- *Corresponding Author:
- C. Gupta
Lloyd Institute of Management and Technology, Greater Noida, Uttar Pradesh 201306, India |
E-mail: chitra.gupta@lloydcollege.in
| Date of Received | 05 May 2021 |
| Date of Revision | 29 April 2023 |
| Date of Acceptance | 21 July 2023 |
| Indian J Pharm Sci 2023;85(4):1126-1136 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Bioanalytical techniques are being widely applied for quantitative estimation of xenobiotics and biotics in biological matrices such as blood, serum, plasma, proteins or urine. They are crucial for supporting new drug applications or biologics license applications. Liquid chromatography-tandem mass spectrometry has become an important tool in pharmaceutical industry as it offers reduced analysis times, improved selectivity and increased throughput in drug bioanalysis. In the present work attempt has been made to develop a novel bioanalytical method for estimation of antihypertensive drug Ramipril and its metabolite Ramiprilat in the plasma samples, by a hyphenated technique which includes liquid chromatography combined with mass spectrometry, using Enalapril and Enalaprilat as internal standards. The developed method was validated as per international council for harmonization guidelines for selectivity, specificity, matrix effect, calibration curve, range, accuracy, precision, dilution integrity and stability etc. It exhibited limit of quantification of 1.09 ng/ml for Ramipril and 1.08 ng/ml for Ramiprilat. The analytes, Ramipril and Ramiprilat were extracted from plasma by liquid chromatography-tandem mass spectrometry using solvent mixtures comprising of acetonitrile, methanol and 0.2 % trifluoro acetic acid as mobile phase and Chromolith speed rod RP 18e gold (50×4.6) column as stationary phase. The validated parameters were within the acceptance criteria as per the regulatory guidelines and the validated calibration curve exhibited r2 value greater than or equal to 0.98 with high recovery. Hence it can be concluded that the developed method was specific, accurate, sensitive, and reliable to quantify Ramipril and its metabolite Ramiprilat in biological samples and can be potentially applied for Pharmacokinetic and bioequivalence studies.
Keywords
Bioanalytical techniques, Ramipril, Ramiprilat, international council for harmonization M10, liquid chromatography-tandem mass spectrometry
Bio-analysis is an emerging sub-discipline of Analytical Chemistry which is widely applied for quantitative estimation of xenobiotics (drugs and their metabolites) and biotics like macromolecules, Deoxyribonucleic Acid (DNA), proteins and metabolites in biological systems[1]. As per the Food and Drug Administration (FDA) guidelines[2,3], a bio-analytical method is a method used for determination of drugs and/or metabolites quantitatively in biological matrices such as blood, serum, plasma, proteins or urine. The development of successful novel pharmaceuticals cannot be achieved without using the data generated via validation of Bio-analytical methods[4,5]. Bio-analytical method validation is the process used to establish the suitability of quantitative analytical method for biochemical applications. Bio-analytical method validation is particularly important for supporting new drug applications or biologics license applications. Pharmacokinetic and bioequivalence studies require very precise[6,7], accurate[8,9], sensitive and reliable assay methods that are well validated to quantify drugs and their metabolites in biological samples. In addition, methods need to be robust and cost effective to conduct specific bioequivalence studies. International Council for Harmonization (ICH M10) provides guideline for validation of bio-analytical methods and perform sample analysis in non-clinical and clinical studies[10,11].
The resemblance of metabolites or more than one medication in a biological sample for the most part requires an advanced detachment for their estimation, particularly, when at least two medications are of comparative physical and chemical nature. In this respect Liquid Chromatography-Mass Spectrometry (LC-MS) has shown promising prospects[12,13]. The method combines the physical partition abilities of fluid chromatography with the mass investigation capacities of mass spectrometry. The recent development of liquid chromatography and mass spectrometry instrumentation has led to reduced analysis times, improved selectivity and increased throughput in drug bioanalysis. It is known that the drug discovery process requires high throughput screening methods and thus Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS) became very important tool in pharmaceutical industry[14]. Different ionization techniques are used for mass spectrometry. In pharmaceutical industry the Electron Spray Ionization (ESI) and Atmospheric Pressure Chemical Ionization (APCI) have been the most commonly used techniques in combination with tandem mass spectrometry[15,16].
In the present study, attempt has been made to develop a bioanalytical method for estimation of Ramipril and its metabolite[17], Ramiprilat[18], using an internal standard Enalapril[19] and its metabolite Enalaprilat by LC-MS-MS[20-22]. Ramipril is a prodrug which is widely used as an effective antihypertensive agent. It is placed with the Angiotensin-Converting Enzyme (ACE) inhibitor class of drugs[23]. It is metabolized into Ramiprilat a dipeptide in the liver and, less significantly, in kidneys[24]. Ramiprilat is an intense, serious inhibitor of ACE[25], the compound responsible for converting Angiotensin (AT)-I to ATII. ATII manages pulse and is a key segment of the Renin-Angiotensin-Aldosterone System (RAAS). Ramiprilat (active metabolite) is used as a cardio protective specialist, a network metalloproteinase inhibitor, and a bradykinin receptor B2 agonist which is utilized in the treatment of hypertension, congestive cardiovascular breakdown, nephropathy, and to lessen the pace of death, myocardial localized necrosis and stroke in people at high danger of cardiovascular failure[26].
