- *Corresponding Author:
- Y. Chen
Department of Pediatrics, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou, Fujian Province 350001
E-mail: ycy2728@163.com
| Date of Received | 25 November 2022 |
| Date of Revision | 13 June 2023 |
| Date of Acceptance | 22 January 2024 |
| Indian J Pharm Sci 2024;86(1):369-375 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The study aimed to explore the effect of the nuclear factor-kappa B signaling pathway on apoptosis of rat renal tubular epithelial cells caused by calcium oxalate monohydrate crystals. After being cultivated in vitro, NRK-52E cells were split into a control group, 1 mm calcium oxalate monohydrate group and 10 μM QNZ+1 mm calcium oxalate monohydrate group. The optical density value was detected via cell counting kit-8 assay, the content of lactate dehydrogenase in cell supernatant was detected via colorimetry, and the expressions of nuclear factor-kappa B signaling pathway-related proteins p65 and p50 and B cell lymphoma-2, tumor necrosis factor receptor 1 and caspase-3 were determined through Western blotting. 1 mm calcium oxalate monohydrate could significantly reduce the proliferation ability of NRK-52E cell and cause cytotoxicity. 1 mm calcium oxalate monohydrate significantly up-regulated the expressions of p65, p50, tumor necrosis factor receptor 1 and caspase-3, and significantly lowered the B cell lymphoma-2. Besides, 10 μM QNZ+1 mm calcium oxalate monohydrate obviously down-regulated the expressions of p65, p50, tumor necrosis factor receptor 1 and caspase-3, and obviously increased the B cell lymphoma. The apoptosis of rat renal tubular epithelial cells in rats with calcium oxalate kidney stones may be promoted through activating the nuclear factor-kappa B signaling pathway. Inhibiting the nuclear factor-kappa B activity can alleviate apoptosis and improve renal cell function.
Keywords
Kidney stones, calcium oxalate monohydrate, nuclear factor-kappa B, apoptosis
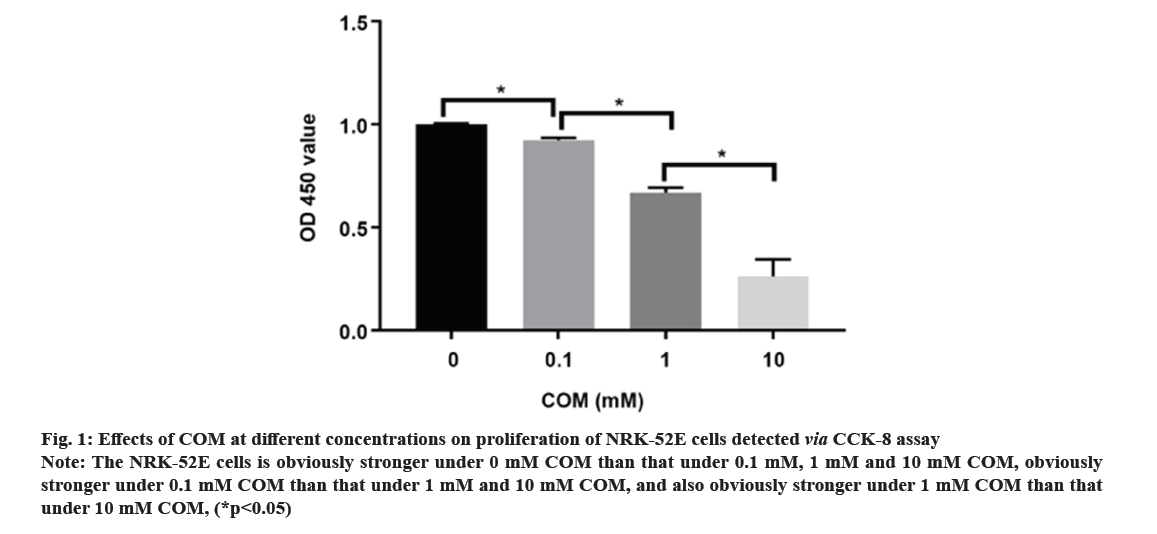
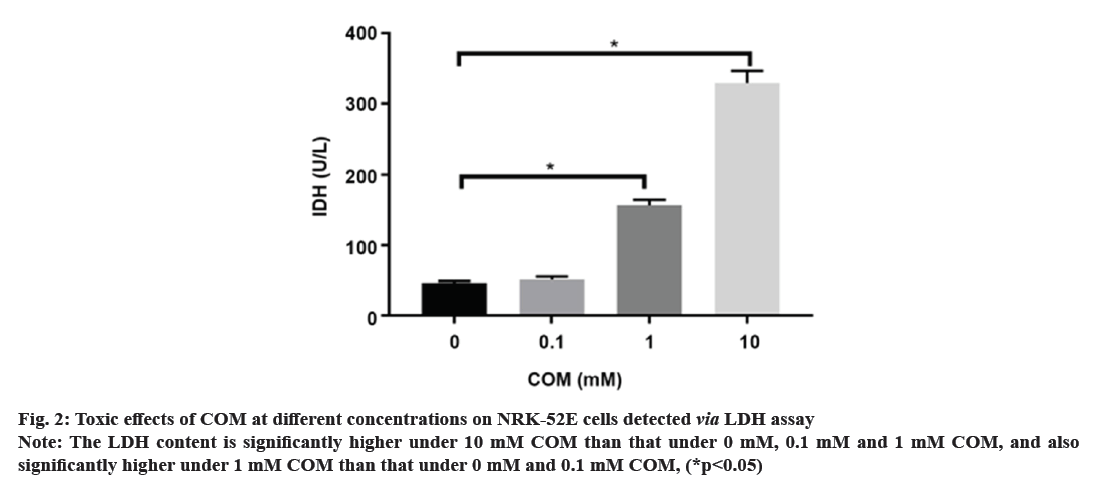
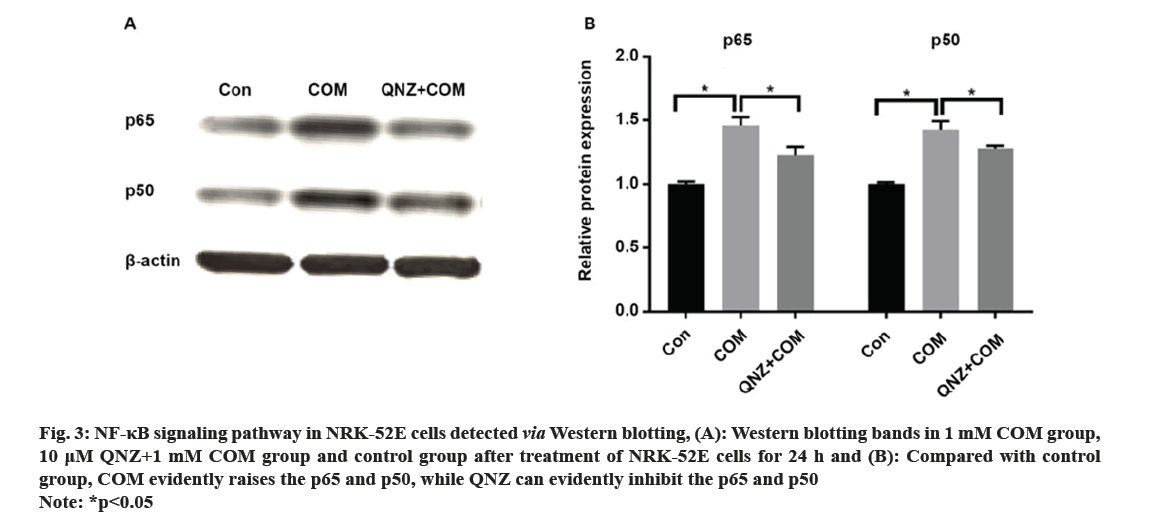
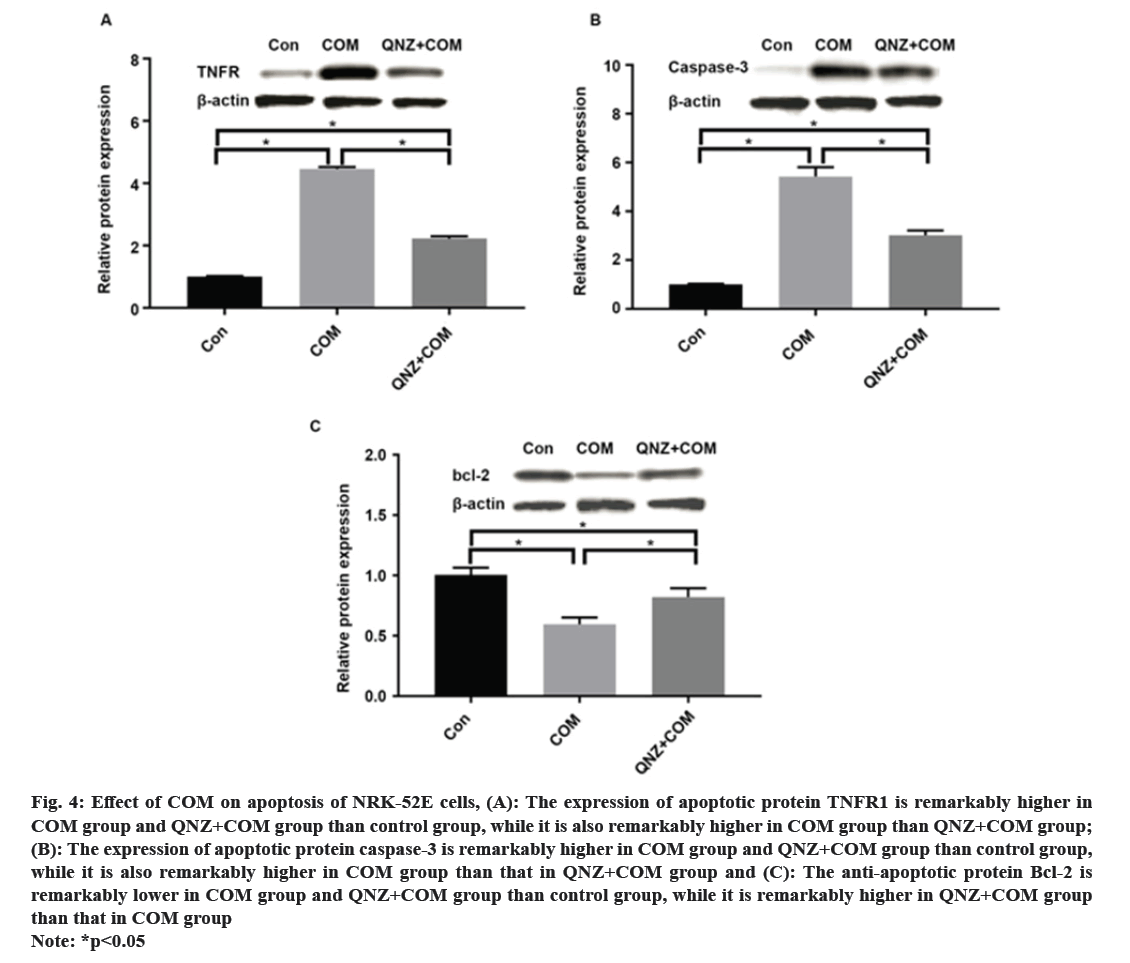
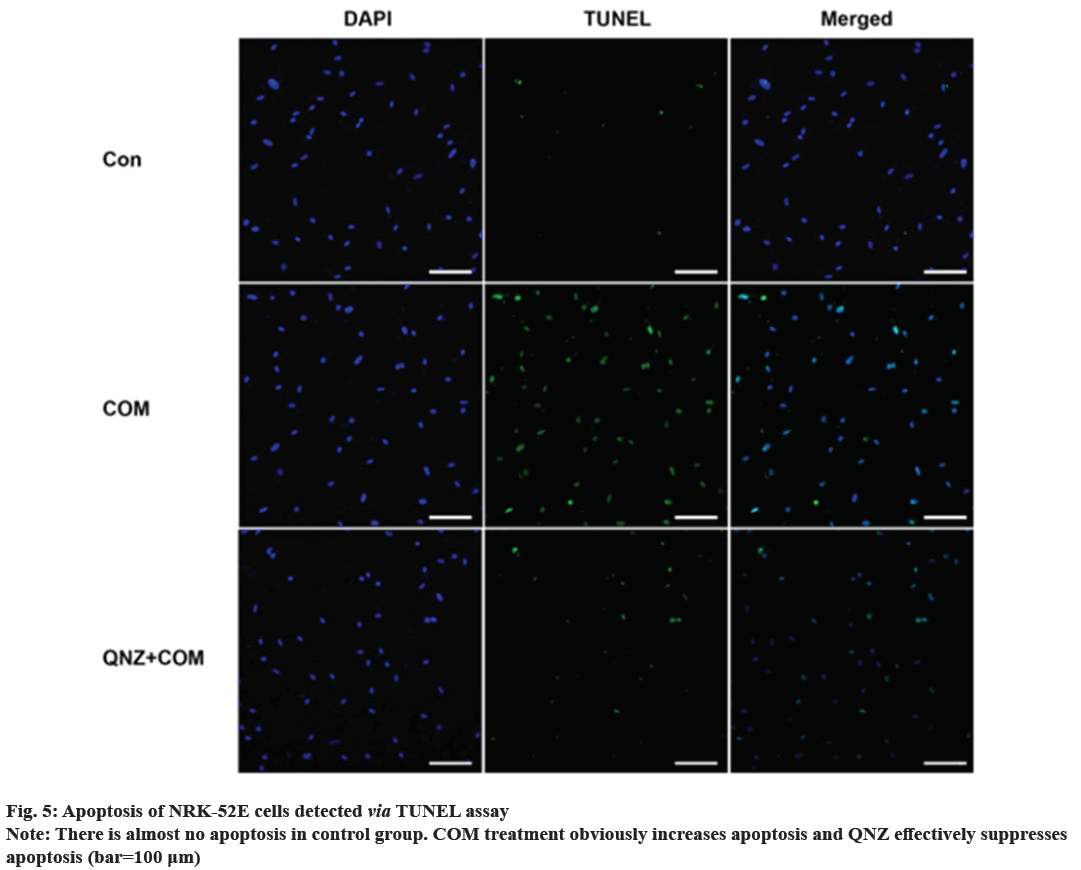
