- *Corresponding Author:
- Kiran Bhise
Allana College of Pharmacy, K. B. Hidayatullah Road, Azam Campus, Pune - 411 001, India
E-mail: prof.kiranbhise@yahoo.com
| Date of Submission | 19 June 2006 |
| Date of Revision | 23 July 2007 |
| Date of Acceptance | 5 September 2007 |
| Indian J Pharm Sci, 2007, 69 (5): 616-625 |
Abstract
The plethora of drug therapies and types of drugs demand different formulations, fabrications conditions and release kinetics. No single polymer can satisfy all the requirements. Therefore there have been tremendous advances in area of biodegradable copolymers over the last 30 years. This article reviews current research on biodegradable polymers, focusing their potential as drug carries. The major classes of polymers are briefly discussed with regard to synthesis, properties and biodegradability, and known degradation modes and products are indicated based on studies reported in the literature. A vast majority of biodegradable polymers studied belongs to the polyester family, which includes polyglycolides and polylactides. Other degradable polymers such as polyorthoesters, polyanhydrides and polyphosphazenes are also discussed and their advantages and disadvantages are summarized.
Keywords
Erosion, polyorthoesters, polyanhydrides, polyphosphazenes
Polymers first developed in search for biodegradable suture materials have been proven to be useful and successful for long-term drug delivery applications. Biodegradable polymers are highly desirable in these situations because they degrade in the body to biologically inert and compatible molecules. By incorporating drugs in biodegradable polymers, dosage forms that release the drug over a prolong length of time can be prepared in variety of shapes and sizes. No surgical procedures are needed after completion of dosage regime since the remaining polymer will degrade and get cleared by the body. As a result, biodegradable polymers offer a novel approach for developing sustained release drug delivery systems that are simple and convenient to patient.
Advantages of Biodegradable Polymers as Drug Carriers
A polymer is a large molecule composed of many smaller units called monomers that are bonded together. In addition to eliminating the necessity of removal, the five key advantages [1] that polymeric drug delivery products can offer are; localized delivery of drug, sustained delivery of drug, stabilization of the drug, release rate which is less dependent of the drug properties and steadier release rate with time. In diffusion controlled systems the release rate typically declines with time. On the other hand, a biodegradable system may yield a constant release even with a simple monolithic device if matrix degradation can compensate for this decline, perhaps with an increase of drug permeability.
Factors Affecting Polymer Selection
If an application requires rapid development and commercialization, than the polymer selection will most likely be made from among those polyesters that have already received regulatory approval. Another factor to be taken into account is choice is choice, whether to use homopolymers consisting of single monomeric repeating unit or copolymers containing multiple monomer species. If copolymers are employed than the relative ratio of the different monomers may be manipulated to change polymer properties listed in Table 1 including bulk hydrophilicity, morphology, structure, and extent of drug polymer interactions. A review that describes in detail the relationship between polymer properties and performance in drug delivery applications have been published [2]. Ultimately all these properties will influence the performance of the drug delivery system via changes to the relative rates of mass transport (e.g., water in and solute or drug out of the system) and the degradation rate of both, the polymer and the device.
| Property | Examples |
|---|---|
| Regulatory and toxicology status | - |
| Monomer or copolymer composition | - |
| Molecular weight | Weight average |
| Number average | |
| Molecular weight distribution | Polydispersity ratio |
| Molecular architecture | Liner polymers |
| Branched polymers | |
| Crosslinked network | |
| Tacticty | Isotactic |
| Syndiotactic | |
| Atactic | |
| Secondary structural attributes | Helicity |
| Beta structure | |
| Amorphous | |
| Morphology | Semi crystalline |
| Crystalline | |
| Melting temperature | |
| Thermal transition temperatures | Glass transition temperature |
| Side-chains | |
| Ionization | Main-chain end groups |
Table 1: Properties Affecting Polymer Selection, Manufacture, And Performance
Polymer Erosion Mechanism
In some cases, the term ‘biodegradation’ is limited to the description of chemical processes (chemical changes that alter either the molecular weight or solubility of the polymer) while ‘bioerosion’ may be restricted to refer to physical processes that result in weight loss of a polymer device. The possibility for a polymer to degrade and to have its degradation by-products assimilated or excreted by living system is designated as bioresorbable.
Degradation by erosion normally takes place in devices that are prepared from soluble polymers. In such instances, the device erodes as water is absorbed into the systems causing the polymer chains to hydrate, swell, disentangle, and ultimately dissolved away from the dosage form. Alternatively, degradation can also result from chemical changes to the polymer including cleavage of covalent bonds, ionization and protonation either along the polymer backbone or on pendent side chains. General factors affecting biodegradation of polymers are listed in Table 2. The erosion mechanism of polymers can be described both physically and chemically.
