- *Corresponding Author:
- M. N. Saraf
Department of Pharmacology, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098, India.
E-mail: saraf@bcpindia.org
| Date of Submission | 25 May 2005 |
| Date of Revision | 10 October 2005 |
| Date of Acceptance | 17 July 2006 |
| Indian J Pharm Sci,2006, 68 (4): 456-460 |
Abstract
The ethanol extract of Tephrosia purpurea Linn. (Family: Leguminosae) was found to significantly inhibit the carbon tetrachloride-induced lipid peroxidation in vivo and superoxide generation in vivo . The ethyl acetate fraction of the same extract was studied for free radical scavenging and antilipid peroxidation activity. The IC50 values in both of these in vitro assays were found to be significantly reduced for ethyl acetate fraction compared with the ethanolic extract of the plant. The observation was further supported by comparing the in vivo antioxidant activity for both the ethanolic extract and its ethyl acetate fraction. The study concluded that the ethanolic extract of T. purpurea exhibits antioxidant activity in vivo and the ethyl acetate soluble fraction has improved antioxidant potential than the extract.
Introduction
Tephrosia purpurea Linn. (Fam: Leguminosae, Syn.: Sanskrit: Sharpunkha; Hindi: Sarponkha; English: Purple Tephrosia] is a perennial herb found throughout the Indian subcontinent. Its aerial parts and roots are used in bronchial asthma [1,2], hepatic ailments [3,4], cutaneous toxicities [5], pain and inflammation [6]. Reactive oxidative species have been implicated in the genesis of these disorders. We have therefore in the earlier studies [7] examined the antioxidant potentials of the ethanolic extract of T. purpurea. The studies revealed that the extract has significant free radical scavenging and antilipid peroxidation activities. However, it failed to scavenge the hydroxyl radicals.
The hydroxyl radical is an extremely reactive oxidizing radical that reacts with most molecules at diffusion-controlled rates [8]. It was, thus, logical to expect ·OH radical to react with the extract that showed significant free radical scavenging and antilipid peroxidation activities. However, since the in vitro model used for determination of hydroxyl radical scavenging potential employed totally aqueous system, it is possible that the relatively less polar active principles of the extract responsible for the antioxidant activity might have precipitated into the aqueous medium and hence could not participate in the reaction. We therefore, examined the effect of fractionation of the extract on the antioxidant potential of the plant extract.
Materials and Methods
Aerial parts of T. purpurea Linn. were obtained from and authenticated by at the department of Dravyaguna (Herbal Pharmacology), Y. M. T. Ayurvedic Medical College, Khargar, Navi Mumbai. The aerial parts of the plant were dried at room temperature, powdered to coarse size and examined for macroscopic and microscopic characteristics. Wistar rats (150-200 g) and Swiss mice (20-25 g) of either sex were housed in clean acrylic cages and allowed free access to standard laboratory chow and water. All animal studies were carried out in accordance with the Guidelines of the Committee for the purpose of control and supervision of experiments on animals by the ministry of social justice and empowerment, Govt. of India. The studies were approved by the Institutional Animal Ethics Committee of Bombay College of Pharmacy.
All chemicals used were of analytical grade. Phorbol-12myristyl acetate (PMA) was purchased from Sigma Chemical Co, USA. Adenine dinucleotide phosphate (ADP) was purchased from S. D. Fine Chemicals, Mumbai. Ascorbic acid and sodium lauryl sulfate (SLS) were purchased from Thomas Baker (Chemicals) Limited, Mumbai. Tris (hydroxymethyl) methylamine (Tris buffer) and carbon tetrachloride (CCl4) were purchased from Loba Chemie Pvt. Ltd., Mumbai. Nitro blue tetrazolium chloride (NBT), 1,1-diphenyl, 2-picrylhydrazyl free radical (DPPH), thiobarbituric acid (TBA) and sodium caseinate were purchased from Himedia laboratories limited, Mumbai.
Extraction of plant material
The coarsely ground powder was extracted with n-hexane in a Soxhlet extractor. The defatted plant material was dried and then extracted with 95% ethanol in a Soxhlet extractor. The ethanolic extract obtained was concentrated under reduced pressure at a bath temperature below 50° to yield a syrupy mass (TP, yield: 9% of starting crude material).
