carefully collected post-euthanasia and fixed using 10 % formalin solution for 2 d. Subsequently, the specimens were routinely embedded in paraffin blocks. 5 μm thin sections of the liver tissues were prepared and were stained with Hematoxylin and Eosin (H&E). Further, they were examined for histopathological changes in its normal hepatic architecture, specifically focusing on parameters such as fatty steatosis, leukocyte infiltration and sinusoidal congestion under light microscope by renowned pathologist. The observations were recorded using highpower field (200X magnification).
Statistical analysis:
GraphPad Prism version 9.3.0 was used to calculate the statistical significance. The values were presented as mean±Standard Deviation (SD); the data was analyzed using one-way Analysis Of Variance (ANOVA) followed by Dunnett’s post hoc test where p≤0.05 was considered to be statistically significant.
Results and Discussion
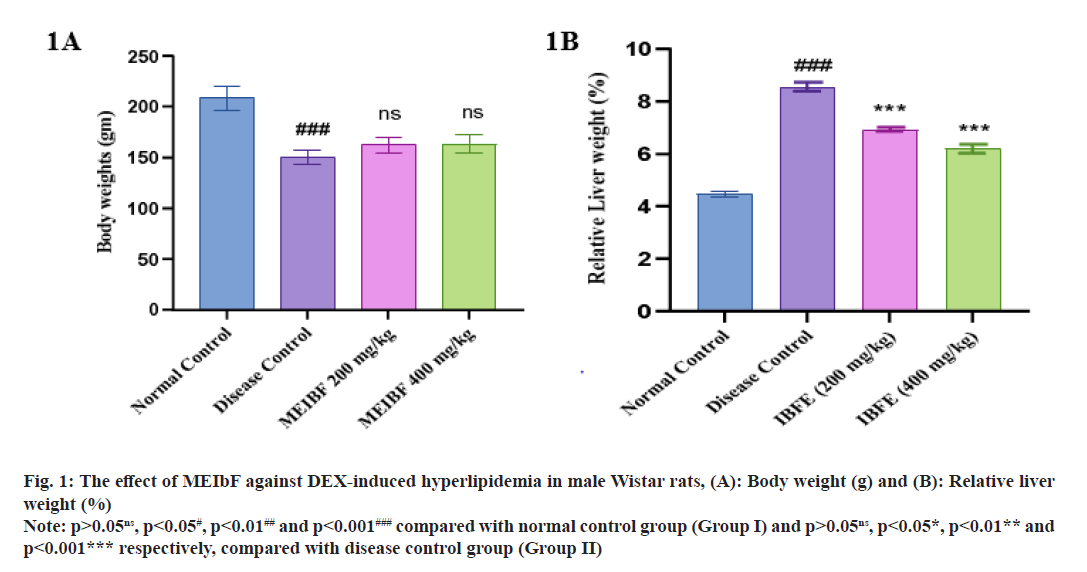
Qualitative phytochemical analysis has revealed the presence of carbohydrates, flavonoids, alkaloids, glycosides, phenols and tannins in MEIbF. Effect of MEIbF on body weight and relative liver weight against DEX induced hyperlipidemia in male Wistar rats.
Body weight of rats showed a significant weight loss in the disease control group compared with the normal control group (p<0.001) (fig. 1A). Rats receiving co-treatment with MEIbF at doses 200 mg/kg and 400 mg/kg showed nonsignificant body weight change compared with the control group (p>0.05). However, there was a significant change in body weight of the rats which were treated with 200 mg/kg and 400 mg/ kg of MEIbF when compared to the normal control group. The relative liver weight of rats in disease control group showed a significant increase in liver weight compared to normal control group (p<0.001) (fig. 1B). Co-treatment with MEIbF at 200 mg/kg showed significant decrease in liver weight (p<0.01) while the co-treatment with 400 mg/kg of MEIbF showed significant decrease in liver weight compared to disease control group (p<0.001) (Table 2).