| Factors |
|---|
| Chemical structure and composition. Distribution of repeat units in multimers. Presents of ionic groups. Presence of unexpected units or chain defects. ConÞguration structure. Molecular weight and Molecular weight distribution. Morphology-amorphous/semicrystalline, microstructures, residual stresses. Presence of low-molecular-weight compounds. Processing conditions. Annealing. Sterilization process. Storage history. Shape. Site of implantation. Adsorbed and absorbed compounds like water, lipids and ions. Physicochemical factors like ion exchange, ionic strength and pH. Physical factors like shape and size changes, variations of diffusion coefficients, mechanical stresses, stress- and solvent-induced cracking. Mechanism of hydrolysis |
Table 2: General Factors Affecting Biodegradation Of Polymers
Chemical erosion
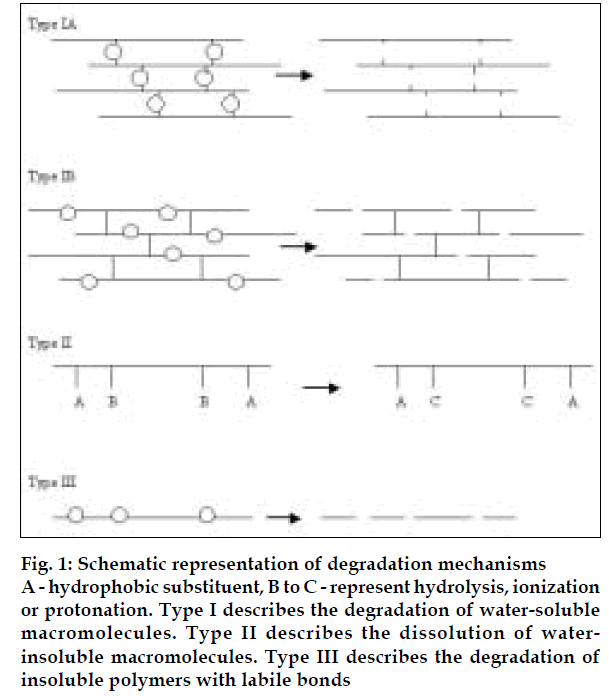
Heller [3] describes three general chemical mechanisms that cause bioerosion (Table 3). Mechanism I describes the degradation of water-soluble macromolecules that are crosslinked to form three-dimensional network. As long as crosslinks remain intact, the network is intact and is insoluble. Degradation in these systems can occur either at crosslinks to form soluble backbone polymeric chains (type IA) or at the main chain to form water-soluble fragments (type IB). Generally, degradation of type IA polymers provide high molecular weight, water-soluble fragments, while degradation of type IB polymers provide low molecular weight, water soluble oligomers and monomers (fig. 1)
| Polymer | Mechanism of erosion |
|---|---|
| Poly (vinyl alcohol) | Type I |
| Gelatin | Type I |
| Collagen | Type I and II |
| Polyanhydrides | Type III |
| Polycaprolactone and its copolymers | Type III |
| Poly (ortho esters)s | Type III |
| Poly (lactic acids)s, | Type III |
| poly (glycolic acid)s and there copolymers |
Table 3: Classification Of Biodegradable Polymers Following The Heller Terms [3].
Fig 1: Schematic representation of degradation mechanisms A - hydrophobic substituent, B to C - represent hydrolysis, ionization or protonation. Type I describes the degradation of water-soluble macromolecules. Type II describes the dissolution of waterinsoluble macromolecules. Type III describes the degradation of insoluble polymers with labile bonds
Mechanism II describes the dissolution of water-insoluble macromolecules with side groups that are converted to water-soluble polymers as a result of ionization, protonation or hydrolysis of the groups. With this mechanism the polymer does not degrade and its molecular weight remains essentially unchanged. Materials displaying type II erosion include cellulose acetate derivatives and partially esterified copolymers of maleic anhydride. These polymers become soluble by ionization of carboxylic group as shown by type II erosion.
Mechanism III describes the degradation of insoluble polymers with labile bonds. Hydrolysis of labile bonds causes scission of the polymer backbone, thereby forming low molecular weight, water-soluble molecules. Polymers undergoing type III erosion include poly (lactic acid), poly (glycolic acid) and their copolymers, poly (ortho esters), polyamides, poly (alkyl-2-cyanoacrylates) and polyanhydrides. The three mechanisms described are not mutually exclusive; combinations of them can occur.
Physical erosion
The physical erosion mechanisms can be characterized as heterogeneous or homogeneous. In heterogeneous erosion, also called as surface erosion, the polymer erodes only at the surface, and maintains its physical integrity as it degrades. As a result drug kinetics are predictable, and zero order release kinetics can be obtained by applying the appropriate geometry. Crystalline regions exclude water. Therefore highly crystalline polymers tend to undergo heterogeneous erosion. Few polymers exhibit heterogeneous erosion. Most polymers undergo homogeneous erosion, means the hydrolysis occurs at even rate throughout the polymeric matrix. Generally these polymers tend to be more hydrophilic than those exhibiting surface erosion. As a result, water penetrates the polymeric matrix and increases the rate of diffusion. In homogeneous erosion, there is loss of integrity of the polymer matrix. Synthetic condensation polymers are generally biodegradable to different extents depending on chain coupling (ester>ether>amide>uret hane), morphology (amorphous>crystalline), molecular weight (lower>higher), and hydrophilic is faster than hydrophobic. However, if a polymer is water soluble, that does not necessarily mean that it is biodegradable [4].
Drug Release Mechanisms
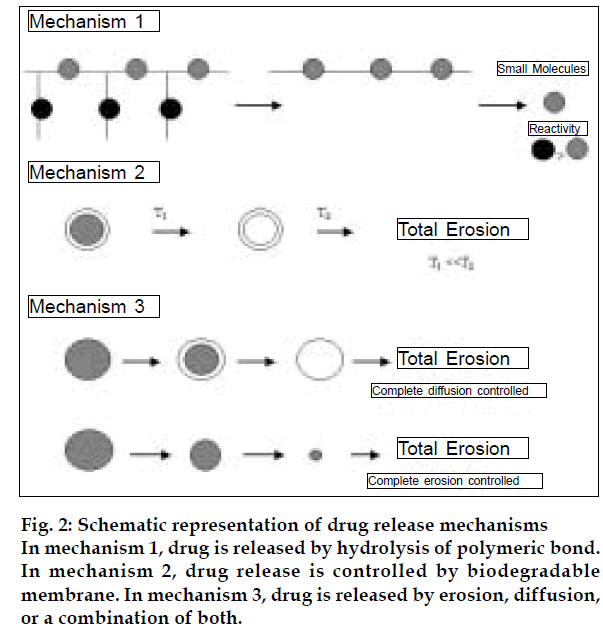
The release of drugs from the erodible polymers may occur by any of the mechanisms presented in (fig. 2) [5]. In mechanism 1, the drug is attached to the polymeric backbone by a labile bond, this bond has a higher reactivity toward hydrolysis than the polymer reactivity to break down. In mechanism 2, the drug is in the core surrounded by a biodegradable rate controlling membrane. This is a reservoir type device that provides erodibility to eliminate surgical removal of the drug-depleted device. Mechanism 3 describes a homogeneously dispersed drug in the biodegradable polymer. The drug is released by erosion, diffusion, or a combination of both.