Materials and Methods
The reference standard of Ramipril and Ramiprilat used in the study were procured from Vivan Life Sciences Pvt. Ltd., Mumbai with the purity of 99.66 % and 99.86 % respectively. The internal standard of Enalapril and Enalaprilat used in study were also procured from Vivan Life Sciences Pvt. Ltd., Mumbai with the purity of 99.83 % and 99.88 %. Apart from these various chemicals and reagents used in the study were Acetonitrile (High Performance Liquid Chromatography (HPLC) grade. T. Baker), Ammonium Acetate (LR grade, Qualigens fine chemicals, India), Trifluoro acetic acid (HPLC grade, Qualigens fine chemicals, India), Water (HPLC grade, Qualigens fine chemicals, India), Dimethyl Sulphoxide (HPLC grade, Qualigens fine chemicals, India), Formic acid (AR grade, Acros organics), Isopropyl alcohol (HPLC grade, Rankem), Methanol (HPLC grade, J.T. Baker) and biological matrix used in the study were Plasma and K3 EDTA as anticoagulant.
Solution preparation:
Two mobile phase solutions were used in the study i.e.[27], mobile phase A: 0.1 % formic acid in 5 mmol ammonium acetate solution and mobile phase B: 0.1 % formic acid in methanol. The diluent solution was prepared by mixing water and methanol which were transferred into the reagent bottle. Trifluoro acetic acid solution was prepared by making up the volume upto 100 ml with water. Extraction solution was prepared by mixing Acetonitrile, methanol and trifluoro acetic acid solution which was shaken well and sonicated. For needle washing solution methanol, acetonitrile, water and isopropyl alcohol were transferred into a reagent bottle, shaken well and sonicated. All the solutions used were freshly prepared.
Standard and internal standard stock solution:
The standard stock solution of Enalaprilat and Ramiprilat with actual concentration of 1110665.60 ng/ml, 1110443.20 ng/ml were prepared as 1 mg/ml solutions in dimethyl sulfoxide and the standard stock solution of Enalapril and Ramipril with actual concentration of 785949.59 ng/ml and 1010552.40 ng/ml respectively were prepared as 1 mg/ml solutions in methanol. Further dilutions of the Internal Standard (IS) stock solutions were prepared by taking a required amount of aliquot and making up the volumes up to 100 ml. For preparation of Quality Control (QC) Samples, these stock solutions were used. The Calibration samples were prepared by adding 1000 µl of plasma with 400 µl of each analytes and 100 µl of internal standard. Samples for the determination of precision and accuracy were prepared by spiking plasma with the analytes at Lower Limit of Quantification (LLOQ), High Quality Control (HQC), Middle Quality Control (MQC) and Low Quality Control (LQC). For checking the stabilities of the sample quality control samples were bulk spiked and stored in deep freezer (-80°) until analysis.
Method and instrument optimization:
An HPLC system (1290 Infinity II, Agilent Technologies, India) interfaced with model MS/MS (6460 Triple quadrupole[28], Agilent Technologies, India) was used for chromatographic analysis and mass spectral quantification of the analytes and internal standard in multiple reaction monitoring quantification using the ESI ionization. Further, the mass spectrometer was supplied with pure nitrogen gas using nitrogen generator (Kemi) during mass analysis. An HPLC system having following components pump (Model no. G7104A), auto sampler (Model no. G7167B) and column oven (Model no. G7116B) were used for chromatographic isolation of the target analytes and internal standard. The entire instrument (HPLC-MS/MS) management and data acquisition was performed using mass hunter workstation software LC/MS data acquisition for 6460 series Triple quadrupole Version B.08.00. Besides common lab equipment’s like weighing balances, pH meter, Micropipette, Sonicator, Refrigerator, Polypropylene tubes etc. were used. A 6460 Triple Quad/LCMS system, 1290 Infinity II HPLC system (Agilent Technologies) was used for the determination of Ramipril and Ramiprilat in human plasma with different mass optimization parameters such as dwell time, collision energy etc.,[29]. Various parameters were optimized for mass spectrometer as given in Table 1.
| Instrument | Ramipril | Ramiprilat | Enalapril | Enalaprilat |
|---|---|---|---|---|
| LC/MS/MS (Agilent 6460) | LC/MS/MS (Agilent 6460) | LC/MS/MS (Agilent 6460) | LC/MS/MS (Agilent 6460) | |
| Ion Source | ESI Positive | ESI Positive | ESI Positive | ESI Positive |
| Capillary voltage | 5500 | 5500 | 5500 | 5500 |
| Gas temperature (°) | 350 | 350 | 350 | 350 |
| Gas flow (l/min) | 10 | 10 | 10 | 10 |
| Nebulizer (Psi) | 50 | 50 | 50 | 50 |
| Sheath gas heater | 400 | 400 | 400 | 400 |
| Sheath gas flow | 11 | 11 | 11 | 11 |
| Parent mass | 417.1 | 389.1 | 377.1 | 349.1 |
| Product mass | 234.1 | 206.1 | 234.1 | 206.1 |
Table 1: Parameters Optimized for Mass Spectrometry
Separation of analytes from the sample was successfully done via liquid chromatography. The sample was optimized for the different chromatographic conditions stated in Table 2.
| Column | Zorbax eclipse XDB-C8 (50×4.6 mm) 5-micron |
|---|---|
| Column oven temperature | 45°±1.0° |
| Injection volume | 10 µl |
| Rinsing solution | Needle washing solution |
| Mobile phase | Mobile Phase A: Mobile Phase B (0.1 % Formic acid in 5 mmol Ammonium acetate Solution: 0.1 % Formic acid in Methanol: 40:60 Isocratic) |
| Flow rate | 0.600 ml/min |
| Run time | 3 min |
| Retention Time | About 2.13 min for Ramipril |
| About 1.30 min for Ramiprilat | |
| About 1.36 min for Enalapril | |
| About 0.98 min for Enalaprilat |
Table 2: Optimized Chromatographic Conditions for the Sample
Sample preparation:
Sample preparation process was accomplished by protein-precipitation method. The required number of calibration curve standards and quality control samples were withdrawn from the deep freezer and thawed at room temperature. Thawed samples were vortexed to ensure complete mixing of contents.0.200 ml of sample was pipetted into micro centrifuge tube and 0.100 ml (from 102.17 ng/ml of Enalapril and 1006.39 ng/ml of Enalaprilat) of internal standard were added. The contents were vortexed for 30 s. 1 ml of extraction solution was added into each sample and contents were vortexed for 10 min. The samples were then centrifuged for 5 min at 14 000 RPM, 4°- 8°. The samples were then transferred into injector vials and 10 µl injected into LC-MS\MS System.