Fig. 1: The effect of MEIbF against DEX-induced hyperlipidemia in male Wistar rats, (A): Body weight (g) and (B): Relative liver weight (%)
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
| Groups | Body weight (g) | Relative liver weight (%) |
|---|---|---|
| Normal control | 208.33±12.11 | 4.46±0.29 |
| Disease control | 150±7.07### | 8.55±0.49### |
| MEIbF (200 mg/kg) | 162.5±7.58ns | 6.92±0.21** |
| MEIbF (400 mg/kg) | 163.33±8.75ns | 6.19±0.48*** |
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
Table 2: Effect of MEIbF on Body Weight and Relative Liver Weight against DEX-Induced Hyperlipidemia in Male Wistar Rats (N=6)
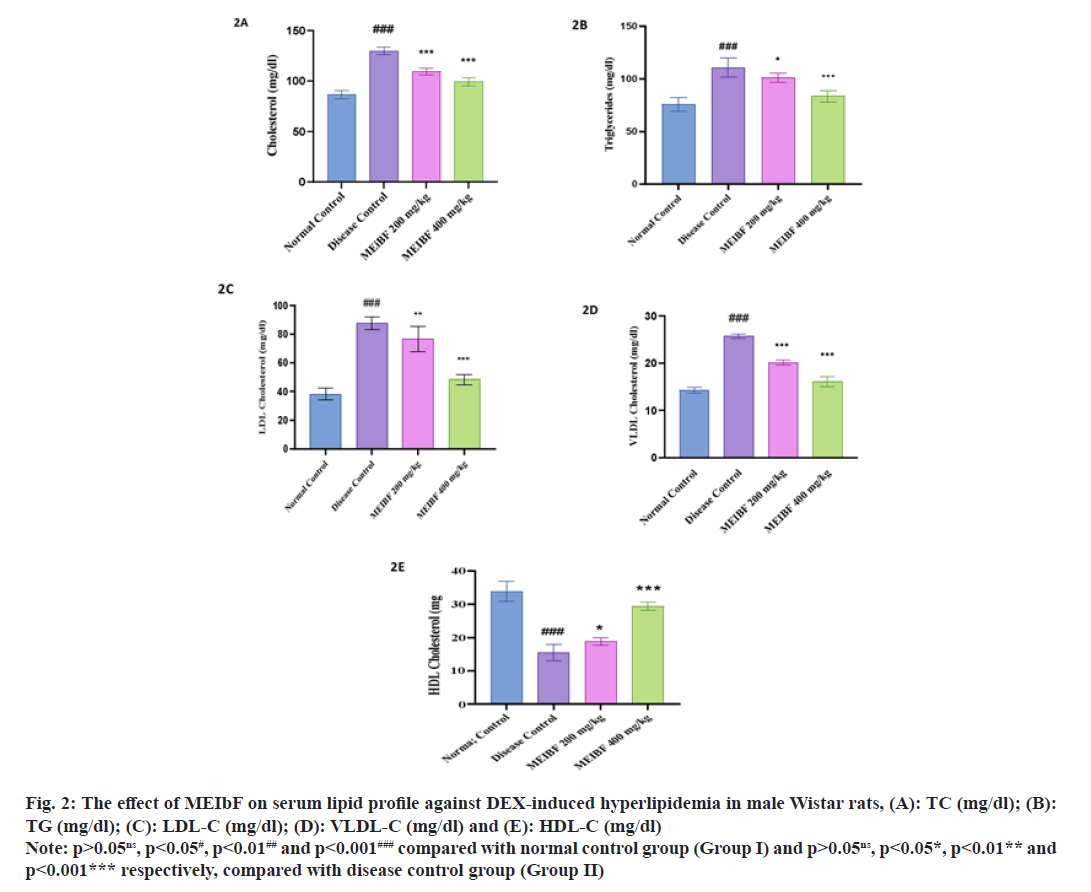
Effect of MEIBF on serum lipid profile against DEX induced hyperlipidemia in male Wistar rats was studied (Table 3). Significant elevation in serum lipid profile which included TC, TG, LDL and VLDL was observed (p<0.001) while reduced serum HDL levels were seen in the DEX-intoxicated group, i.e., the disease control group. However, co-treatment with MEIbF in DEX-intoxicated groups, i.e., in groups III and IV which were treated using 200 mg/kg and 400 mg/kg doses, respectively, has significantly decreased (p<0.001). Further, it was also found that the serum TC levels elevated compared with the control group (fig. 2A).
| Groups (n=6) | TC (mg/dl) | TG (mg/dl) | HDL (mg/dl) |
|---|---|---|---|
| Normal control | 86.79±3.84 | 75.33±6.50 | 33.83±2.99 |
| Disease control | 130±3.64### | 110.8±9.30### | 15.50±2.43### |
| MEIbF (200 mg/kg) | 109±3.43*** | 100.66±3.08* | 18.83±1.17* |
| MEIbF (400 mg/kg) | 99±3.89*** | 83.5±7.13*** | 29.33±1.38*** |
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
Table 3: Effect of MEIbF on Serum TC, TG and HDL (mg/dl) against DEX-Induced Hyperlipidemia in Male Wistar Rats (N=6)
Co-treatment with MEIbF in the DEX-intoxicated group, i.e., group III whose dose was 200 mg/kg showed significant decrease in elevated serum TG (p<0.05) and with MEIbF at 400 mg/kg dose, i.e., group IV showed more significant decrease in elevated serum TG levels (p<0.001) compared to the disease control group (fig. 2B). Co-treatment with MEIbF in the DEX-intoxicated group at dose 200 mg/kg, i.e., group III showed significant decrease in elevated serum LDL levels (p<0.01) and at dose 400 mg/kg, i.e., group IV, showed statistically more significant decrease in elevated serum LDL levels compared to the disease control group (p<0.001) (fig. 2C). Similar to TC, highly significant decrease was seen with elevated serum VLDL levels with MEIbF co-treatment in DEXintoxicated groups at both doses of 200 mg/kg and 400 mg/kg, i.e., groups III and IV, respectively, compared to the disease control group (p<0.001) (fig. 2D) (Table 4). DEX intoxication showed a statistically significant (p<0.001) decrease in serum HDL levels compared to the normal control group. Co-treatment with MEIbF in the DEXintoxicated group at dose 200 mg/kg showed a significant (p<0.01) increase in serum HDL, i.e., group III, and co-treatment with MEIbF at dose 400 mg/kg in DEX-intoxicated group (group IV) showed statistically more significant increase in serum HDL levels compared to the disease control group (p<0.001) (fig. 2E).