Effect of TP on PMA-induced superoxide generation in vivo
Swiss mice of either sex (20-25 g) were injected with sodium caseinate (0.2 ml of 0.5% aqueous solution) to elicit the macrophages. On the day 5, mice were administered with 100 μg of PMA intraperitoneally [9]. The test agent (TP) was administered 1 h before PMA in different doses. Animals were sacrificed 2 h after the PMA administration. Phosphate buffered saline (PBS, 2 ml) was injected intraperitoneally and the abdomen was massaged for 3-4 min. The peritoneal fluid was withdrawn by lavage and centrifuged. The pellet was suspended in phosphate buffered saline (1 ml). The macrophages count in the suspension was adjusted to 4×106 cells/ml using 0.1% crystal violet containing 0.024% citric acid as the stain. The cell suspension (1 ml) was incubated with a mixture (1 ml) of nitro blue tetrazolium chloride (0.2% in PBS), 5% dextrose and sterile Hank’s solution (3:1:2, respectively). The mixture was kept for 3-4 h in dark and then centrifuged at 1300 g for 15 min. The pellet was mixed with 1,4-dioxane (3 ml) and boiled for 15 min. The mixture was cooled and centrifuged. The absorbance of supernatant was measured at 530 nm.
Effect of TP on CCl4-induced lipid peroxidation in vivo
Wistar rats (150-200 g) of either sex were orally administered with carbon tetrachloride (50% solution in arachis oil) in a dose of 4 ml/kg. TP was given (25–100 mg/kg) orally 1 h before CCl4 administration. Rats were sacrificed 24 h after CCl administration and the livers 4 were quickly removed and chilled in ice-cold 0.9% NaCl. The malondialdehyde (MDA) content was estimated in the liver homogenate using the method of Ohkawa et al.[10]
Fractionation of ethanol extract (TP)
TP (10 g) was suspended in 100 ml of distilled water. This suspension was exhaustively extracted with less polar solvents viz. benzene, chloroform or ethyl acetate separately. The upper organic layer was separated and evaporated below 50 under reduced pressure to obtain the respective fraction. The ethyl acetate soluble fraction obtained above is referred henceforth as EA (yield: 6.37% w/w of starting crude material).
Effect of EA on free radicals
The ability of EA to scavenge the free radicals was determined by an in vitro assay method using a stable free radical DPPH (1,1-diphenyl, 2-picrylhydrazyl) [7,11,12]. Effect of different concentrations of EA (10-60 μg/ml of the final volume) on the lipid peroxidation in rat liver homogenate [7,11] was measured in vitro in terms of formation of thiobarbituric acid reactive substances (TBARS) [12,13]. The effect of EA on superoxide generation and on CCl4-induced lipid peroxidation in vivo was investigated in doses of 100-400 mg/kg and 25-100 mg/kg, respectively, using the procedures explained earlier for TP.
Statistical analysis
The results are given as mean±SD. The data obtained were analyzed by one-way analysis of variance (ANOVA) followed by Student’s t test. Differences were considered significant at the 5% level. The IC50 value was calculated using linear regression analysis of the percent inhibition obtained using different concentrations. The regression equation was obtained and the concentration required to produce 50% inhibition (IC50) was calculated.
Results and Discussion
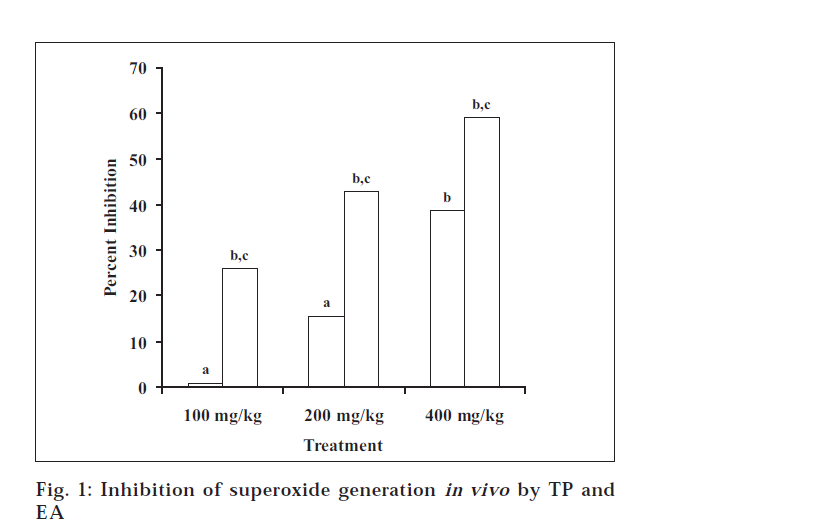
Ethanol extract of T. purpurea (TP) exhibits free radical scavenging and antilipid peroxidation properties in the in vitro studies, as reported earlier [7]. However, it becomes pertinent to probe if the same extract exhibits antioxidant activity in vivo also. In the present study, ethanol extract was studied for its effects on the superoxide generation in peritoneal macrophages in vivo. Peritoneal macrophages, when elicited chemically, have been found to produce superoxide radical [14]. PMA, a potent cocarcinogen and allergen, was used to induce the superoxide generation in the peritoneal macrophages [9]. In the PMA-induced superoxide generation assay, TP at the doses of 100, 200 and 400 mg/kg showed inhibition of superoxide generation to the extent of 0.9, 15.7 and 38.7%, respectively (fig. 1). The inhibition was significant at the dose of 400 mg/kg only.