Kidney Stones (KS) are one of the prevalent illnesses of the urinary system, characterized by a high morbidity rate and proneness to recurrence. With the development of disease, the normal kidney function will be seriously affected, harming the patient’s physical and mental health and reducing the social productivity[1]. The pathogenesis of urinary system stones remains unclear, which involves the uptake of stone formation-related substances, formation of stone crystals, crystal adhesion and kidney injury[2]. Oxalate, a metabolic end product, is primarily excreted by the kidneys and is implicated in various pathological conditions. This organic dicarboxylate undergoes filtration in the glomeruli and is subsequently transported bidirectionally into the Renal Tubules (RT)[3]. The most common urinary system stones are calcium oxalate stones, whose main component is Calcium Oxalate Monohydrate (COM). Under normal physiological conditions, the urine in the RT has a high flow rate and there is less adhesion of various substances. Once supersaturation occurs in the urine, crystals may form in the urine, and crystal sedimentation may cause damage to the Renal Tubular Epithelial (RTE) cells[4]. Due to the physical adhesion and the recognition of cell surface molecules, crystals can adhere firmly to renal tubular epithelium, which, as a result, will greatly promote the formation of stones[5]. According to related studies, crystal adhesion is a key initiator for crystal growth and its damage to RTE cells[6]. The transcription factor known as Nuclear Factor Kappa B (NF-κB) plays a crucial part in mediating inflammatory and immune responses by stimulating the production of diverse cytokines and growth factors[7]. In the realm of vertebrates, a total of five members of the NF-κB family have been successfully identified, specifically RelA (p65), NF-κB1 (p50/p105), RelB, c-Rel, and NF-κB2 (p52/p100). These proteins collectively possess the Rel Homology Domain (RHD), which is highly conserved and spans 300 amino acids in the N-terminal region. The RHD is accountable for various crucial functions, including Deoxyribonucleic Acid (DNA) binding, dimerization, nuclear translocation, and interaction with inhibitory κB protein[8]. Previous research has demonstrated that NF-κB is able to promote apoptosis in different stimuli and specific cell types[9]. The induction of NF-κB signaling pathway has been observed to trigger apoptosis in mice suffering from acute