Method validation:
The developed method for bioanalysis of Ramipril and its metabolite was validated as per (International Council For Harmonization) ICH M10 guideline[30-34]. The method validation included determination of various parameters like blank screening and selectivity, sensitivity, linearity, precision, accuracy, recovery, stability, matrix factor, extended precision and accuracy batch, re-injection reproducibility, ruggedness, carry-over test. Selectivity was done by analyzing the blank samples of the biological matrix which were obtained from the six individual sources. Each blank sample was tested for interference, and selectivity was ensured at the LLOQ. Sensitivity (LLOQ and Upper LOQ (ULOQ)) was quantitatively determined with an acceptable precision and accuracy, which was assessed using three calibration curve standards. The linearity was checked within the concentration range of 1.09 ng/ml to 108.71 ng/ ml and 1.08 ng/ml to 107.56 ng/ml for Ramipril and Ramiprilat weighting least square regression analysis of standard plot associated with eight-point standard curve. The accuracy was measured as the absolute value of the mean values of LLOQ, LQC, MQC1, MQC2 and HQC samples to their respective nominal values, expressed as percentage. Precession was measured with percent coefficient of variance using concentrations of QC samples.
Accuracy was expressed in percent for an absolute ratio of the mean value of calculated concentration of LLOQ, LQC, MQC1, MQC2 and HQC samples to their nominal values. Precession was expressed as percent Coefficient of Variation (% CV) which is the estimation of disperse for concentration acquired for replicate samplings of a homogenous sample. Recovery was determined by comparing the detector response of the Ramipril and ramiprilat and IS from an extracted sample to the detector response of the analytes from an un-extracted sample representing the 100 % recovery. Six replicates of low, medium and high quality control samples were extracted. Enalapril and enalaprilat were added to the quality control samples during processing, concurrently un-extracted samples of the pure authentic standard were prepared, for Ramipril and ramiprilat at concentration representing 100 % extraction of LQC, MQC and HQC samples and Enalapril and enalaprilat concentration representing 100 % extraction. The chemical or physical stability of an analytes in given matrix under specific conditions for given time intervals was measured. The Analytes stability in plasma was determined from various method including freeze-thaw stability in which the stability of the spiked plasma samples was determined during four freeze-thaw cycles. Four replicate numbers of LQC and HQC samples (stability samples) were kept at -70°±15° and were analyzed after fourth freeze thaw cycle against freshly spiked calibration curve standards and freshly spiked QC samples (comparison samples). Bench top stability or short- term temperature stability was determined by analyzing four replicates of LQC and HQC stability samples, which had been kept at room temperature for 5 h 55 min against the freshly spiked calibration curve standards and freshly spiked QC samples (comparison samples). In-injector stability (extracted samples/ auto sampler tray) was determined by analyzing four replicates of LQC and HQC stability samples, which had been processed and kept in Auto sampler for 66 h 25 min and were analyzed against freshly spiked calibration curve standards and freshly spiked QC samples (comparison samples). For determination of Reinjection Reproducibility, all the samples of accepted precession and accuracy batch exercise (reference solution, calibration standards and QC samples) were re-injected after at least 48 h of last injection of QC sample of accepted batch\ruggedness batch exercise. The concentrations obtained were tabulated. Mean concentration, standard deviation, % CV and % nominal values for all re-injected QC samples were determined and the % difference between original concentration obtained and the concentration obtained upon re-injection of each QC sample was calculated. The ruggedness of the method was evaluated by running the Precession and Accuracy batch, employing the same instrument and different analyst. Standard calibration curve were generated, and the concentration of quality control samples was calculated. Carry over effect in matrix was determined for following samples from CC of any accepted batch double blank sample (first injection), LOQ sample (STD-1), ULOQ sample (STD-8), double blank sample (second injection from the same vial used for first injection), double blank sample (third injection from the same vial used for first injection). Then the peak area response at the Retention Time (RT) of the analytes (s) and IS was evaluated by comparing response in all double blank samples against the peak area response of analytes (s) in the extracted LOQ sample.
Data processing:
Chromatograms were obtained using the computer- based software Mass Hunter Workstation Software version B.08.00 supplied by Agilent. The concentration of the unknown was calculated by using regression analysis of spiked calibration standards with appropriate weighting factor i.e., y=mx+b. Where, y=peak area ratio of Ramipril and Ramiprilat to Enalapril and Enalaprilat (IS), m=slope of the calibration curve, x=concentration of Ramipril and Ramiprilat, b=y-axis intercept of the calibration curve.