Characteristics Of Some Major Biodegradable Polymers
Polyanhydrides
Historically, polyanhydrides were developed in textile industry during the first half of the 20th century as alternate fiber materials. The anhydride linkages of these polymers are, in general, more hydrolytically liable than the polyester bond. In order to achieve a surface eroding mechanism, polymers are generally prepared from very hydrophobic monomers in order to minimize water penetration into the bulk of the device6. By doing this, hydrolysis of liable anhydride linkages would be restricted to the outer exposed surfaces of the polymer device. A wide variety of aromatic and aliphatic monomers are used to prepare surface eroding polyanhydride polymers [7,8].
Polymers with increasing hydrophobicity can be made from aromatic monomers including phthalic acid and various carboxyphenoxyalkanes such as poly[1-bis(p-carboxyphenoxy)methane] (CPM), poly[1,3-bis(p-carboxyphenoxy)propane] (CPP) and poly[1,6-bis(p-carboxyphenoxy)hexane] (CPH). High-molecular-weight polyanhydrides are usually synthesized by first converting the dicarboxylic acid monomer to mixed anhydride prepolymers using acetic anhydride followed by polymerization of prepolymers using polycondensation reaction in the melt.
Typically, homopolymers are not studied because they posed unfavorable characteristics rendering their handling and manufacture difficult. Therefore, polyanhydrides are often prepared as copolymers of aliphatic and/or aromatic monomers. The most common copolymers under investigation in drug delivery include poly[fatty acid dimmer (FAD)-sebacic acid (SA)] and poly(CPP-SA).
Studies on aliphatic polyanhydrides have shown that increasing the alkyl chain length (from n=4 to 12) of the dicarboxylic acid monomers increases polymer hydrophobicity resulting in a decrease in both polymer degradation and drug release rates [8]. By varying the ratio of the hydrophobic moiety CPP and sebacic acid (SA), controlled degradation rates, from days to years, have been achieved.
The release of number of drugs from polyanhydride matrices has been studied including p-nitroaniline, ciprofloxacin [8], cortisone acetate, insulin [9] and variety of proteins. The only commercial product that has received regulatory approval is Gliadel® which contains carmustine.
Poly(ortho esters)
Poly(ortho esters) have been under development since 1970 [10,11]. Poly(ortho esters) are mainly divided in three major families; Poly(ortho ester) I, II and III. Poly(ortho ester) I, the first such polymer prepared, has been developed at Alza Corporation. This polymer was originally designated as Chronomer™ but the name was later changed to Alzamer®. Poly(orthoester) II was developed at Stanford Research Institute also known as SRI International.
Poly(ortho ester) I is hydrolysed when placed in an aqueous environment. Because the ortho ester linkages are sensitive and hydrolysis of this polymer produces γ-butyrolactone, which rapidly opens to γ-hydroxybutyric acid, the polymer must be stabilized with a base such as Na2CO3 to avoid an uncontrolled, autocatalytic hydrolysis reaction [12].
The use of a crosslinked poly(ortho ester) to release 5- fluorouracil (5-FU) and Luteinizing hormone releasing hormone (LHRH) analog intended for glaucoma filtration surgery and contraception, respectively, has been described [13]. The crosslinking density and a readily erodable component, 9,10-dihydroxystearic acid, in the polymer controls the release kinetics. A near-constant release is observed in some cases as a result of the increasing permeation rate caused by the cleavage of the crosslinking bonds. Release of LHRH on the other hand follows a biphasic profile. The initial release corresponds to diffusional release, and later to the hydrolytic liberation of the protein that has been chemically bound to the matrix during fabrication. Studies focusing on the erosion characteristics of catalyzed poly(ortho ester) matrices [14] and the effect of crosslinking and base incorporation on the polymer degradation behavior, have also been reported [15].
Poly (ortho ester) III is a semisolid material that has been shown to be highly biocompatible and is currently being investigated as an adjunct to glaucoma filtering surgery and other ocular applications. However, the polymerization is difficult to control and is not readily scaled up. Now-a-days poly(ortho ester) IV can also be easily prepared in a highly reproducible manner. It is currently under development for a variety of applications, such as ocular delivery, protein release, post-operative pain treatment and post-operative cancer treatment [16].
Phosphorus containing polymers
There are two major classes of phosphorus containg polymers, the phosphazenes and phosphoesters [17]. The class of phosphoesters based polymers includes polyphosphonates, polyphosphates and polyphosphites. The copolymer, poly(lactide-co-phosphate) exhibited faster degradation as the phosphate content increased. This polymer is currently being investigated by Guilford Pharmaceuticals for delivery of the chemotherapeutic agent paclitaxel by the name Paclimer®.
The class of poly(phosphazenes) were first explored as drug carriers in 1983 to deliver naproxen [18]. Previous studies suggested that the degradation products of this polymer consist of ammonia, glycine or alanine, ethanol or benzyl alcohol, phosphate, and the side group substituents. It appears that polymers would be attractive candidates for pendant delivery system [19].
Polyesters
The first biodegradable synthetic polymer and bioabsorbable suture material was poly(glycolic acid) (PGA), which appeared in 1954 belongs to this class [20,21]. So far, drug delivery devices based only on polymers and copolymers deriving from lactic acid (LA) enantiomers, glycolic acid, and ∈-caprolactone (PLA, PGA, and PCL, respectively) has been commercialized. The prospective applications include devices to treat cancer, drug addiction, and infection, as well as drugs for contraception, vaccination, tissue regeneration and cartilage tissue engineering. A number of products are commercially available such as Decapeptyl®, Lupron Depot®, Zoladex®, Adriamycin®, and Capronor®.
The use of PGA homopolymer is limited to suture material because of its high crystallinity and absence of practical solvent. There are two main routes to synthesize these aliphatic polyesters [22], polycondensation of bifunctional hydroxy acids and ring opening polymerization of cyclic esters monomers. The main route to synthesize high molar mass PLA, PLA, and PCL are the ring opening polymerization of heterocyclic monomers, namely lactide, glycolide or ∈-caprolactone.