kidney injury, while the specific blockage of NF-κB can effectively reduce renal cell apoptosis[10]. However, the association between the expression of NF-κB signaling pathway and the apoptosis of RTE cells in COM-induced KS is rarely studied. Therefore, the primary objective of this study is to investigate the impact of COM on the expression of the NF-κB signaling pathway and apoptosis in RTE cells. The findings of this research endeavor aim to establish a theoretical foundation and potentially offer a therapeutic approach for the investigation and clinical management of KS. NRK-52E cells (Shanghai Cell Bank), Dulbecco’s Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS) and Phosphate-Buffered Saline (PBS) (Gibco), COM (Sigma), p65, p50, B-Cell Lymphoma 2 (BCL-2), Tumor Necrosis Factor Receptor 1 (TNFR1), caspase-3, β-actin antibodies and Horseradish Peroxidase (HRP)-labeled secondary antibodies (Abcam), QNZ (Selleck), TRIzol (Invitrogen), Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) apoptosis assay kit (Beyotime), and 0.22 μm pinhole filter (Millipore). NRK-52E cell culture including the NRK-52E cells were cultured in DMEM supplemented with 10 % FBS under standard conditions of 5 % Carbon dioxide (CO2) at 37°. Upon reaching 90 % confluence, the cells were subjected to two washes with PBS and subsequently digested using 0.25 % trypsin, followed by passage (1:3). Treatment of NRK-52E cells with COM crystal suspension including the COM was resuspended in DMEM containing 10 % FBS and prepared into COM crystal suspension at concentrations of 0.1 mM, 1 mM and 10 mM. NRK-52E cells cultured in the culture plate were added with the above COM crystal suspension at different concentrations. Cell Counting Kit-8 (CCK-8) assay cells were cultured and treated with COM crystal suspension at concentrations of 0.1 mM, 1 mM and 10 mM for 24 h, with those cultured in normal medium as control group at 24 h after different treatment. Subsequently, the supernatant was gathered, and the Lactate Dehydrogenase (LDH) concentration was assessed employing the LDH assay kit. NRK-52E cells were cultured in the 6-well plate and treated with COM crystal suspension at concentrations of 0.1 mM, 1 mM and 10 mM for 24 h, with those cultured in normal medium as control group. At 24 h after different treatment, the