Results and Discussion
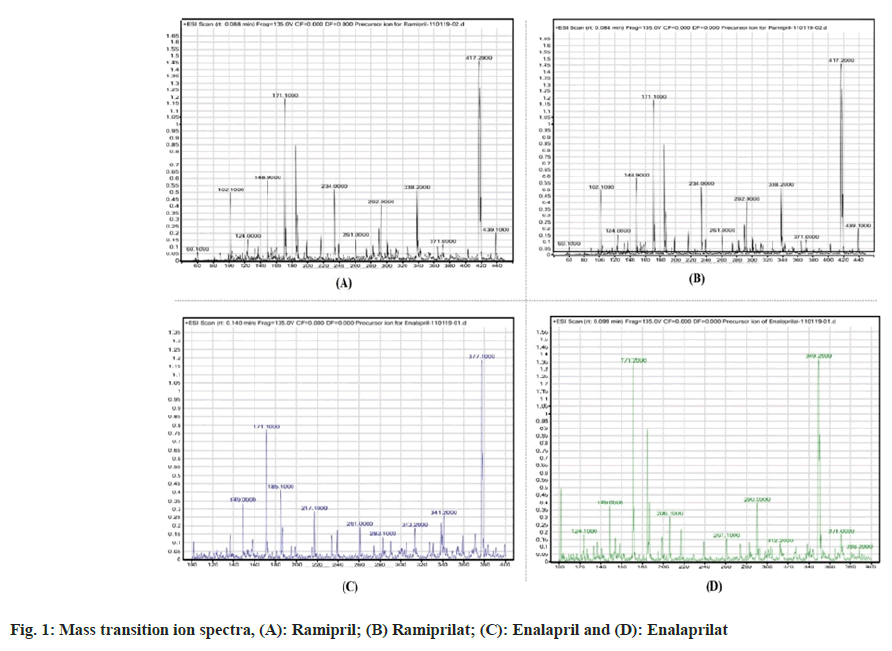
Method development involved the detection of ions of Ramipril, its metabolite Ramiprilat and the internal standards Enalapril and Enalaprilat using mass spectrometry and extraction of Ramipril and Ramiprilat from plasma by Protein precipitation method. Ramipril and Ramiprilat are acidic drugs. Accordingly, a positive ion monitoring mode was adopted in LC-MS assay. The fragmentor voltage was adjusted to different values to obtain different base peaks. It was found that upon increasing the voltage, the intensity of daughter ion at m/z 234.1, 206.1 also increased and it became the base peak at high voltage. Thus, higher sensitivity was achieved at higher voltage selecting the daughter ion at m/z 234.1, 206.1. The mass transition ion spectra of Ramipril, Ramiprilat, Enalapril and Enalaprilat was as given in fig. 1. Various compounds having similar structure and physicochemical properties as that of analytes were tried as internal standards, but the best results were obtained with Enalapril and Enalaprilat which were subsequently used as Internal Standards in the study. Various sample-processing techniques were tested for effective separation of the drug components from endogenous biological matrix and the best result was obtained with protein precipitation method. Hence, it was selected as the optimum extraction technique. Different columns viz. Chromolith performance C18 (100 mm×4.6 mm), Ascentis RP Amide (150×4.6 mm; 5 μm), Hypersil C8 (100×4.6; 5 µm) were used but they demonstrated low response and bad chromatography. But Zorbax Eclipse XDB-C8 (50×4.6 mm) 5-micron was found to be appropriate as it provided a particularly good response. For mobile phase selection, different mobile phases were tried like Acetonitrile (CAN): AA (10 mmol, pH- 6.5) 80: 20 % v/v, ACN: 0.15 % formic acid in 10 mmol A: 90: 10 % v/v, but the chromatographic peak shapes were not good and ion suppression was observed with these mobile phase compositions. Finally, it was decided to use mobile phase B (0.1 % formic acid in 5 mmol ammonium acetate solution: 0.1 % formic acid in methanol: 40:60 Isocratic) as it exhibited good peak shapes, consistency and reproducibility. Different extraction solutions were used like Sodium hydroxide (NaOH), Ammonia with ACN, and the final solution which showed good response was ACN, methanol, 0.5 % Tri fluoro acetic acid.
Different rinsing solutions were used to modify the problem of carry over. Finally, ACN:HPLC grade water: 80:20 % v/v was selected as rinsing solution.
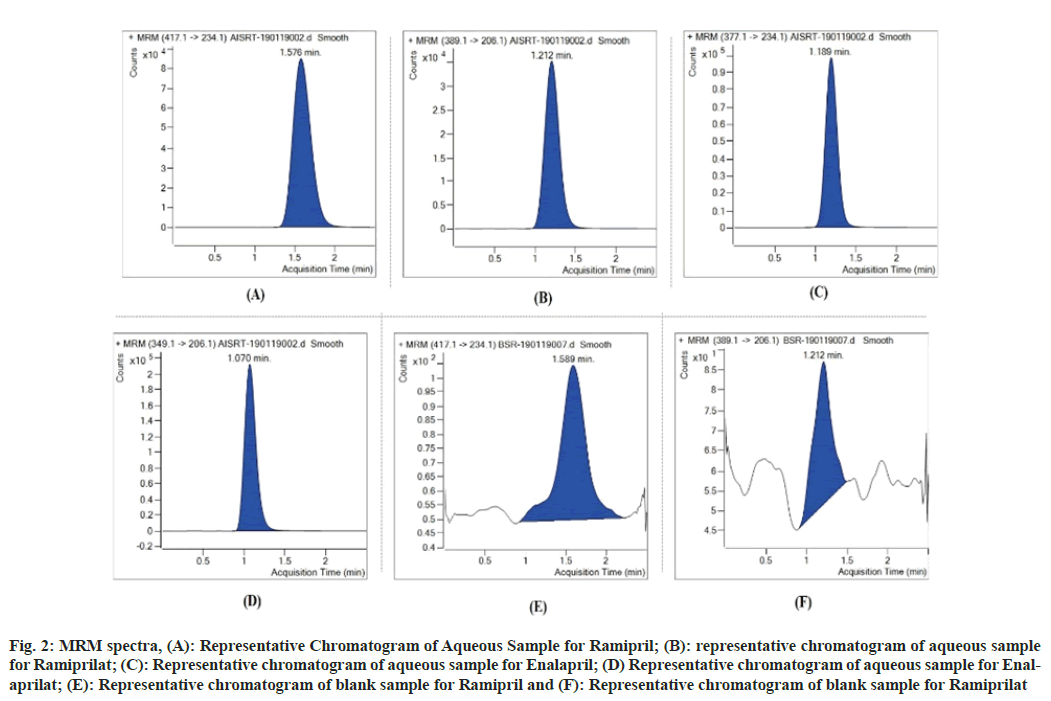
The carry over test is very important to know the sample passing from previous sample to next sample in any analysis either from higher to lower concentration. No peak area was observed at RT of Ramipril, Ramiprilat, Enalapril and Enalaprilat during method validation showing nil carry over effect in matrix as depicted in Table 3, which is as per ICH M10 guidelines. Plasma samples were evaluated, and none showed significant interfering peaks at the retention time of Ramipril, Ramiprilat, Enalapril and Enalaprilat (IS) as seen in fig. 2.