Fig. 1: Inhibition of superoxide generation in vivo by TP and EA Values are expressed as mean±SD of 6 observations; TP (![]() ) represents ethanol extract of Tephrosia purpurea; EA (
) represents ethanol extract of Tephrosia purpurea; EA (![]() ) represents ethyl acetate soluble fraction of ethanol extract of Tephrosia purpurea; ‘a’ means not significant compared with control and F value (ANOVA) > Fcrit (4.9646); ‘b’ means P<0.05 compared with control; ‘c’ means P<0.05 compared with TP.
) represents ethyl acetate soluble fraction of ethanol extract of Tephrosia purpurea; ‘a’ means not significant compared with control and F value (ANOVA) > Fcrit (4.9646); ‘b’ means P<0.05 compared with control; ‘c’ means P<0.05 compared with TP.
Macrophages have been involved in the processes of inflammation, allergy and asthma. It is known that during inflammation and associated processes, there is an increased production of superoxide ions [15]. T. purpurea has been reported to have mild antiinflammatory [16], antiallergic and antiasthmatic activities [1,2,17]. It may be possible that the inhibition of superoxide generation in peritoneal macrophages is related to the antiinflammatory activity of the plant T. purpurea.
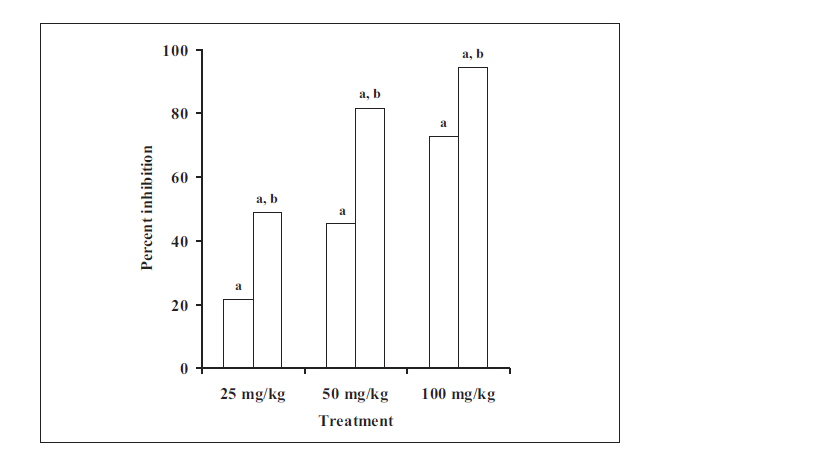
TP at dose of 25, 50 and 100 mg/kg produced significant inhibition of lipid peroxidation induced by CCl4 to the extent of 21.7%, 45.4% and 72.7%, respectively (fig. 2). CCl4 is known to cause liver damage in vivo by generation of free radicals. The homolytic cleavage of the haloalkane, occurring mainly at the hepatic microsomal cytochrome P450 site, gives rise to the trichloromethyl radical (CCl3), which, in turn, rapidly reacts with molecular oxygen yielding the trichloromethylperoxyl radical (CCl3O2·) [18-20]. These free radicals are generally considered to cause the damage to the liver. The present study showed that ethanolic extract of T. purpurea inhibited the lipid peroxidation in vivo. It is also reported to inhibit lipid peroxidation in vitro [7]. It may thus be possible that the hepatoprotective activity of T. purpurea is due to antilipid peroxidation activity.
Fig. 2: Inhibition of lipid peroxidation in vivo by TP and EA Values are expressed as mean±SD of 6 observations; TP ( ![]() ) represents ethanol extract of Tephrosia purpurea; EA (
) represents ethanol extract of Tephrosia purpurea; EA (
![]() ) represents ethyl acetate soluble fraction of ethanol extract of Tephrosia purpurea; ‘a’ means P<0.05 compared with control and F value (ANOVA) > Fcrit (4.9646); ‘b’ means P<0.05 compared with TP.