Fig. 2: The effect of MEIbF on serum lipid profile against DEX-induced hyperlipidemia in male Wistar rats, (A): TC (mg/dl); (B): TG (mg/dl); (C): LDL-C (mg/dl); (D): VLDL-C (mg/dl) and (E): HDL-C (mg/dl)
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
| Groups (n=6) | LDL (mg/dl) | VLDL (mg/dl) |
|---|---|---|
| Normal control | 38.34±4.93 | 14.3±0.62 |
| Disease control | 87.54±4.58### | 25.7±0.49### |
| MEIbF (200 mg/kg) | 76.28±8.87** | 20.06±0.53*** |
| MEIbF (400 mg/kg) | 49.34±4.86*** | 15.9±0.83*** |
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
Table 4: Effect of MEIbF on Serum LDL and VLDL (mg/dl) against DEX-Induced Hyperlipidemia in Male Wistar Rats (N=6)
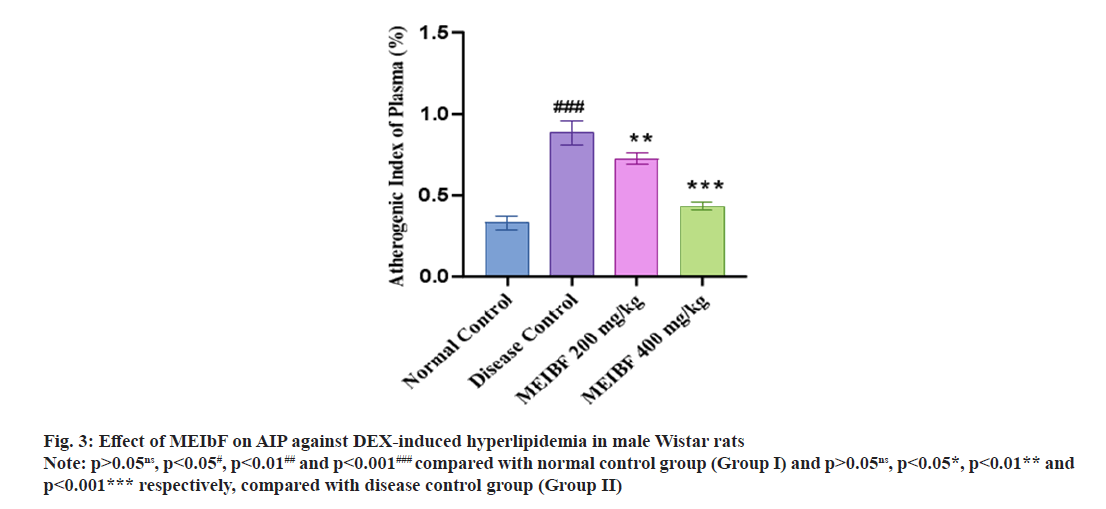
Similarly, the effect of MEIbF on Atherogenic Index of Plasma (AIP) against DEX induced hyperlipidemia in male Wistar rats (Table 5). In DEX-intoxicated group i.e., group II significantly elevated AIP was observed compared to the normal control group (p<0.001). However, co-treatment with MEIbF with 200 mg/kg dose in DEXintoxicated group i.e., group III showed decreased AIP compared to the disease control group (p<0.01). Co-treatment with MEIbF at 400 mg/ kg dose in DEX-intoxicated i.e., group IV showed more significant decrease in AIP compared to the disease control group (p<0.001) (fig. 3).
| Groups (n=6) | AIP (%) |
|---|---|
| Normal control | 0.33±0.04 |
| Disease control | 0.89±0.07### |
| MEIbF (200 mg/kg) | 0.72±0.03** |
| MEIbF (400 mg/kg) | 0.43±0.02*** |
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
Table 5: Effect of MEIbF on AIP against DEX-Induced Hyperlipidemia in Male Wistar Rats (N=6)
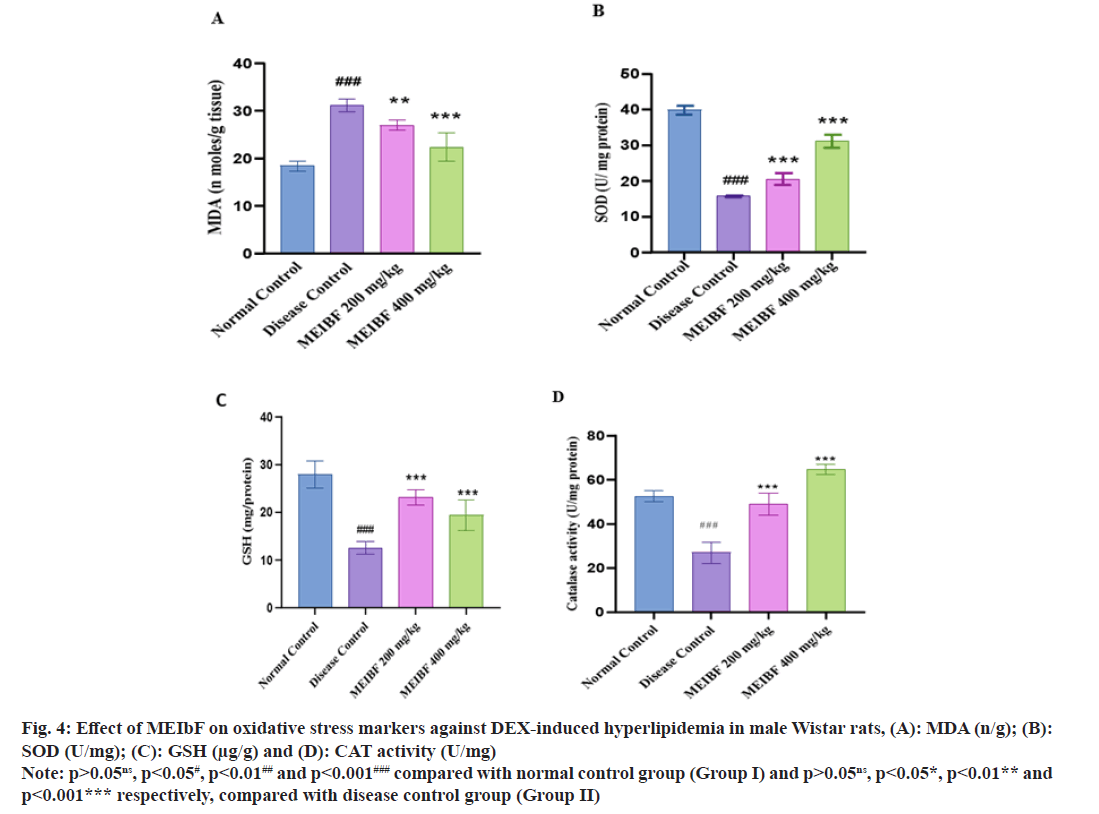
Effect of MEIbF on oxidative stress markers was studied (Table 6). The enzymatic antioxidant levels like SOD, CAT and GSH were significantly reduced in the DEX-intoxicated group when compared with the normal control group (p<0.001). Cotreatment with MEIbF has significantly restored the antioxidant enzyme activity in dose-dependent manner. Co-treatment with MEIbF at 200 mg/kg dose increased the levels of SOD, GSH and CAT (p<0.001). The percentage protection offered by 200 mg/kg of MEIbF on SOD, GSH and CAT was found to be 68.12 %, 61.15 % and 81.65 %, respectively, compared to the disease control group; whereas co-treatment with MEIbF at 400 mg/kg dose in group IV showed more statistically significant elevation of SOD, GSH and CAT (p<0.001). Percentage protection offered by 400 mg/kg of MEIbF on SOD, GSH and CAT was 151.39 %, 79.33 % and 139.95 %, respectively, compared with the disease group (fig. 4A-fig. 4C).
| Groups (n=6) | MDA (n/g) | SOD (U/mg protein) | GSH (µg/g of tissue) | CAT activity (U/mg protein) |
|---|---|---|---|---|
| Normal control | 18.36±1.06 | 9.02±2.00 | 21.77±2.29 | 52.65±2.47 |