| Name of the analyte | Absence of Matrix | Presence of Matrix | ||
|---|---|---|---|---|
| LQC | HQC | LQC | HQC | |
| Ramipril | 0.0129# | 1.4996# | 1.0085±0.01443* (1.4) | 1.0046±0.00553* (0.6) |
| Ramiprilat | 0.0054# | 0.5791# | 0.9994±0.03195* (3.2) | 0.9844±0.03886* (3.4) |
Note: #Mean area ratio (n=6) and *mean±S.D. (% CV) (n=10)
Table 3: The Effect of Absence of Matrix and Presence of Matrix on Ramipril and Ramiprilat
Fig. 2: MRM spectra, (A): Representative Chromatogram of Aqueous Sample for Ramipril; (B): representative chromatogram of aqueous sample for Ramiprilat; (C): Representative chromatogram of aqueous sample for Enalapril; (D) Representative chromatogram of aqueous sample for Enalaprilat; (E): Representative chromatogram of blank sample for Ramipril and (F): Representative chromatogram of blank sample for Ramiprilat
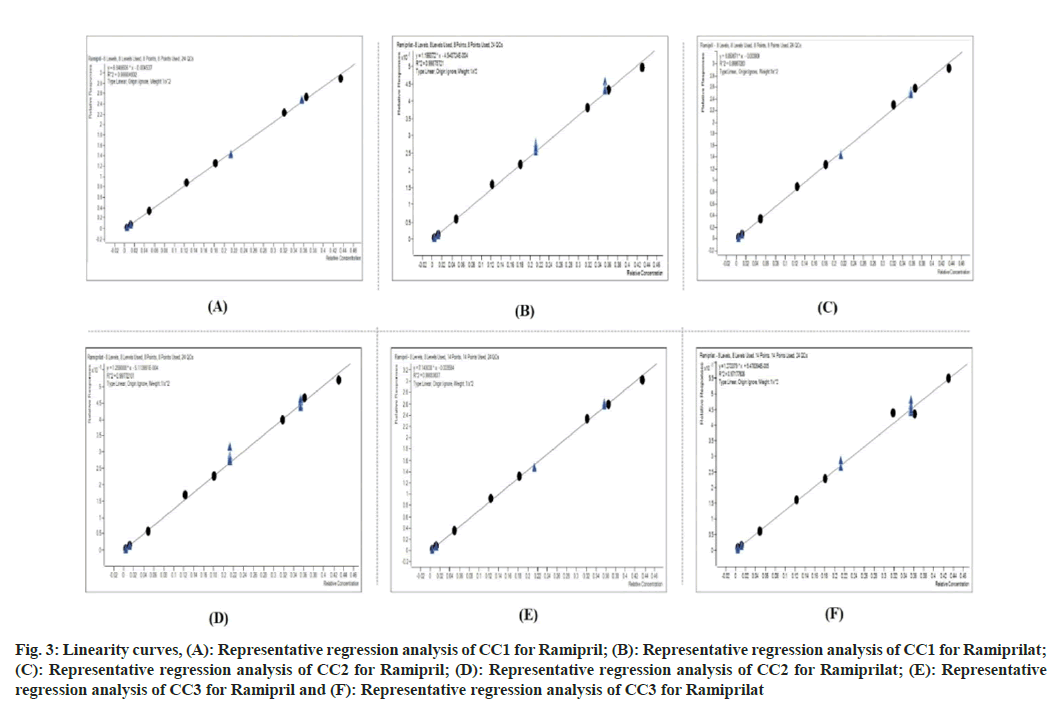
The LOQ was 1.09 ng/ml and 1.08 ng/ml for Ramipril and for Ramiprilat, respectively. The between batch precision and accuracy at LLOQ concentration for Ramipril and Ramiprilat using internal standard ratio method was 2.70 %, 101.12 %, 7.37 % and 99.64 %, respectively. The calibrations were found to be linear in range of 1.09 ng/ml, 108.71 ng/ml, 1.08 ng/ ml and 107.56 ng/ml for Ramipril and Ramiprilat respectively as can be seen in fig. 3. The best–fit calibration lines of chromatographic response vs. concentration were determined by weighted least square regression analysis with weighting factor of 1/Concentration2. The coefficient of determination (r2) was seen to be consistently greater than or equal to 0.98 while validation, which is within limit i.e., should be not less than 0.98.