With regard to monomer synthesis, the synthesis of lactides and glycolide consists of two steps, Lactic acid or glycolic acid is first polycondensed to yield low molar mass oligomers. Then, oligomers are thermally depolymerised to form the corresponding cyclic diester, which is recovered by distillation at low pressure. Catalysts such as zinc metal or zinc oxide are used in the second step to improve the yield. For polymer synthesis, the conversion of cyclic monomers to polymer chains requires the use of initiators or catalysts. Two compounds are used industrially, namely Tin (II) 2-ethyl hexanoate (stannous octoate) and Zn metal. Stannous octoate is approved by USFDA for surgical and pharmacological applications, although it is very unstable and usually contains impurities. Polymerization techniques used are, ring opening polymerization, anionic polymerization, cationic polymerization and insertion-coordination polymerization. The copolymers of lactic and glycolic acids (PLGA) remain a popular choice as the biodegradable drug carrier. In an attempt to avoid the use of catalysts, bulk polycondensation techniques are used to obtain low molecular weight PLGA (MW = 1600 to 3300) [23].
Manufacturing methodology
The various drug delivery devices can be divided into two types: monolithic and reservoir type [24]. These devices can be classified into two categories, implantables and injectable devices. Implantable large devices such as cylinders, pellets, slabs, disks, and films thicker than 0.1 mm are usually prepared by compression molding an intimate polymer-drug mixture. However, there is risk of thermal degradation. Tubings and needle like implants can be obtained by extrusion [25]. Implantable 1,3-bis(2-chloroethyl)- 1-nitrosourea (BCNU) loaded PLGA wafers are developed by compressing molding of mixtures of BCNU and PLGA [26]. The wafers produced are 5 mm (diameter) × 1 mm (thickness) in size with a flat surface.
Among the various injectable drug delivery systems, microparticles [27] are the most widely investigated. There are basically three methods to manufacture monolithic microparticles: grinding, phase separation, and solvent evaporation. Grinding method is suitable for water-soluble drugs [28]. The phase separation method is successfully used for highly water-soluble peptides into PLGA copolymers [29]. The solvent evaporation method has been largely used to encapsulate lipophilic drugs [30]. Surfactants used during emulsification are poly(vinyl alcohol), poly(vinylpyrrolidone), alginates, and gelatins. PLGA provides the additive advantages of being fully biodegradable and subsequent obviating the problems associated with permanent implants based on non-biodegradable polymers [31]. Especially, the biocompatibility of PLGA was proven in the brain of rodents and human [32].
In examining the effect of gamma-sterilization on the stability and degradation of the copolymers in microsphere form, it is found that sterilization decreases the molecular weight by 30 to 40% as determined by gel permeation chromatography. The polymer undergoes catastrophic disintegration when the molecular weight is approximately 25000. Even on storage at room temperature the molecular weight of these gamma-irradiated samples declines, upto approximately 40% of their original value in 9 months. This decline of molecular weight caused by sterilization in turn affects the release profile [33].
The bulk erosion mechanism of biodegradation established for PLGA is detrimental to protein molecules due to the fact that the acid-catalyzed hydrolysis of the PLGA ester bonds throughout the formulation results in an accumulation of acidic PLGA chains within the center of the formulation. This result in an acidic microenvironment, which can damage or denature protein molecules [34]. In addition, DNA was damaged in the acidic environment created by PLGA degradation products [35]. Another drawback of PLGA is the extreme hydrophobicity of the polymer along with triphasic protein release kinetics [36]. Several encapsulation techniques, mainly using biodegradable PLGA, have been reported, such as spray-drying and modified double emulsion methods [35], all of which utilize high-speed homogenization or sonication. These shear forces are found to compromise plasmid integrity and bioactivity [37-39].
To overcome the drawbacks of PLGA, a polymer that is more hydrophilic in nature than PLGA was created to modify the release kinetics of proteins from the formulation [40]. Several types of modified polymers were synthesized, most prominently the block copolymers consisting of PLA or PLGA alternating with hydrophilic molecules, such as poly (ethylene oxide) (PEO) or poly (ethylene glycol) (PEG) [41,42]. Branched structures were also developed, including star-branched copolymers using sugars or dextran as backbones grafted with several PLGA chains [43].
Branched polyesters
Branched polyesters consisting of poly (vinyl alcohol) (PVA) grafted with chains of poly (lactic-co-glycolic acid) (PLGA) represent a new class of biodegradable polymers [44]. The amphiphilic character and the resulting increase in hydrophilicity of this class of polymers provide advantages when packaging sensitive drug molecules, such as proteins, peptides or DNA. Furthermore, the PVA backbone can be modified, for example, with sulfobutyl moieties or amine structures, to create polymers with negative or positive charges. The ability to modify not only the backbone but also the length of the PLGA side chains results in an extremely flexible polymer system, which can be adapted to meet the needs of almost any drug substance. Further, the rate of biodegradation may also be manipulated through polymer modification to achieve half-lives ranging from several hours to several weeks. The three major groups of branched polyesters based upon poly (vinyl alcohol)-grafted poly (lactic-co-glycolic acid) (PVA-g-PLGA) are, the neutrally charged PVA-g-PLGA, the negatively charged sulfobutyl-modified PVA-g-PLGA and the positively charged amine-modified PVA-g-PLGA. These polymers may also be formulated into several different types of drug delivery vehicles, including nanoparticles [45], microspheres [46], stent coatings, implants, tablets and in situ forming devices [47].