| Disease control | 31.17±1.36 | 2.51±0.11 | 10.89±0.87 | 26.93±4.75 |
| MEIbF (200 mg/kg) | 26.96±1.07** (15.61↓) | 4.22±0.90*** (68.12↑) | 17.55±3.31*** (61.15↑) | 48.92±4.92*** (81.65↑) |
| MEIbF (400 mg/kg) | 21.33±1.32*** (31.56↓) | 6.31±0.75*** (151.39↑) | 19.53±2.06*** (79.33↑) | 64.62±2.27*** (139.95↑) |
Note: (↓): Decreased and (↑): Increased, p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
Table 6: Effect of MEIbF on Oxidative Stress Markers against DEX-Induced Hyperlipidemia in Male Wistar Rats (N=6)
Extent of lipid peroxidation was quantified by estimating the MDA levels. DEX-intoxicated group i.e., group II significantly (p<0.001) increased the lipid peroxidation which resulted in higher levels of MDA compared to normal control group, whereas co-treatment with MEIbF had reversed the condition. MEIbF at 200 mg/kg dose significantly decreased the MDA (p<0.01) and offered 15.61 % protection compared to disease control group while dose of 400 mg/kg of MEIbF significantly decreased the MDA and offered 31.56 % protection compared to disease control group (p<0.001) (fig. 4D).
Fig. 4: Effect of MEIbF on oxidative stress markers against DEX-induced hyperlipidemia in male Wistar rats, (A): MDA (n/g); (B): SOD (U/mg); (C): GSH (μg/g) and (D): CAT activity (U/mg)
Note: p>0.05ns, p<0.05#, p<0.01## and p<0.001### compared with normal control group (Group I) and p>0.05ns, p<0.05*, p<0.01** and p<0.001*** respectively, compared with disease control group (Group II)
CVD remains as a predominant cause of mortality in both developed and developing nations. Elevated lipid levels exacerbated by oxidative stress, stand as primary risk factors[26] enhancing the onset and advancement of these conditions. Hyperlipidemia which is a significant contributor plays a pivotal role in the development of atherosclerosis and its associated complications, including coronary heart disease, ischemic cerebrovascular disease and peripheral vascular diseases. Hyperlipidemia and hypercholesterolemia, coupled with low levels of HDL-C, emerge as significant contributors to increased atherogenic risk. This complex scenario is influenced by a combination of genetic factors and lifestyle choices, with high calorie diets, saturated fat and cholesterol playing a pivotal role in its manifestation across developed nations worldwide[27]. Despite the prevalence of hyperlipidemia, effective remedies remain elusive. However, there is a growing recognition of the potential therapeutic benefits offered by herbal drugs. Many of these natural remedies not only expedite the reduction of cholesterol levels but also align with healthy dietary intake. Their efficacy is attributed to rich therapeutic properties and the appeal lies in their 100 % natural composition.