Fig. 3: Linearity curves, (A): Representative regression analysis of CC1 for Ramipril; (B): Representative regression analysis of CC1 for Ramiprilat; (C): Representative regression analysis of CC2 for Ramipril; (D): Representative regression analysis of CC2 for Ramiprilat; (E): Representative regression analysis of CC3 for Ramipril and (F): Representative regression analysis of CC3 for Ramiprilat
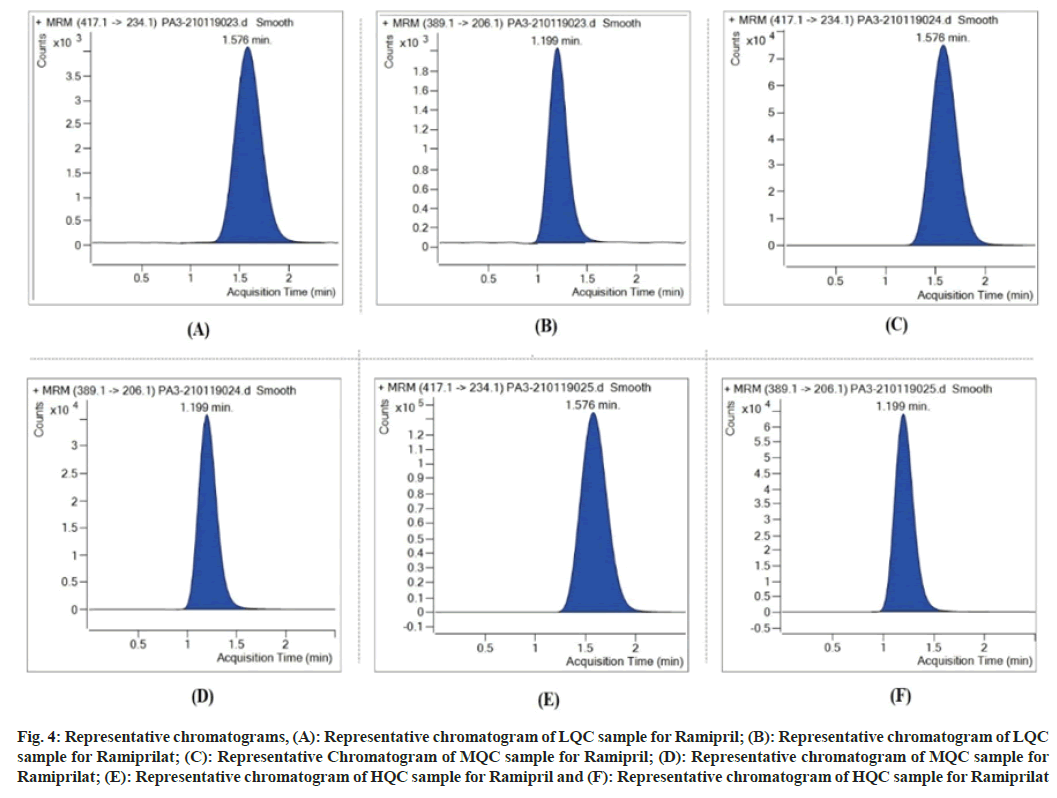
Accuracy as well as precision were measured as between batch, within batch and extended batch. Between batch accuracy using internal standard area ratio method ranged from 94.10 %, 101.95 %, 92.41 % and 104.30 % for Ramipril and Ramiprilat. Within batch accuracy using internal standard area ratio method ranged from 93.67 %, 103.82 %, 89.09 % and 106.61 %, respectively for Ramipril and Ramiprilat. The extended batch accuracy using internal standard area ratio method ranged from 86.94 %, 98.20 %, 98.61 % and 111.92 %, respectively for Ramipril and Ramiprilat. The precision of the assay was measured by the percent coefficient of variation over the concentration range of LLOQC, LQC, MQC and HQC samples of Ramipril and Ramiprilat. The between batch precision using internal standard area ratio method ranged from 0.45 %, 2.70 %, 2.72 % and 7.37 % respectively, for Ramipril and Ramiprilat. The within batch precision using internal standard area ratio method ranged from 0.41 %, 2.65 %, 1.55 % and 7.53 %, respectively for Ramipril and Ramiprilat. The extended batch precision using internal standard area ratio method ranged from 1.64 %, 2.10 %, 12.16 % and 12.86 % for Ramipril and Ramiprilat respectively as reflected in Table 4 and fig. 4.
| Name of analyte | % Nominal (accuracy) | Mean | Mean (SD) | % CV (precession) |
|---|---|---|---|---|
| Intra- batch or within- batch precision and accuracy | ||||
| Ramipril | LLOQC | 0.308 | 0.0065 | 2.65 |
| 101.12 | ||||
| LQC | 0.809 | 0.0149 | 1.8 | |
| 90.9 | ||||
| MQC | 42.832 | 0.609 | 1.4 | |
| 103.7 | ||||
| HQC | 84.231 | 1.2101 | 1.4 | |
| 102 | ||||
| Ramiprilat | LLOQC | 0.215 | 0.0162 | 7.37 |
| 99.64 | ||||
| LQC | 0.0623 | 0.0293 | 4.7 | |
| 102.2 | ||||
| MQC | 24.074 | 1.52 | 6.3 | |
| 98.5 | ||||
| HQC | 60.582 | 3.0346 | 5 | |
| 96.7 | ||||
Table 4: Accuracy and Precession of Ramipril and Ramiprilat
Fig. 4: Representative chromatograms, (A): Representative chromatogram of LQC sample for Ramipril; (B): Representative chromatogram of LQC sample for Ramiprilat; (C): Representative Chromatogram of MQC sample for Ramipril; (D): Representative chromatogram of MQC sample for Ramiprilat; (E): Representative chromatogram of HQC sample for Ramipril and (F): Representative chromatogram of HQC sample for Ramiprilat
The Percent Recovery of Ramipril and Ramiprilat at LQC, MQC and HQC samples were 79.83 %, 81.66 %, 80.19 %, 82.02 %, 86.22 % and 87.05 % respectively. The percentage CV for recovery of inter quality control sample for Ramipril and Ramiprilat was 1.20 % and 3.17 % respectively. The percent mean of recovery was 80.560 % and 85.097 % for Ramipril and Ramiprilat respectively while for Enalapril and Enalaprilat (IS) was 83.58 % and 102.75 % as shown in Table 5.