cell supernatant was collected and subjected to centrifugation in order to eliminate any impurities. Subsequently, the supernatant was obtained following the centrifugation process, and the LDH content was assessed utilizing the LDH assay kit. Western blotting including the after cells were normally cultured and treated with 1 mM COM and 10 uM QNZ+1 mM COM for 24 h. Then the protein was separated via 8 % Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), transferred onto Polyvinylidene Difluoride (PVDF) membrane, sealed with 5 % skim milk powder and 0.1 % tris-buffered saline with Tween-20, incubated with p65, p50, BCL-2, TNFR1 and caspase-3 primary antibodies at 4° overnight, and then incubated again with HRP-labeled secondary antibodies. Finally, the protein was detected using the Enhanced Chemiluminescence (ECL) reagent, with β-actin as the control. NRK-52E cells were cultured in the 6-well plate, and normally cultured and treated with 1 mM COM and 10 μM QNZ+1 mM COM for 24 h. Then apoptosis was determined according to the instructions of the one-step TUNEL apoptosis assay kit. Finally, the cells were enclosed using an anti-fluorescence quenching blocking buffer and subsequently imaged utilizing a fluorescence microscope. The Statistical Package for the Social Sciences (SPSS) 20.0 was employed to conduct the analysis, specifically utilizing the Chi-square (χ2) test for count data, and was used for measurement data. The t-test was employed to conduct a comparison between two distinct groups, statistical significance was observed (p<0.05). Following a 24 h treatment of cells with varying concentrations of COM, the cell proliferation was assessed utilizing the CCK-8 kit. As shown in fig. 1, the proliferation of NRK-52E cells was suppressed with the increase of COM concentration, which was obviously weaker under 10 mM COM than that under 1 mM COM. Furthermore, the toxic effect of COM on NRK-52E cells was detected via LDH assay, and the LDH content in cell supernatant was detected after COM treatment for 24 h. As shown in fig. 2, the LDH content in supernatant was increased with the increase of COM concentration, which was obviously higher under 10 mM COM than that under 0 mM, 0.1 mM and 1 mM COM, and also obviously higher under 1 mM COM than that under 0 mM and 0.1 mM COM. After NRK-52E