In this context, herbal interventions stand out as promising avenues for addressing hyperlipidemia, providing potential bridge between lifestyle modifications and effective cholesterol management. DEX is a potent synthetic corticosteroid with anti-inflammatory and immunosuppressive properties. It belongs to the glucocorticoid class of hormones which is widely used in medical practice for its therapeutic effects. This corticosteroid is a synthetic analogue of prednisolone which is known for its potent antiinflammatory actions, making it valuable in the treatment of various conditions. In the context of the study mentioned earlier, DEX was utilized to induce hyperlipidemia in experimental rat models. DEX-induced model of hyperlipidemia in vivo has been established well. A major advantage of using DEX is that the hyperlipidemic state can be generated in a relatively short period of time and has a lot of relevance to clinical hyperlipidemia condition in vivo. Synthesis of triacylglycerol in the liver is stimulated by the injection of glucocorticoid in rats and consequently may lead to the accumulation of free fatty acids leading to hyperlipidemia[28]. The stimulation of TG production could lead to increased secretion of VLDL-C. Increasing VLDL-C secretion has been reported when DEX is injected for several days in rats[29,30]. It was reported that DEX administration (10 mg/kg body weight) increases TG level, inducing imbalance in lipid metabolism leading to hyperlipdemia[31]. In this study (2-3) mo old male Wistar rats were used and not female rats as (2-3) mo old female rats were shown to be protected from the consequences of DEX on glucose tolerance and thus hyperlipidemia[32]. DEX-induced hyperlipidemia is a specific model of hyperlipidemia reflecting the hyperlipidemia in the state of hypercortisolism, over dose of exogenous glucocorticoids drugs and does not summarize all hyperlipidemic models. The present study focused on the treatment of MEIbF against DEX-induced hyperlipidemia due to rich abundance of flavonols (kaempferol, quercetin and potentially myricetin) in the extract. Rats injected with DEX exhibited decrease in body weight and reduced food consumption compared to normal control group; this observation aligned with earlier research findings[33]. It was proposed that the DEX-induced attenuation in gain of body weight may be linked to elevated plasma leptin levels following high doses of corticoids. The prolonged increase in leptin levels could contribute to the DEX-induced restriction in food intake and weight gain. Numerous studies have substantiated that Corticotropin Releasing Hormone (CRH) reduces body weight.
Leptin is known to enhance CRH secretion, thereby reducing the body weight set point[34]. Co treatment with MEIbF (at doses 200 mg/kg and 400 mg/kg) did not show any improvement in body weight. Administration of DEX showed significant increase in TC, TG, FFA and reduced HDL-C. The observed increase in TG and cholesterol may be due to the increase in plasma VLDL. Co-treatment with MEIbF at doses 200 mg/kg and 400 mg/ kg reduced TC and TG in plasma. The possible reason is kaempferol blocks nuclear translocation of Sterol Regulatory Element-Binding Protein-1 (SREBP-1)[35] and thus lowers hepatic and plasma TG concentration. Another mechanism of action is that kaempferol exerts its TG lowering effect via Protein kinase B (Akt) inhibition and activation of Peroxisome Proliferator Activated Receptor (PPAR) α and PPAR Gamma (δ)[36]. The order of potency to decrease the serum TC and TG levels were found to be MEIbF (200 mg/kg)<MEIbF (400 mg/kg). DEX treatment to rats for 8 d significantly reduced the serum HDL whose mechanism is unknown. Co-treatment with MEIbF significantly increased their levels in serum. The possible mechanism of action is that kaempferol directly binds to Liver X Receptor Beta (LXR-β) and activates it in order to reduce the glucose and to increase HDL-C concentration as well as increasing faecal cholesterol and bile acids excretion[35]. The order of potency to increase serum HDL-C levels was found to be MEIbF (200 mg/kg)<MEIbF (400 mg/kg). Corticoid treatment is recognized for its role in increasing VLDL secretion by the liver, with additional potential stimulation of VLDL formation in the intestine[31]. Moreover, the administration of glucocorticoids has been shown to reduce the activity of hepatic lipoprotein lipase in rats[37]. This inhibition of hepatic lipoprotein lipase function impedes the removal of VLDL from plasma, contributing to an elevation in plasma VLDL levels. Hepatic lipoprotein lipase selectively hydrolyses VLDL triglycerides, forming partial glycerides and free fatty acids[38]. The diminished activity of liver lipoprotein lipase could be implicated in the increased VLDL and TG levels observed in DEX administered rats[31]. However, co-treatment with MEIbF effectively played crucial role in preventing the escalation of VLDL levels in the plasma. The order of potency to decrease serum VLDL cholesterol levels was found to be MEIbF (200 mg/kg)<MEIbF (400 mg/ kg). The overall impact of MEIbF on lipid profile can be correlated with the presence of flavanols[36].
Flavanols have the ability to reduce LDL-C and increase HDL-C, inhibit cholesterol biosynthesis, absorption and modifying the activity of lipogenic and lipolytic enzymes, leading to reduced lipid metabolism. So, in this study, flavanols present in the MEIbF extract might be the reason for reducing TC, LDL-C and increasing HDL-C among 400 mg/kg treated rats. The elevation in liver weights may be ascribed to the rapid mobilization of stored fat influenced by glucocorticoids, making more FFAs accessible for deposition in the form of triglycerides[33]. Concurrent administration of MEIbF notably decreased the relative weight of the liver and potentially attributed to the presence of flavonols in the extract. The comparative efficacy in reducing the relative weight of the liver was observed (MEIbF 200 mg/kg<MEIbF 400 mg/ kg); DEX showed an elevation in AIP compared to normal control group. This supported the previous study, which reported that higher dose of DEX (10 mg/kg) significantly, elevated AIP as compared to control rats which may increase the risk of atherogenicity[39]. This study also reported that DEX treatment predisposes thickening or aortas relative to control rats. Our study was consistent with the previous study and co-treatment with MEIbF reduced the AIP values significantly which can be attributed to the presence of kaempferol, quercetin and myricetin[16]. The order of potency to decrease AIP was found to be MEIbF 200 mg/kg< MEIbF 400 mg/kg. Oxidative stress is recognized as a key factor in the pathogenesis of CVD induced by glucocorticoids[40]. Overproduction of free radicals and hepatic oxidative damage has been documented in DEX-induced hyperlipidemia[39].