| LQC | MQC | HQC | ||||
|---|---|---|---|---|---|---|
| Extracted | Non-Extracted | Extracted | Non-Extracted | Extracted | Non-Extracted | |
| Ramipril (n=6) | ||||||
| Mean | 80620.2 | 98794.7 | 1122538 | 1385399 | 2161289 | 2648151 |
| S.D. | 1348.68 | 808.49 | 15703.1 | 7525.37 | 25954.2 | 14406 |
| C.V (%) | 1.67 | 0.82 | 1.4 | 0.54 | 1.2 | 0.54 |
| % Recovery | 79.83 | 81.66 | 80.19 | |||
| Overall recovery | 0.8056 | |||||
| Ramipril (n=6) | ||||||
| Mean | 28075.5 | 32834.5 | 379262 | 442548 | 733613 | 852474 |
| S.D. | 1322.87 | 341.89 | 12497.2 | 4050.48 | 20647.9 | 9458.38 |
| C.V (%) | 4.71 | 1.04 | 3.3 | 0.92 | 2.81 | 1.11 |
| % Recovery | 82.02 | 86.22 | 87.05 | |||
| Overall recovery | 0.85097 | |||||
Table 5: % Recovery of Ramipril and Ramiprilat from Spiked Plasma Samples
Spiked samples were evaluated for Freeze thaw, bench top and in-injector stability. The samples were found to be stable for 4 freeze thaw cycles at -70°±15 °, the comparative stability ranged from 99.34 %, 101.09 %, 100.09 % and 104.01 % for Ramipril and Ramiprilat respectively. They exhibited satisfactory bench top stability (7 h 36 min) and it was found to be 98.95 %, 101.08 %, 96.62 % and 105.40 % for Ramipril and Ramiprilat, respectively. The in- injector, for 107 h 33 min, was found to be 102.18 %, 108.30 %, 111.52 % and 114.37 % for Ramipril and Ramiprilat respectively as seen in Table 6.
| Name of the stability parameter | Ramipril | Ramiprilat | ||
|---|---|---|---|---|
| LQC | HQC | LQC | HQC | |
| Freeze thaw (4 cycles) at-70°±15° | 2.885±0.0100 | 89.940±0.4391 | 2.890±0.1707 | 92.448±7.1935 |
| (0.36) (95.21) | (0.49) (100.90) | (5.91) (96.01) | (7.78) (104.48) | |
| Bench top (07 h 36 min) | 2.870±0.0432 | 89.600±0.8612 | 2,790±0.0622 | 93.685±4.9927 |
| (1.51) (94.72) | (0.96) (100.52) | (2.23) (92.69) | (5.33) (105.88) | |
| In-Injector (107 h 33 min) | 3.075±0.0238 | 92.523±1.1571 | 3.303±0.1112 | 99.120±8.8856 |
| (0.77) (101.49) | (1.25) (103.79) | (3.37) (109.72) | (8.96) (112.03) | |
Table 6: Stability of Ramipril and Ramiprilat at Different Conditions
For long term stability-1, the stability at -20° and -70° ranged from 95.64 %, 97.86 %, 99.43 %, 100.53 %, 97.70 %, 98.11 %, 100.78 % and 100.81 % for Ramipril and Ramiprilat respectively. The stock solution of Ramipril and Ramiprilat and Enalapril and Enalaprilat (IS) were found to be stable for both analytes and IS, stored at room temperature for 7 h. The percent stability of the Stock solution for Ramipril, Ramiprilat and Enalapril and Enalaprilat (IS) was 99.84 %, 99.76 %, 99.30 % and 100.44 %, respectively. The accuracy for two-times diluted concentration was 96.99 % and 95.28 % and four- times diluted concentration was 104.79 % and 106.95 % for Ramipril and Ramiprilat, respectively. The precision for the two times diluted concentration were 0.96 % and 6.99 % for Ramipril and Ramiprilat respectively and the four-times diluted concentration were 1.03 % and 7.59 % for Ramipril and Ramiprilat respectively. The re-injected batch met the acceptance criteria; the percentage difference for 100.00 % and 91.67 % of the re-injected QC samples of Ramipril and Ramiprilat was within 20 (the acceptable limit). Ruggedness tested as the within batch accuracy ranged from 93.89 % to 102.31 % and 92.03 % to
107.56 % for Ramipril and Ramiprilat respectively. The within batch precision ranged from 0.44 % to 1.17 % and 2.29 % to 7.11 % for Ramipril and Ramiprilat, respectively. The results indicated that the observations met the acceptance criteria of linearity, precision and accuracy which were within the limits as per the ICH M10 guideline. Hence, the developed analytical method for determination of Ramipril and Ramiprilat (over a range of 1.09 ng/ml to 108.71 ng/ml and 1.08 ng/ml to 107.56 ng/ml) in human plasma was found to be valid.
Acknowledgements:
The authors are grateful to Arbro Pharmaceuticals Private Limited, New Delhi for providing the necessary facilities to complete the research work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Howard H. Development of bioanalysis-A short history. Bioanalysis 2009;1(1):1-3-7.