cells were treated with 1 mM COM and 10 μM QNZ+1 mM COM for 24 h, the protein expressions of p65 and p50 were detected via Western blotting. The findings indicated that in comparison to the control group, COM evidently raised the p65 and p50, while QNZ could evidently inhibit the p65 and p50 (fig. 3). After NRK-52E cells were treated with 1 mM COM and 10 μM QNZ+1 mM COM for 24 h, the TNFR1, caspase-3 and BCL-2 were detected via Western blotting. The finding revealed that compared with control group, COM remarkably increased the expressions of apoptotic proteins TNFR1 and caspase-3, while QNZ could remarkably decrease the expressions of TNFR1 and caspase-3 (fig. 4A and fig. 4B). Besides, COM remarkably decreased the anti-apoptotic protein BCL-2, while QNZ could remarkably increase the expression of BCL-2 (fig. 4C). The results of TUNEL assay showed that COM treatment increased apoptosis and QNZ could suppress COM-induced apoptosis (fig. 5). The formation of KS is often complicated, and one major cause is that the microcrystals are separated out due to super saturation of urine, and adhere to the RTE cells, thus further forming aggregates, the main component of which is usually COM[11]. The mechanism of adhesion between COM crystals and RTE cells is considered to be mediated by anion molecules or urine macromolecules[12]. The adhesion of COM crystals to RTE cells can cause cell damage, lead to massive production of free radicals in cells and result in death of renal tubular surface cells. After cell death, the adhesion of COM crystals is further promoted, ultimately leading to development of KS[13]. In this experiment, it was found that a large amount of COM adhered to the surface of NRK-52E cells after treatment with COM crystals, and a large number of adherent crystals could still be observed even after being washed with PBS. Studies have demonstrated that COM crystals can aggregate in cluster on the cell surface, and they are wrapped by microvilli and move towards cells under the further movement of microvilli[14], suggesting that the adhesion of COM crystals to cells may be one of the causes of the development of KS. The NF-κB signaling pathway assumes a pivotal role in regulating diverse cellular physiological processes, for instance, cell apoptosis, growth, and differentiation[11]. NF-κB