DEX induction led to reduction of SOD, GSH and CAT, indicating enzyme inactivation by Reactive Oxygen Species (ROS) and subsequent protein damage. Treatment with the MEIbF extract demonstrated potent antioxidant activity, contributing to its preventive effects on hypolipidemic activity by elevating antioxidant levels. DEX induction resulted in significant increase in TBARS or MDA in liver tissue compared to normal control group. These findings aligned with the previous in vivo and in vitro studies showing elevated ROS levels upon DEX treatment[41].
MDA levels, a surrogate marker for lipid peroxidation, reflect the presence of excess reactive free radicals. In this study, MEIbF administration for 8 d restored the balance between oxidants and antioxidants compared to the disease control group, suggesting its antioxidant effect. MEIbF significantly and dose-independently attenuated DEX-induced elevation in MDA levels in liver tissue. Additionally, it increased SOD, GSH and CAT levels in the same tissues. These effects are attributed to the presence of flavonols in the extract, consistent with findings from previous studies. The potential mechanism of MEIbF's antioxidant activity lies in the active compounds, flavonols, acting as electron donors, terminating oxidation chain reactions by reducing oxidized intermediates into stable forms[42]. Another known mechanism involves phenols or polyphenols’ chelating redoxactive metal ions, inactivating lipid free radical chains and preventing hydroperoxide conversion into reactive oxyradicals. This allows them to function as reducing agents, hydrogen donors and singlet-oxygen scavengers[43,44]. MEIbF's ability to bring oxidative stress markers to normal levels suggests that its antioxidant activity is at least in part, which is responsible for its antihyperlipidemic effects.
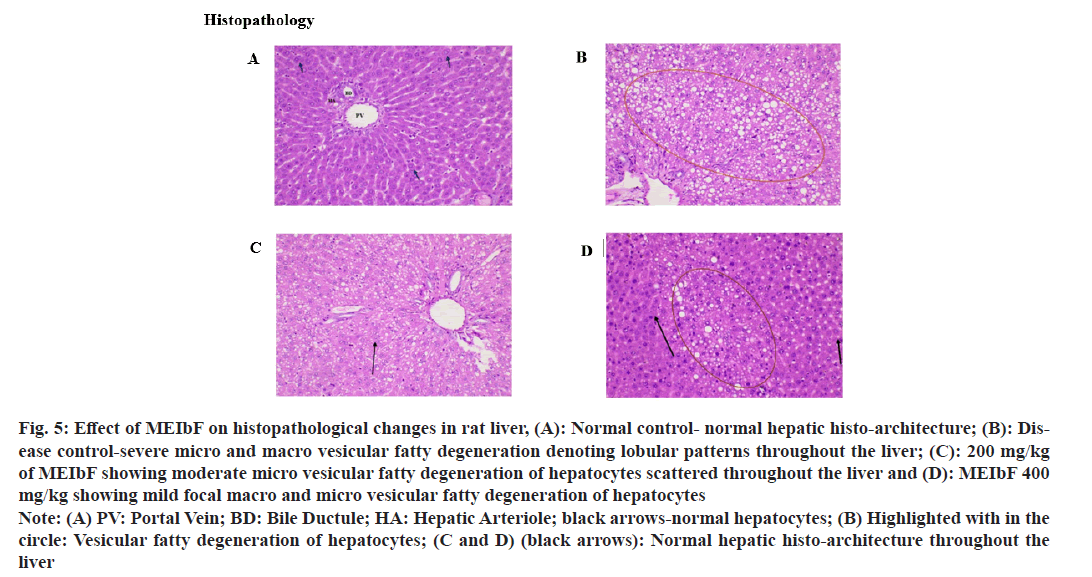
Histopathology studies revealed that administration of DEX for 8 d consecutively resulted in severe macro and micro vesicular fatty degeneration throughout the liver tissue in the disease control group where as co-treatment with MEIbF at 200 mg/ kg dose showed micro vesicular fatty degeneration throughout the liver (fig. 5). However, cotreatment with MEIbF at 400 mg/kg dose showed focal micro and macro vesicular fatty degeneration and normal liver architecture throughout the liver.