Multiblock poly(ether-ester)s:
Recently, multiblock poly(ether-ester)s based on poly(ethylene glycol) (PEG), butylene terephthalate (BT) and butylene succinate (BS) segments have been developed as a new series of degradable polymers for controlled release applications [48]. These poly(etherester) s are a modification of poly(ethylene glycol) terephthalate (PEGT)/poly(butylene terephthalate) (PBT) copolymers, which have been successfully applied as matrix in controlled release systems both in vitro and in vivo [49-54]. However, for controlled release application requiring frequently repeated injections, the degradation rate of some PEGT/PBT copolymer compositions might be too slow [55]. Substitution of the aromatic terephthalate units by aliphatic succinate units was shown to increase the degradation rate of the copolymers [48,56]. In addition, in vivo studies showed no signs of bioincompatibility for these new materials [56]. Preliminary release studies on a selected PEG(T/S)/PB(T/S) polymer composition showed the complete release of model proteins within hours up to several weeks, depending on the size of the solute [48]. As release mechanism, protein diffusion due to a combination of swelling and degradation of the matrix was proposed but this required further investigation [57].
Naturally occurring polymer–Chitosan
Polysaccharide-based polymers represent a major class of biomaterials, which includes agarose, alginate, carageenan, dextran, and chitosan. One of the most important naturally occurring polymer that is being investigated widely is chitosan. Chitosan, β(1,4)2-amino-2-D-glucose, is a cationic biopolymer produced by alkaline N-deacetylation of chitin [58], which is the main component of the shells of crab, shrimp and krill. Chitosan has many biomedical applications, including tissue engineering, owing to its biocompatibility, low toxicity, and degradation in the body by enzymes such as chitosanase and lysozyme [59], which has opened up avenues for modulating drug release in vivo in the treatment of various diseases. These chitosan-based delivery systems range from microparticles to nanoparticles [60], gels [61] and films [62]. Further, gels and films of chitosan have been used for oral delivery of chlorhexidine digluconate in the treatment of fungal infections [63]. In addition, chitosan has been extensively evaluated as a carrier of various antineoplastic agents such as 5-FU, mitoxantrone [64] and cytarabine [65].
The film-forming property of chitosan has found many applications in tissue engineering and drug delivery by virtue of its mechanical strength and rather slow biodegradation [66]. Some drug-loaded chitosan films are emerging as novel drug delivery systems and appear to have potential for local sustained delivery of cancer chemotherapeutic agents. Following surgical removal of tumor, these implantable systems may be placed in the resection cavity to elicit a local response at the biophase; further, they may be secured by suturing at the site to prevent any displacement problems. Combination of chitosan with other naturally occurring biodegradable polymers like alginate-chitosan beads [67], albumin-chitosan microspheres [68] and alginate-agarose microcapsules [69] are also reported.
Responsive Systems
Although controlled release systems represent a significant improvement over the conventional mode of drug administration, it is still not the ideal delivery system. The ideal drug delivery system would be one, which responds to physiological needs. Therefore, attention is shifted to the self regulated or trigged delivery systems [70].
One such disease that has received a great deal of attention because of the potential for therapies using controlled drug delivery is diabetes. Most of the systems under study for insulin delivery are based on the control of delivery responding to the environment created by reaction of glucose with glucose-oxidase in blood. Such environment can be created in drug delivery system with glucose-oxidase immobilized in polymer. Work with biodegradable polymers has also yielded polyorthoesters that are pH sensitive and that will degrade more quickly in acidic environment. A poly(ortho ester) system is designed with a tertiary amine function to render the degradation of poly(ortho ester) more sensitive to pH changes [71]. Such polymers have been studied as the central core of a drug delivery system in which the polymer-insulin matrix is surrounded by a membrane containing grafted glucose oxidase, which provides the reaction substrate and the change in pH necessary to enhance biodegradation and subsequent insulin delivery.
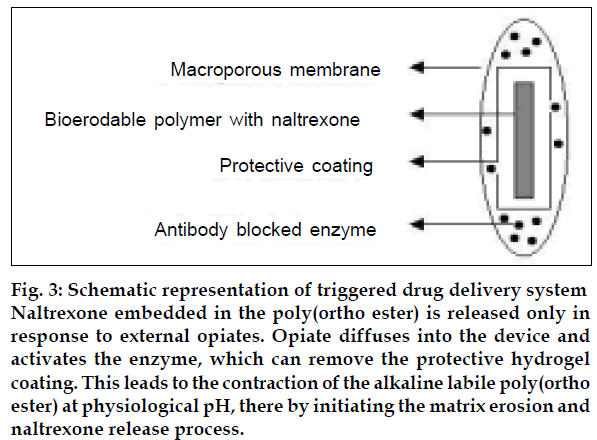
A different approach to release narcotic antagonists, trigged by opiates, involves a more elaborate design. As shown in fig. 3, the naltrexone embedded in the poly(ortho ester) is released only in response to external opiates [71]. According to this scheme, the opiate diffuses into the device and activates the enzyme, which can remove the protective hydrogel coating. This leads to the contraction of the alkaline labile poly(ortho ester) at physiological pH, there by initiating the matrix erosion and naltrexone release process. The use of haptane-antibody interaction to suppress enzymatic degradation and permeability of polymeric reservoirs or matrix drug delivery system is also reported by Pitt [72].
Fig 3: Schematic representation of triggered drug delivery system Naltrexone embedded in the poly(ortho ester) is released only in response to external opiates. Opiate diffuses into the device and activates the enzyme, which can remove the protective hydrogel coating. This leads to the contraction of the alkaline labile poly(ortho ester) at physiological pH, there by initiating the matrix erosion and naltrexone release process.
Overview Of Different Technologies And Products Based On Biodegradable Polymers
Zoladex®
Zoladex® (AstraZeneca) is supplied as a sterile, biodegradable product containing goserelin acetate equivalent to 3.6 mg of goserelin [73]. Zoladex® is designed for subcutaneous injection with continuous release over a 28 d period. Goserelin acetate is dispersed in a matrix of D, L-lactic and glycolic acids copolymer (13.3-14.3 mg/dose) containing less than 2.5% acetic acid. The encapsulated drug is released by a combination of diffusion and erosion-controlled mechanisms. However, because the delivery device is a monolithic, heterogeneous hydrolysis is thought to be the predominant erosion process.