can be induced by various exogenous stimuli, encompassing established carcinogens[15]. It has been found in studies that the NF-κB signaling pathway is activated in kidney injury caused by ischemia-reperfusion[10]. Therefore, there is speculation that the adherence of COM crystals to the surface of NRK-52E cells could potentially lead to the activation of the NF-κB signaling pathway. In this study, the expressions of NF-κB signaling pathway-related proteins were determined, and the results manifested that COM crystals activated the NF-κB signaling pathway and promoted the expressions of p65 and p50 in the pathway. Moreover, as the concentration of COM crystals increases, the proliferation ability of NRK-52E cells declined or apoptosis even occurred. According to the LDH assay, cytotoxicity caused by COM crystals was enhanced with the increase of concentration. The above research demonstrate that COM crystals may cause damage to NRK-52E cells, and the cell injury pathway may be mediated by the activation of the NF-κB due to COM crystals, which may further cause apoptosis. To verify the association between NF-κB signaling pathway and apoptosis, the expressions of apoptosis-related genes were further detected. According to related studies, the NF-κB signaling pathway has a bidirectional regulatory effect, which can promote or resist apoptosis under different stimulation conditions. Research revealed that the activation of NF-κB in RTE cells, induced by elevated glucose levels, can potentially enhance the expression of X-Linked Inhibitor of Apoptosis Protein (xIAP), thus inhibiting apoptosis. NF-κB also activates the release of Tumour Necrosis Factor Alpha (TNF-α), and promotes the expression of caspase-8, leading to apoptosis[16]. In the study on Kupffer cells, elastase-induced activation of NF-κB can up-regulate Fas/Fas Ligand (FasL) and induce apoptosis, but it has no influence on TNF, while inhibiting the NF-κB activity can reduce apoptosis[17]. In the experiments conducted both in vivo and in vitro, the focus was on Human Immunodeficiency Virus (HIV)-induced nephritis, the up-regulation of Fas/FasL expression is observed, causing severe apoptosis, while inhibiting the NF-κB activity can lower the activity of FasL promoter in renal epithelial cells, reduce the mRNA expression of Fas