Fig. 5: Effect of MEIbF on histopathological changes in rat liver, (A): Normal control- normal hepatic histo-architecture; (B): Disease control-severe micro and macro vesicular fatty degeneration denoting lobular patterns throughout the liver; (C): 200 mg/kg of MEIbF showing moderate micro vesicular fatty degeneration of hepatocytes scattered throughout the liver and (D): MEIbF 400 mg/kg showing mild focal macro and micro vesicular fatty degeneration of hepatocytes
Note: (A) PV: Portal Vein; BD: Bile Ductule; HA: Hepatic Arteriole; black arrows-normal hepatocytes; (B) Highlighted with in the circle: Vesicular fatty degeneration of hepatocytes; (C and D) (black arrows): Normal hepatic histo-architecture throughout the liver
In conclusion, the findings of this study underscore the potential health benefits associated with the MEIbF. Notably, the extract demonstrated noteworthy capacity to lower blood lipid levels which was evidenced by the reduction of serum lipids. The extract also exhibited antioxidant activity, manifested by the decrease in lipid peroxides within liver tissue, coupled with an increase in the levels of SOD, CAT and GSH in the same liver tissue. Moreover, the observed improvements in liver histopathological changes in DEX-induced hyperlipidemic rats further highlight the potential therapeutic value of the MEIbF. This suggests its possible role as an intervention to alleviate the global burden of metabolic syndrome of hyperlipidemia and related conditions. The comprehensive impact on various facets of metabolic health positions this herbal medicine as a promising candidate for further exploration and potential integration into preventive healthcare strategies. Nevertheless, while these findings are promising, it is crucial to emphasize the need for additional trials to robustly assess the efficacy of MEIbF in managing hyperlipidemia. Furthermore, exploring and identifying all the bioactive phytochemicals within the extract and elucidating the underlying mechanisms contributing to its lipid-lowering actions are imperative for a more comprehensive understanding of its therapeutic potential. Continued research in these areas will contribute to the development of evidence-based interventions, which could broaden the range of natural treatments available for metabolic disorders.
Acknowledgments:
The authors gratefully acknowledge the Principal of Andhra University College of Pharmaceutical Sciences, Visakhapatnam for providing the necessary facilities. Additionally, they also thank the principal, management and staff of the Department of Pathology, Andhra Medical College, Visakhapatnam, for conducting the histopathological studies.
Conflict of interests:
The authors declared no conflict of interests.
References
- Suanarunsawat T, Boonnak T, Ayutthaya WN, Thirawarapan S. Anti-hyperlipidemic and cardioprotective effects of Ocimum sanctum L. fixed oil in rats fed a high fat diet. J Basic Clin Physiol Pharmacol 2010;21(4):387-400.
[Crossref] [Google Scholar] [PubMed]
- Sheik SM, Bakthavatchalam P, Shenoy RP, Hadapad BS, Nayak D, Biswas M, et al. Anti-hyperglycemic, anti-hyperlipidemic, and anti-inflammatory effect of the drug Guggulutiktaka ghrita on high-fat diet-induced obese rats. J Ayur Integrat Med 2022;13(3):1-10.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Wang H, Zhang X, Yang ST. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit Rev Food Sci Nutr 2014;54(9):1180-201.
[Crossref] [Google Scholar] [PubMed]
- Mansbach CM, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastroint Liver Physiol 2007;293(4):645-50.
[Crossref] [Google Scholar] [PubMed]
- El-Tantawy WH, Temraz A. Natural products for controlling hyperlipidemia. Arch Physiol Biochem 2019;125(2):128-35.
[Crossref] [Google Scholar] [PubMed]
- Subramaniam S, Ramachandran S, Uthrapathi S, Gnamanickam VR, Dubey GP. Antihyperlipidemic and antioxidant potential of different fractions of Terminalia arjuna Roxb. bark against PX407 induced hyperlipidemia. Indian J Exp Biol 2011;49(4):282-8.
[Google Scholar] [PubMed]
- Watts GF, Karpe F. Triglycerides and atherogenic dyslipidaemia: Extending treatment beyond statins in the high-risk cardiovascular patient. Heart 2011;97(5):350-6.
[Crossref] [Google Scholar] [PubMed]
- Dillard CJ, German JB. Phytochemicals: Nutraceuticals and human health. J Sci Food Agricult 2000;80(12):1744-56.
- Elkhalifa AE, Alshammari E, Adnan M, Alcantara JC, Awadelkareem AM, Eltoum NE, et al. Okra (Abelmoschus Esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules 2021;26(3):1-6.
[Crossref] [Google Scholar] [PubMed]
- Ji X, Shi S, Liu B, Shan M, Tang D, Zhang W, et al. Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed Pharmacother 2019;118:1-10.
[Crossref] [Google Scholar] [PubMed]
- McGill Jr HC. Relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man. Am J Clin Nutr 1979;32(12):2664-702.
[Crossref] [Google Scholar] [PubMed]
- Sanidas E, Grassos C. The role of nutraceuticals in the treatment of primary dyslipidemia. Hellenic J Cardiol 2020;61(1):60-2.
[Crossref] [Google Scholar] [PubMed]
- Impatiens balsamina. Edible Medicinal and Non-Medicinal Plants. Springer 2013;7:537-47.
[Google Scholar] [PubMed]
- Li Q, Zhang X, Cao J, Guo Z, Lou Y, Ding M, et al. Depside derivatives with anti-hepatic fibrosis and anti-diabetic activities from Impatiens balsamina L. flowers. Fitoterapia 2015; 105:234-9.
[Crossref] [Google Scholar] [PubMed]
- Yadav RN, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol 2011;3(12):10-4.
- Mahendran P, Devi CS. Effect of Garcinia cambogia extract on lipids and lipoprotein composition in dexamethasone administered rats. Indian J Physiol Pharmacol. 2001;45(3):345-50.
[Google Scholar] [PubMed]
- Imam MZ, Nahar N, Akter S, Rana MS. Antinociceptive activity of methanol extract of flowers of Impatiens balsamina. J Ethnopharmacol 2012;142(3):804-10.