Lupron depot®
The first FDA-cleared PLGA product was the Lupron Depot® drug-delivery system (TAP Pharmaceutical Inc.). Lupron Depot® is a microsphere formulation based on the biodegradable polymers of polylactic acid (PLA) and poly(lactic/glycolic acid) [73]. The double-emulsion process is used to manufacture Lupron Depot®. The primary emulsion consists of leuprorelin acetate in an aqueous solution containing gelatin dispersed in a solution of PLG in methylene chloride. The water-in-oil emulsion is then emulsified in a solution of poly(vinyl alcohol) (surfactant and stabilizer). The microspheres are formed by evaporation of the methylene chloride, which is the continuous phase of the primary emulsion.
Gliadel® wafer
Gliadel® Wafer is a white, dime-sized wafer made up of a biocompatible polymer that contains the cancer chemotherapeutic drug, carmustine (BCNU). On removal of a high-grade malignant glioma, up to eight Gliadel Wafers are implanted in the cavity where the tumor resided [73]. Once implanted, the Gliadel Wafers slowly dissolve, releasing high concentrations of BCNU into the tumor site targeting microscopic tumor cells that sometimes remain after surgery. Gliadel wafer was the first new treatment of this kind or brain cancer introduced in over 20 years. In the field of local delivery, carmustine-loaded Gliadel wafer (Guilford Pharmaceuticals, Baltimore, MD) fabricated from poly(carboxyphenoxy propane: sebacic acid) proved very promising in clinical trials for the treatment of malignant glioma, increasing both survival and safety [74]. BCNU-loaded Gliadel wafer provides localized delivery of chemotherapy directly to the site of the tumor (as an adjunct therapy) and is the only FDA approved brain cancer treatment capable of doing so [75].
Alzamer® depot technology
Alzamer® technology offers a non-aqueous polymer solution for sustained delivery of small molecules and biopharmaceuticals for periods of weeks to months, both locally and systemically. Protein stability is maintained in Alzamer® depot formulations by isolating the drug in a solid particle. This particle is suspended in the nonaqueous polymer solution to prevent premature exposure to water. Processing of both the gel and protein particles is simple, incorporating standard lyophilization and aseptic blending techniques. No reconstitution or additional mixing is required.
Atrigel® in situ implant system
This system can be used for both parentral and sitespecific drug delivery. It contains a biodegradable polymer dissolved in a biocompatible carrier. When the liquid polymer system is placed in the body using conventional needles and syringes, it solidifies upon contact with aqueous body fluids to form a solid implant. If a drug is incorporated into the polymer solution, it gets entrapped within the polymer matrix as it solidifies, and is slowly released as the polymer biodegrades. The Atrigel® system is protected by more than 140 patents in the United States and the rest of the world. Both the basic technologies as well as process improvements are covered in these patents. Seven products have already been approved by the FDA using the Atrigel technology like Eligard® and the Atridox® [78-81].
The poly(DL-lactide), lactide/glycolide copolymers, and lactide/caprolactone copolymers are most often used because of their degradation characteristics and their approval by the FDA [22,76]. The solvents employed in the Atrigel system to dissolve the polymers range from the more hydrophilic solvents, such as N-methyl-2-pyrrolidone (NMP), polyethylene glycol, tetraglycol and glycol furol, to the more hydrophobic solvents, such as triacetin, ethyl acetate and benzyl benzoate [77].
Future Thrust
New tailor-made copolymers with desirable functional groups, are being created by researchers who envision their use not only for innovative drug delivery systems but also as potential linings for artificial organs, substrates for cell growth, chemical reactors, agents in drug targeting and immunology testing. The most exciting opportunities in controlled drug delivery lie in the arena of responsive delivery systems. We expect that, in future even more than today, device designers and physicians will have a wealth of products using biodegradable polymers that will help speedy patient recovery and eliminate follow-up surgeries. All things considered, total or near total use of biodegradable polymers is within reach in near future.
References
- Leong KW. Biodegradable polymers as drug delivery systems. In: Tarcha, PJ, editors. Polymers for Controlled Drug Delivery. CRC Press: Boca Raton; 1991. p. 128.
- Heller J. Fundamentals of Polymer Science. In: Robinson JR, Lee VHL, editors. Controlled Drug Delivery: Fundamentals and Applications. 2nd ed. Marcel Dekker: New York; 1987. p. 164.
- Heller J. Controlled release of biologically active compounds from bioerodible polymers. Biomaterials 1980;1:51-7.
- Mathiowitz E. Encyclopedia of controlled drug delivery. New York; 1999. p. 570.
- Heller J. Controlled drug release from poly(orthoesters)-A surface eroding polymer. J Control Release 1985;2:167-77.
- Tamada J, Langer R, The development of polyanhydrides for drug delivery applications. J Biomater Sci Polym Ed 1992;3:315-53.
- Leong KW, Brott BC, Langer R. Bioerodible polyhydrides as drug-carrier matrixes. J Biomed Mater Res 1985;19:941-55.
- Domb AJ, Nudelman R. In vivo and in vitro and elimination of aliphatic polyanhydrides. Biomaterials 1995;16:319-23.
- Leong KW, Mathiowitz E, Langer R. Polyanhydrides for controlled release of bioactive agents. Biomaterials 1986;7:364-71.
- Heller J. Controlled drug release from poly(ortho esters)-A surface eroding polymer. J Control Release 1985;2:167-77.
- Heller J. Development of poly(ortho esters): A historical overview. Biomaterials 1990;1:659-65.
- Heller J, Sparer RV, Zenter GM. Poly(ortho esters), biodegradable polymers as drug delivery systems. In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. Marcel Dekker: New York; 1990. p. 121.
- Heller H, Ng SY, Penhale DW, Fritzinger BK, Sander LM, Burns RA, et al. Use of poly(ortho esters) for the controlled release of 5-fluorouracil and a LHRH analogue. J Control Release 1987;6:217-312.
- Nguyen TH, Higuchi T, Himmelstein KJ. Erosion characteristics of catalyzed poly(ortho ester) matrices. J Control Release 1987;5:1-6.
- Chow AW, Hamlin RD, Heller J. Cure behavior of neat and drug-loaded poly(ortho ester) Bioerodible Implants. J Control Release 1989;9:123-6.