and relieve apoptosis[18]. In the present study, NF-κB activated could promote the TNFR1 and caspase-3, and decrease the BCL-2, while suppressing the NF-κB activity could decrease the expressions of TNFR1 and caspase-3, and increase the BCL-2. The results of TUNEL assay further confirmed the results of apoptosis assay. In conclusion, the apoptosis of RTE cells in rats with calcium oxalate KS may be promoted through activating the NF-κB. The inhibition of NF-κB activity has the potential to mitigate apoptosis and enhance renal cell function.
Fig 1: Effects of COM at different concentrations on proliferation of NRK-52E cells detected via CCK-8 assay
Note: The NRK-52E cells is obviously stronger under 0 mM COM than that under 0.1 mM, 1 mM and 10 mM COM, obviously stronger under 0.1 mM COM than that under 1 mM and 10 mM COM, and also obviously stronger under 1 mM COM than that under 10 mM COM, (*p<0.05)
Fig 3: NF-κB signaling pathway in NRK-52E cells detected via Western blotting, (A): Western blotting bands in 1 mM COM group, 10 μM QNZ+1 mM COM group and control group after treatment of NRK-52E cells for 24 h and (B): Compared with control group, COM evidently raises the p65 and p50, while QNZ can evidently inhibit the p65 and p50
Note: *p<0.05
Fig 4: Effect of COM on apoptosis of NRK-52E cells, (A): The expression of apoptotic protein TNFR1 is remarkably higher in COM group and QNZ+COM group than control group, while it is also remarkably higher in COM group than QNZ+COM group; (B): The expression of apoptotic protein caspase-3 is remarkably higher in COM group and QNZ+COM group than control group, while it is also remarkably higher in COM group than that in QNZ+COM group and (C): The anti-apoptotic protein Bcl-2 is
remarkably lower in COM group and QNZ+COM group than control group, while it is remarkably higher in QNZ+COM group than that in COM group
Note: *p<0.05
Conflict of interests:
The authors declared no conflict of interests
References
- Baumann JM, Affolter B. From crystalluria to kidney stones, some physicochemical aspects of calcium nephrolithiasis. World J Nephrol 2014;3(4):256-67.
[Crossref] [Google Scholar] [PubMed]
- Vervaet BA, Verhulst A, de Broe ME, D’Haese PC. The tubular epithelium in the initiation and course of intratubular nephrocalcinosis. Urol Res 2010;38(4):249-56.