) represents ethyl acetate soluble fraction of ethanol extract of Tephrosia purpurea; ‘a’ means P<0.05 compared with control and F value (ANOVA) > Fcrit (4.9646); ‘b’ means P<0.05 compared with TP.
In the next part of the study, benzene, chloroform and ethyl acetate soluble fractions were obtained and subjected to DPPH screening assay, in which the ethyl acetate fraction showed best activity [data not shown]. The ethyl acetate fraction was therefore chosen for further studies and results were compared with that reported for ethanolic extract [7].
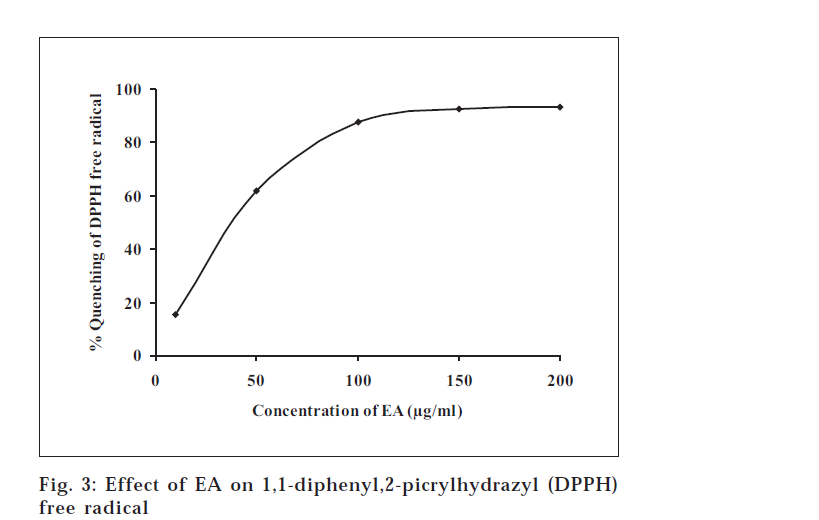
In the free radical scavenging assay, EA was found to interact significantly with the DPPH free radical (fig. 3). EA at concentrations of 10, 50, 100, 150 and 200 μg/ml scavenged the free radical DPPH to the extent of 15.4, 61.9, 87.5, 92.7 and 93.1%, respectively. It is obvious from the results that the free radical activity reached to a saturation level at the concentration of 200 μg/ml. In the lipid peroxidation assay, where MDA production was taken as an index of lipid peroxidation, EA at the concentrations of 10, 20, 40 and 60 mg/ml produced significant inhibition of lipid peroxidation to the extent of 14.2, 32.7, 55.0 and 102.2%, respectively (Table 1).
| Treatment | Malondialdehyde | Percent |
|---|---|---|
| (µg/ml) | (µmol/mg protein ± s.d*) | inhibition |
| Control | 0.963±0.031 | - |
| EA 10 | 0.827±0.020a | 14.18 |
| EA 20 | 0.649±0.075a | 32.65 |
| EA 40 | 0.433±0.019a | 55.01 |
| EA 60 | -0.022±0.018a | 102.24 |
| *Values are expressed as mean±SD of 4 observations. ‘a’ means P<0.05 compared with control and F value (ANOVA) >Fcrit (5.9873); EA represents ethyl acetate soluble fraction of ethanol extract of Tephrosiapurpurea | ||
Table 1: Effect Of Ea On The Lipid Peroxidation In Vitro
In both these assays, the activity of EA was found to be more profound than that reported for TP7. An analysis of the concentration giving 50% inhibition (IC50) illustrates a clearer comparison. In the DPPH assay, the IC50 of EA was found to be 32.9 μg/ml compared to 62.1 μg/ml reported for TP. While in lipid peroxidation assay, The IC50 was 31.9 μg/ml compared to 77.3 μg/ml reported for TP[7]. Thus, we inferred that the EA fraction shows better antioxidant activity than the extract TP in both of these assays.
To substantiate above in vitro observations, the in vivo antioxidant activity of fraction was estimated and compared with TP. Ethyl acetate soluble fraction was subjected to in vivo studies viz. carbon tetrachloride-induced lipid peroxidation in rats and superoxide generation in mice and the corresponding results were compared.
In the superoxide generation assay, EA at the doses of 100, 200 and 400 mg/kg showed significant inhibition of 25.9, 42.8 and 58.9%, respectively. When the activity was compared with that of TP, it was found that TP significantly inhibited the PMA-induced superoxide generation only at higher dose, whilst EA produced better inhibition at the similar doses. There was a significant difference between the inhibition caused by TP and EA (fig. 1).