- Heller J, Barr J, Ng SY, Shen HR, Schwach-Abdellaoui K, Emmahl S, Rothen-Weinhold A, et al. Poly(ortho esters)-their development and some recent applications. Eur J Pharm Biopharm 2000;50:121-5.
- Leong KW. Biodegradable polymers as drug delivery systems. In: Tarcha PJ, editors. Polymers for Controlled Drug Delivery. CRC Press: Boca Raton; 1991. p. 142.
- Grolleman CW, de Visser AC, Wolke JG, Klein H, van der Goot CP, Timmerman H. Studies on a bioerodible drug carrier system based on polyphosphazene. J Control Release1986;3:143-54.
- Allcock HR. Polyphosphazene drug delivery systems for antitumor treatment. In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. Marcel Dekker: New York; 1990. p. 163.
- Charles EL, Buffalo NY. US Patent No , US2668162, 1954.
- Schmitt EE, Polistina RA. US Patent No , US3297033, 1967.
- Lewis DH. Poly-caprolactone and its copolymers. In: Chasin M, Langer R. editors, Biodegradable polymers as drug delivery systems. Marcel Dekker: New York; 1990. p. 1.
- Asano M, Fukuzaki H, Yoshida M, Mashimo MK, Yuasa H, Imai K, et al. In vivo characteristics of low molecular weight copoly(-lactic acid/glycolic acid) formulations with controlled release of luteinizing hormone-releasing hormone agonist. J Control Release 1989;9:111-5.
- Wood DA. Biodegradable drug delivery systems. Int J Pharm 1980;7:1-4.
- Kaetsu I, Yoshida M, Asano M, Yamanaka H, Imai K, Yuasa H, et al. Biodegradable implant composites for local therapy. J Control Release 1987;6:249-63.
- Lee JS, An TK, Chae GS, Jeong JK, Cho SH, Lee HB, et al. Evaluation of in vitro and in vivo antitumor activity of BCNU-loaded PLGA wafer against 9L gliosarcoma. Eur J Pharm Biopharm 2005;59:169-75.
- Arshady R. Preparation of porous and nonporous biodegradable polymeric hollow microspheres. J Control Release 1991;17:1-22.
- Mauduit J, Bukh N, Vert M. Gentamicin poly lactic acid blends aimed at sustained release local antibiotic therapy administered per operatively. J Control Release 1993;23:221-30.
- Sanders LM, Kent JS, McRae GI, Vickery BH, Tice TR, Lewis DH. Controlled release of a luteinizing hormone-releasing hormone analogue from poly(d,l-lactide-co-glycolide) microspheres. J Pharm Sci 1984;73:1294-7.
- Spenlehauer G, Verllard M, Benoit JP. Formation and characterization of cisplation loaded polymethyl methacrylate microspheres. J Pharm Sci 1986;75:750-5.
- Hutchison FG, Furr ABJ. Biodegradable polymer systems for the sustained release of polypeptides. J Control Release 1990;13:279-94.
- Kou JH, Emmett C, Shen P, Aswani S, Iwamoto T, VaghefiF, et al. Bioerosion and biocompatibility of poly(d,l-lactic-co-glycolic acid) implants in brain. J Control Release 1997;43:123-30.
- Spenlehauer G, Vert M, Benoit JP, Chabot F, Veillard M. Biodegradable cisplatin microspheres prepared by the solvent evaporation method. J Control Release 1988;7:217-29.
- Weert M, Hennnink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) Microparticles. Pharm Res 2000;17:1159-67.
- Walter E, Moelling K, Pavlovic J, Merkle HP. Microencapsulation of DNA using poly(DL-lactide-co-glycolide): Stability issues and release characteristics. J Control Release 1999;61:361-74.
- Sanders LM, Kell BA, McRae GI, Whitehead GW. Prolonged controlled-release of narfarelin from biodegradable polymeric implants. J Pharm Sci 1986;75:356-60.
- Tinsley-Bown AM, Fretwell R, Dowsett AB, Davis SL, Farrar GH. Formulation of poly(D,L-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. J Control Release 2000;66:229-41.
- Ando S, Putnam D, Pack DW, Langer R. PLGA microspheres containing plasmid DNA: preservation of supercoiled DNA via cryopreparation and carbohydrate stabilization. J Pharm Sci 1999;88:126-30.
- Evans RK, Xu Z, Bohannon KE, Wang B, Bruner MW, Volkin DB. Evaluation of degradation pathways for plasmid DNA in pharmaceutical formulations via accelerated stability studies. J Pharm Sci 2000;89:76-87.
- Breitenbach A, Li Y, Kissel T. Branched biodegradable polyesters for parenteral drug delivery systems. J Control Release 2000;64:167-78.
- Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci 2003;92:1343-55.
- Otsuka H, Nagasaki Y, Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Del Rev 2003;55:403-19.
- Pistel KF, Bittner B, Koll H, Winter G, Kissel T. Biodegradable recombinant human erythropoietin loaded microspheres prepared from linear and star-branched block copolymers: Influence of encapsulation technique and polymer composition on particle characteristics. J Control Release 1999;59:309-25.
- Dailey LA, Wittmar M, Kissel T. The role of branched polyesters and their modiÞcations in the development of modern drug delivery vehicles. J Control Release 2005;101:137-49.
- Crommelin DJ, Storm G, Jiskoot W, Stenekes R, Mastrobattista E, Hennink WE. Nanotechnological approaches for the delivery of macromolecules. J Control Release 2003;87:81-8.
- Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release 2003;90:261-80.
- HatefiA, Amsden B. Biodegradable injectable in-situ forming drug delivery systems. J Control Release 2002;80:9-28.
- Métairie S, Dijkhuizen-Radersma R, Roosma JR, Kaim P, Péters FLAMA, de Wijn J, et al. Biodegradable poly(ether-ester) multiblock copolymers for controlled release applications. J Biomed Mater Res 2003;67A:1294-304.