[Crossref] [Google Scholar] [PubMed]
- Food and Drug Administration. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products-general considerations. Food and Drug Administration, Washington, DC. 2003.
- Meesters R, Voswinkel S. Bioanalytical method development and validation: From the USFDA 2001 to the USFDA 2018 guidance for industry. J Appl Bioanal 2018;4(3):67-73.
- Tiwari G, Tiwari R. Bioanalytical method validation: An updated review. Pharm Methods 2010;1(1):25-38.
[Crossref] [Google Scholar] [PubMed]
- Moein MM, El Beqqali A, Abdel-Rehim M. Bioanalytical method development and validation: Critical concepts and strategies. J Chromatogr B. 2017;1043:3-11.
[Crossref] [Google Scholar] [PubMed]
- Brahankar DM, Jaiswal SB. Biopharmaceutics and Pharmacokinetics: A treatise. Vallabh Prakashan; 2009.
- Prabu SL, Suriyaprakash TN, Ruckmani K, Thirumurugan R. Biopharmaceutics and Pharmacokinetics. Published online; 2015.
- Center for drug evaluation and research, US. Food and drug administration. ER/in vitro/in vivo correlations. Bioavailability and Bioequivalence; 1997.
- Central drugs standard control organization, directorate general of health services, government of India. Guidelines for bioavailability and bioequivalence-studies 2005;1-33.
- International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. Development safeties update report-E2F, ICH Triplicate efficacy guidelines. Version 4; 2010.
- International Council for Harmonisation Multidisciplinary Guidelines.
- Moreau S, Schad GJ. Application of an LC–MS/MS Library for the Identification and Quantitation of Pesticides in Food Safety Using MRM Spectrum Mode. Column 2017;13(12):12-6.
- Xu QA, Madden TL, editors. LC-MS in drug bioanalysis. Springer Science and Business Media; 2012.
- Sushmaa B, Vijayaraja S. Bioanalytical techniques–an overview. Pharma Tutor 2015;3(9):14-24.
- de Hoffmann E, Stroobant V. Mass spectrometry: Principles and applications. 2nd ed. John Wiley and Sons; 2007.
- Niessen WM. Liquid chromatography-mass spectrometry.3rd ed. CRC press; 2006. p. 50-90.
- Ramipril. DrugBank; 2020.
- Ramiprilat. DrugBank; 2020.
- Enalapril. DrugBank; 2020.
- Enalaprilat. DrugBank; 2020.
- Meisel S, Shamiss A, Rosenthal T. Clinical pharmacokinetics of ramipril. Clin Pharm 1994;26(1):7-15.
[Crossref] [Google Scholar] [PubMed]
- MacFadyen RJ, Meredith PA, Elliott HL. Enalapril clinical pharmacokinetics and pharmacokinetic-pharmacodynamic relationships: An overview. Clinical Pharm 1993;25(4):274-82.
[Crossref] [Google Scholar] [PubMed]
- Levitt DG, Schoemaker RC. Human physiologically based pharmacokinetic model for ACE inhibitors: Ramipril and ramiprilat. BMC Clin Pharmacol 2006;6(1):1-27.
[Crossref] [Google Scholar] [PubMed]
- Nassar AF. Drug metabolism handbook: Concepts and applications. John Wiley and Sons; 2009. p. 800.
- Knütter I, Wollesky C, Kottra G, Hahn MG, Fischer W, Zebisch K, et al. Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther 2008;327(2):432-41.
[Crossref] [Google Scholar] [PubMed]
- Tripathi KD. Essentials of medical pharmacology. JP Medical Ltd; 2013.
- Tan A, Fanaras JC. Use of high‐pH (basic/alkaline) mobile phases for LC–MS or LC–MS/MS bioanalysis. Biomed Chromatogr 2019;33(1):e4409.
[Crossref] [Google Scholar] [PubMed]
- Agilent 6460 Triple quad Mass Spectrometer (K6460) System. Agilent Technologies.
- Chatwal RG, Anand KS. Instrumental method of chemical analysis, Himalaya publishing house. 3rd ed. Mumbai; 2007. p. 2.566-2.302.
- Tan A, Jin W, Deng F, Hussain S, Musuku A, Masse R. Bioanalytical method development and validation using incurred samples-Simultaneous quantitation of ramipril and ramiprilat in human EDTA plasma by LC–MS/MS. J Chromatogr B 2009;877(29):3673-80.
[Crossref] [Google Scholar] [PubMed]
- Yuan B, Wang X, Zhang F, Jia J, Tang F. Simultaneous determination of ramipril and its active metabolite ramiprilat in human plasma by LC–MS–MS. Chromatographia 2008;68:533-9.
[Crossref] [Google Scholar] [PubMed]
- Zhu Z, Vachareau A, Neirinck L. Liquid chromatography–mass spectrometry method for determination of ramipril and its active metabolite ramiprilat in human plasma. J Chromatogr B. 2002;779(2):297-306.
[Crossref] [Google Scholar] [PubMed]
- Gupta VK, Jain R, Lukram O, Agarwal S, Dwivedi A. Simultaneous determination of ramipril, ramiprilat and telmisartan in human plasma using liquid chromatography tandem mass spectrometry. Talanta 2011;83(3):709-16.
[Crossref] [Google Scholar] [PubMed]
- Lu XY, Shen-Tu JZ, Liu J. High-performance liquid chromatography–mass spectrometric analysis of ramipril and its active metabolite ramiprilat in human serum: Application to a pharmacokinetic study in the Chinese volunteers. J Pharm Biomed Anal 2006;40(2):478-83.