In carbon tetrachloride-induced lipid peroxidation assay (fig. 2), EA at dose of 25, 50 and 100 mg/kg produced significant inhibition to the extent of 48.8, 81.6 and 94.5%, respectively. On comparing the effect of TP and EA on the CCl4-induced lipid peroxidation, it was found that although both of them inhibited the lipid peroxidation significantly (fig. 2), it was EA that showed better antioxidant activity at similar doses. A significant difference was observed between the inhibition of MDA production by EA and TP.
Thus, in the present investigation, EA had consistently shown better antioxidant activity than TP in different models. The possible reason for these observations may be the fractionation of active constituents. The TP may contain both polar and non-polar constituents. On ethyl acetate fractionation, only the non-polar antioxidant principles might be getting extracted leaving behind the polar components. This fact, in turn may be responsible for the ethyl acetate soluble fraction of ethanolic extract (EA) exhibiting a better antioxidant profile than the ethanol extract (TP).
This indicates that the ethyl acetate soluble fraction may provide an important lead in progressing towards isolation of the antioxidant principles from the plant T. purpurea. The fraction can be further fractionated and the individual components separated out to isolate the most active compound. These sub-fractions or individual components can be exploited in the treatment of various diseases or design of agents with better antioxidant activity.
Acknowledgements
We are thankful to the All India Council for Technical Education, New Delhi, India for providing a research grant and fellowships to two of the authors (KS and PSK). Thanks are extended to Dr. Aashish Phadke for his help in supply and authentication of the plant material for the study. We are also thankful to Dr. Krishna Iyer, Asst. Prof, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai, for his valuable suggestions and advices at different stages of this study.
References
- Gokhale, A.B. and Saraf, M.N., Indian Drugs, 2000, 37, 553.
- Gokhale, A.B. and Saraf, M.N., Indian Drugs, 2000, 37, 346.
- Chauhan, C.K., Nanivadekar, S.A. and Billimoria, F.R., Indian J. Pharmacol., 1992, 24, 107.
- Upadhyay, Y.N., Shukla, K.P., Shankaran, P.S. and Pathak, S.N., J. Med. Sci. Banaras Hindu University, 1964, 5, 97.
- Saleem, M., Alam, A., Ahmed, S., Iqbal, M. and Sultan, S., Pharm. Pharmacol. Commun. 1999, 5, 455.
- Singh, N., Nath, R., Agarwal, A.K. and Kohli, R.P., J. Res. Indian Med. Yoga Homeopathy, 1978, 13, 58.
- Soni, K., Suresh Kumar, P. and Saraf, M.N., Indian J. Pharm.Sci., 2003, 65, 27.
- Cheeseman, K.H. and Slater, T.F., Brit. Med. Bull., 1993, 39, 481.
- Anto, R.B., Kuttan, G., Dinesh Babu, K.V., Rajasekharan, K.N. and Kuttan, R., Pharm. Pharmacol. Commun.,1988, 4, 103.
- Ohkawa, H., Ohishi, N. and Yagi, K., Anal. Biochem., 1979,95, 351.
- Blois, M.S., Nature, 1958, 181, 1199.
- Prashanth Kumar, V., Shashidhara, S., Kumar, M.M. and Sridhara,B.Y., J. Pharm. Pharmacol., 2000, 52, 891.
- Buege, J.A. and Aust, S.D., Methods Enzymol.,1978, 52, 302.
- Pick, E. and Keisari, Y., Cell Immunol., 1981, 39, 301.
- Winrow, V.R., Winyard, P.G., Morris, C.J. and Blake, D.R., Brit.Med. Bull., 1993, 39, 506.
- Singh, N., Nath, R., Agarwal, A.K. and Kohli, R.P., J. Res. Indian Med. Yoga Homeopathy, 1978, 13, 58.
- Raghunathan, K. and Mitra, R., Eds., In; Pharmacognosy of IndigenousDrugs, Vol. II. Central Council for Research in Ayurveda and Siddha,New Delhi, 1982, 938.
- Packer, J.E., Slater, T.F. and Willson, R.L., Life Sci., 1978, 23, 2617.
- Albano, E., Lott, K.A.K., Slater, T.F., Stier, A., Symons, M.C.R. andTomasi, A., Biochem. J., 1982, 204, 593.
- Slater, T.F., Biochem. J., 1984, 222, 1.