- Dijkstra PJ, Bezemer JM, Radersma R, Grijpma DW, Feijen J, van Blitterswijk CA. Zero-order release of lysozyme from poly(ethylene glycol)/ poly(butylene terephthalate) matrices. J Control Release 2000;64:179-92.
- Bezemer JM, Radersma R, Grijpma DW, Dijkstra PJ, van Blitterswijk CA, Feijen J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers I Influence of preparation techniques on particle characteristics and protein delivery. J Control Release 2000;67:233-48.
- Grijpma DW, Bezemer JM, Radersma R, Dijkstra PJ, van Blitterswijk CA, Feijen J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers II Modulation of release rate. J Control Release 2000;67:249-60.
- Bezemer JM, Grijpma DW, Dijkstra PJ, van Blitterswijk CA Feijen J. Control of protein delivery from amphiphilic poly(ether ester) multiblock copolymers by varying their water content using emulsiÞcation techniques. J Control Release 2000;66:307-20.
- Stienstra NA, Péters FL, Grijpma DW, Dijkhuizen-Radersma R, Feijen J, de Groot K, et al. Control of vitamin B12 release from poly(ethyleneglycol)/poly(butylenes-terephthalate) multiblock copolymers. Biomaterials 2002;23:1527-36.
- Dijkhuizen-Radersma R, Wright SJ, Taylor LM, John BA, de Groot K, Bezemer JM. A new series of multiblock poly(ether-ester)s based on poly(ethylene glycol) from poly(ether-ester) microspheres. Pharm Res 2004;21:484-91.
- de Groot K, Hesseling SC, Kaim PE, Dijkhuizen-Radersma R, Bezemer JM. Biocompatibility and degradation of poly-(ether-ester) microspheres: In vitro and in vivo evaluation. Biomaterials 2002;23:4719-29.
- Dijkhuizen-Radersma R, Roosma JR, Sohier J, Péters FL, van den Doel M, van Blitterswijk CA, et al. Biodegradable poly(ether-ester) multiblock copolymers for controlled release applications: An in vivo evaluation. J Biomed Mater Res 2004;71A:118-27.
- van Dijkhuizen-Radersma R, Metairie S, Roosma JR, de Groot K, Bezemer JM. Controlled release of proteins from degradable poly(ether-ester) multiblock copolymers. J Control Release 2005;101:175-86.
- Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J Control Release 2003;89:151-65.
- Pangburn SH, Trescony PV, Heller J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials 1982;3:105-8.
- Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release 2001;73:255-67.
- Ruel-Gariepy E, Chenite A, Chaput C, Guirguis S, Leroux JC. Characterization of thererapeutically active chitosan gels for the sustained delivery of drugs. Int J Pharm 2000;203:89-98.
- Muzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G. Biological activity of chitosan- ultrastructural study. Biomaterials 1998;9:247-52.
- Senel S, Ikinci G, Kas S, Yousefi-Rad A, Sargon MF, Hincal AA. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int J Pharm 2000;193:197-203.
- Jameela SR, Jayakrisnan A. Glutaraldehyde cross-linked chitosan microspheres as a long acting biodegradable drug delivery. Biomaterials 1995;16:769-75.
- Blanco MD, Gómez C, Olmo R, Muñiz E, Teijón JM. Chitosan microspheres in PLG films as devices for cytarabine release. Int J Pharm 2000;202:29-39.
- Ma J, Wang H, He B, Chen J. A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan scaffold and chitosan-collagen scaffold. Biomaterials 2001;22:331-6.
- Bhaskaran S. Preparation and evaluation of alginate - Chitosan beads. Indian J Pharm Sci 2002;64:389-91.
- Muthushamy K, Shibi KP, Ravi TK. Preparation and evaluation of albumin-chitosan microshphere containing theophylline. Indian J Pharm Sci 2004;66:245-8.
- Orive G, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Survival of different cell lines in alginate-agarose microcapsules. Eur J Pharm Sci 2003;18:23-30.
- Talegaonkar S, Mishra PR. Recent advances in modulated drug delivery systems. Indian J Pharm Sci 2002;64:515-24.
- Heller J. Chemically self-regulated drug delivery systems. J Control Release 1988;8:111-25.
- Pitt CG, Gu ZW, Hendren RW, Thompson J, Wani MC. Triggered drug delivery systems. J Control Release 1985;2:363.
- Sah H, Chien YW, In: Hillery AM, Lloyd AW, Swarbrick J, editors. Drug delivery and targeting for pharmacist and pharmaceutical scientist. Taylor and Francis: London; 2001. p. 101.
- Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Control Release 2001;74:63-67.
- Dang WB, Daviau T, Ying P, Zhao Y, Nowotnik D, Clow CS, et al. Effects of Gliadel wafer initial molecular weight on the erosion of wafer and release of BCNU. J Control Release 1996;42:83-92.
- Pitt CG, Poly (ε-caprolactone) and its copolymers. In: Chasin M, Langer R, editors. Biodegradable polymers as drug delivery systems. Marcel Dekker: New York; 1990. p. 71.
- Spiegel AJ, Noseworthy MM. Use of non-aqueous solvents in parenteral products. J Pharm Sci 1963;52:917-27.
- Polson AM, Dunn RL, Fulfs JC, Godowski KC, Polson AP, Southard GL, et al. Periodontal pocket treatment with subgingival doxycycline from a biodegradable system. J Dental Res 1993;72:360-4.
- Polson A, Garrett S, Stoller N, Bandt C, Haner P, Killoy W, et al. Multicenter comparative evaluation of subgingivally deliveredsanguinarine and doxycycline in the treatment of periodontitis, II: Clinical results. J Periodontol 1997;68:119-26.
- Ravivarapu HB, Moyer KL, Dunn RL. Parameters affecting the efÞcacy of a sustained release polymeric implant of leuprolide. Int J Pharm 2000;194:181-91.
- Dunn RL, Moyer KL, Ravivarapu HB. Sustained activity and release of leuprolide acetate from an in situ forming polymeric implant of leuprolide acetate. J Pharm Sci 2000;89:732-41.