[Crossref] [Google Scholar] [PubMed]
- Soren P, Sharma R, Kharwal M, Singh R, Singh B, Mal G. Effect on the body weight, organ weight and haematology during sub-chronic lantadene toxicity and its amelioration with Berberis lycium and Picrorhiza kurroa in guinea pigs. J Pharmaco Phytochem 2019;8(6):373-7.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18(6):499-502.
[Crossref] [Google Scholar] [PubMed]
- Dobias OM, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin Biochem 2001;34(7):583-8.
[Crossref] [Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265-75.
[Google Scholar] [PubMed]
- Tirkey N, Pilkhwal S, Kuhad A, Chopra K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol 2005;5:1-8.
[Crossref] [Google Scholar] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170-5.
[Google Scholar] [PubMed]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70-7.
- Catalase activity. In Handbook methods for oxygen radical research. Taylor & Francis 2018. p. 1-2.
- Sharma N, Garg V. Antidiabetic and antioxidant potential of ethanolic extract of Butea monosperma leaves in alloxan induced diabetic mice. Indian J Biochem Biophys 2009;46(1):99-105.
[Google Scholar] [PubMed]
- Loscalzo J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison’s principles of internal medicine. 6th Ed 2008.
- Krausz Y, Bar-On H, Shafrir E. Origin and pattern of glucocorticoid-induced hyperlipidemia in rats dose-dependent bimodal changes in serum lipids and lipoproteins in relation to hepatic lipogenesis and tissue lipoprotein lipase activity. Biochimica Biphysica Acta 1981;663(1):69-82.
[Crossref] [Google Scholar] [PubMed]
- Brader ED, Lee PC, Raff H. Dexamethasone treatment in the newborn rat: Fatty acid profiling of lung, brain and serum lipids. J Appl Physiol 2005;98(3):981-90.
[Crossref] [Google Scholar] [PubMed]
- Kaur N, Sharma N, Gupta AK. Effects of dexamethasone on lipid metabolism in rat’s organ. Indian J Biochem Biophys 1989;26(6):371-6.
[Google Scholar] [PubMed]
- dos Santos C, Ferreira FB, Goncalves-Neto LM, Taboga SR, Boschero AC, Rafacho A. Age-and gender related changes in glucose homeostasis in glucocorticoid-treated rats. Can J Physiol Pharmacol 2014;92(10):867-78.
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology 1997;138(9):3842-8.
[Crossref] [Google Scholar] [PubMed]
- Badmus OO, Olatunji LA. Glucocorticoid exposure causes disrupted glucoregulation, cardiac inflammation and elevated dipeptidyl peptidase-4 activity independent of glycogen synthase kinase-3 in female rats. Arch Physiol Biochem 2019;125(5):414-22.
[Crossref] [Google Scholar] [PubMed]
- Michel C, Cabanac M. Effects of dexamethasone on the body weight set point of rats. Physiol Behav 1999;68(1-2):145-50.
[Crossref] [Google Scholar] [PubMed]
- Lee MJ, Wang Y, Ricci MR, Sullivan S, Russell CD, Fried SK. Acute and chronic regulation of leptin synthesis, storage, and secretion by insulin and dexamethasone in human adipose tissue. Am J Physiol Endocrinol Metab 2007;292(3):858-64.
[Crossref] [Google Scholar] [PubMed]
- Hoang MH, Jia Y, Mok B, Jun HJ, Hwang KY, Lee SJ. Kaempferol ameliorates symptoms of metabolic syndrome by regulating activities of liver X receptor-β. J Nutr Biochem 2015;26(8):868-75.
[Crossref] [Google Scholar] [PubMed]
- Hoang MH, Jia Y, Lee JH, Kim Y, Lee SJ. Kaempferol reduces hepatic triglyceride accumulation by inhibiting Akt. J Food Biochem 2019;43(11):1-13.
[Crossref] [Google Scholar] [PubMed]
- Mangiapane EH, Brindley DN. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very low-density lipoproteins and lysophatidylcholinge by monolayer cultures of rat hepatocytes. Biochem J 1986;233(1):151-60.
[Crossref] [Google Scholar] [PubMed]
- Berkhout TA, Havekes LM, Pearce NJ, Groot PH. The effect of (-)-hydroxy citrate on the activity of the low-density-lipoprotein receptor and 3-hydroxy-3-methylglutaryl-CoA reductase levels in the human hepatoma cell line Hep G2. Biochem J 1990;272(1):181-6.
[Crossref] [Google Scholar] [PubMed]
- Borradaile NM, de Dreu LE, Barrett PH, Behrsin CD, Huff MW. Hepatocyte apoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry 2003;42(5):1283-91.
[Crossref] [Google Scholar] [PubMed]
- Nayak IN, Chinta R, Jetti R. Anti-atherosclerotic potential of aqueous extract of Cinnamomum zeylanicum bark against glucocorticoid induced atherosclerosis in wistar rats. J Clin Diagn Res 2017;11(5):19-23.
[Crossref] [Google Scholar] [PubMed]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440(7086):944-8.
[Crossref] [Google Scholar] [PubMed]
- Koodkaew I, Sukonkhajorn P. Anti-tyrosinase and antioxidant activities of Impatiens balsamina L. Songklanakarin J Sci Technol 2019;41(3):686-92.
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int 2011;44(1):391-6.