[Crossref] [Google Scholar] [PubMed]
- Kuo SM, Aronson PS. Pathways for oxalate transport in rabbit renal microvillus membrane vesicles. J Biol Chem 1996;271(26):15491-7.
[Crossref] [Google Scholar] [PubMed]
- Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF. Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int 2005;68(4):1543-53.
[Crossref] [Google Scholar] [PubMed]
- Smith CL, St Peter JV. The effect of traditional risk factors for stone disease on calcium oxalate crystal adherence in the rat bladder. Urol Res 2007;35(5):243-6.
[Crossref] [Google Scholar] [PubMed]
- Khan SR. Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron Exp Nephrol 2004;98(2):e55-60.
[Crossref] [Google Scholar] [PubMed]
- Ashizawa M, Miyazaki M, Abe K, Furusu A, Isomoto H, Harada T, et al. Detection of nuclear factor-κB in IgA nephropathy using Southwestern histochemistry. Am J Kidney Dis 2003;42(1):76-86.
[Crossref] [Google Scholar] [PubMed]
- Chaturvedi LS, Koul S, Sekhon A, Bhandari A, Menon M, Koul HK. Oxalate selectively activates p38 mitogen-activated protein kinase and c-Jun N-terminal kinase signal transduction pathways in renal epithelial cells. J Biol Chem 2002;277(15):13321-30.
[Crossref] [Google Scholar] [PubMed]
- Angileri FF, Aguennouz MH, Conti A, La Torre D, Cardali S, Crupi R, et al. Nuclear factor-κB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer 2008;112(10):2258-66.
[Crossref] [Google Scholar] [PubMed]
- Lee JW, Kim SC, Ko YS, Lee HY, Cho E, Kim MG, et al. Renoprotective effect of paricalcitol via a modulation of the TLR4-NF-κB pathway in ischemia/reperfusion-induced acute kidney injury. Biochem Biophys Res Commun 2014;444(2):121-7.
[Crossref] [Google Scholar] [PubMed]
- Tozawa K, Yasui T, Okada A, Hirose M, Hamamoto S, Itoh Y, et al. NF–κB activation in renal tubular epithelial cells by oxalate stimulation. Int J Urol 2008;15(10):924-8.
[Crossref] [Google Scholar] [PubMed]
- Kumar V, Farell G, Yu S, Harrington S, Fitzpatrick L, Rzewuska E, et al. Cell biology of pathologic renal calcification: Contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med 2006;54(7):412-24.
[Crossref] [Google Scholar] [PubMed]
- Sheng X, Jung T, Wesson JA, Ward MD. Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc Natl Acad Sci 2005;102(2):267-72.
[Crossref] [Google Scholar] [PubMed]
- Yasui T, Fujita K, Tozawa K, Asai K, Soji T, Kato T, et al. Calcium oxalate crystal attachment to cultured rat kidney epithelial cell, NRK-52E. Urol Int 2001;67(1):73-6.
[Crossref] [Google Scholar] [PubMed]
- Matthews CP, Birkholz AM, Baker AR, Perella CM, Beck Jr GR, Young MR, et al. Dominant-negative activator protein 1 (TAM67) targets cyclooxygenase-2 and osteopontin under conditions in which it specifically inhibits tumorigenesis. Cancer Res 2007;67(6):2430-8.
[Crossref] [Google Scholar] [PubMed]
- Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, et al. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol 2004;15(1):85-7.
[Crossref] [Google Scholar] [PubMed]
- Peng Y, Gallagher SF, Haines K, Baksh K, Murr MM. Nuclear Factor-κB mediates kupffer cell apoptosis through transcriptional activation of Fas/FasL1,2. J Surg Res 2006;130(1):58-65.
[Crossref] [Google Scholar] [PubMed]
- Ross MJ, Martinka S, D’Agati VD, Bruggeman LA. NF-κB regulates Fas-mediated apoptosis in HIV-associated nephropathy. J Am Soc Nephrol 2005;16(8):2403-11.
[Crossref] [Google Scholar] [PubMed]