- *Corresponding Author:
- Haibo Zhou
Chongqing Tongliang District Hospital of Traditional Chinese Medicine,
Chongqing 402560,
China,
Email: df198347@163.com
| Date of Submission | 25 November 2020 |
| Date of Revision | 24 September 2021 |
| Date of Acceptance | 15 April 2022 |
| Indian J Pharm Sci 2022;84(2):493-500 |
Abstract
The protective mechanism of ginsenoside Rg1 for Alzheimer’s disease induced by Amyloid β-Protein has rarely been reported. To evaluate the cerebroprotective effects of ginsenoside Rg1 on Alzheimer’s disease rats. Male Sprague Dawley rats were randomly divided into 5 groups (n=10). Alzheimer’s disease model was established by injecting Amyloid β-Protein into the hippocampal CA1 area. Rg1 group, specific phosphoinositide 3-kinase inhibitor LY294002 group and Rg1 plus LY294002 group were intraperitoneally injected with corresponding drugs, once daily for 28 consecutive d. The spatial learning and memory abilities were studied by water maze and platform tests respectively. The pathological changes of hippocampal CA1 area were observed by hematoxylin and eosin staining. Neuronal apoptosis was detected by Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling assay. Protein kinase B and Glycogen synthase kinase 3 beta protein expressions were measured by Western blotting. Compared with model group, the learning and memory abilities of Rg1 group were significantly improved (p<0.05), and the abnormal morphology of neurons in Rg1 group and their apoptosis were significantly relieved (p<0.05). Compared with model group, the superoxide dismutase and glutathione peroxidase levels of Rg1 group increased significantly, whereas that of Malondialdehyde decreased significantly (p<0.05). Compared with model group, the Protein kinase B and Glycogen synthase kinase 3 beta phosphorylation levels of Rg1 group significantly increased (p<0.05). LY294002 and Rg1+LY294002 groups had similar results (p>0.05). Ginsenoside Rg1 may mitigate Amyloid β-Protein induced Alzheimer’s disease by activating the phosphoinositide 3-kinase/Protein kinase B/ Glycogen synthase kinase 3 beta signaling pathway, inhibit neuronal apoptosis in the hippocampus and attenuate oxidative stress, exerting cerebroprotective effects.
Keywords
Ginsenoside Rg1, amyloid β-protein, Alzheimer’s disease, phosphoinositide 3-kinase, Glycogen synthase kinase 3 beta
Alzheimer’s Disease (AD) is one of the most common neurodegenerative diseases among the elderly in clinical practice[1]. Nowadays, there are more than 100 million patients with AD all over the world and the incidence is on the rise year by year with the worldwide acceleration of the global aging process[2], so the research regarding AD is one of the hotpots in this area. The amyloid cascade hypothesis is the classical theory for the pathogenesis of AD. The mechanism of neuron loss in the brain of patients with AD is related to cell apoptosis and the massive disposition of Amyloid β (Aβ) protein can induce neural apoptosis, which leads to AD[3]. Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/Akt) signaling pathway is important for the regulation of related proteins during apoptosis, and can transport the membrane receptor signals into the cells to maintain cell proliferation and to inhibit apoptosis[4]. As a substrate of Akt, Glycogen Synthase Kinase- 3β (GSK-3β) is a key element in the regulation of apoptosis[5]. Ginseng is a traditional valuable Chinese herbal medicine containing various active components, among which ginsenoside Rg1 acquires the properties including improvement of immune function, antitumor, anti-inflammatory and antioxidant roles, as well as protective effects on impaired neural functions, the last of which has been widely reported and applied in clinical treatment of cerebrovascular diseases[6]. However, the protective mechanism of ginsenoside Rg1 on the brain of elderly AD induced by Amyloid β-Protein (Aβ1-42) has rarely been reported. In this study, we established an Aβ1-42-induced AD model of rats, explored the mechanism for the protective effects of ginsenoside Rg1 on the brain, and assessed its regulatory effects on the PI3K/Akt/GSK-3β signaling pathway, aiming to provide an experimental foundation for further studies.
Materials and Methods
Main reagents:
Ginsenoside Rg1 (batch number: 070319812; purity >98 %) was purchased from Kunming Pharmaceutical Corporation (China), diluted with normal saline (pH 7) before use, calculated according to body weight and stored at 4°. Superoxide Dismutase (SOD), Malondialdehyde (MDA) and Glutathione Peroxidase (GSH-Px) kits were bought from Beijing Solarbio Science & Technology Co., Ltd. (China). TUNEL kit was obtained from Roche (Germany). LY294002 was provided by Shanghai Biochempartner Co., Ltd. (China). Aβ1-42 was purchased from Sigma (USA), and diluted to 2 μg/μl with normal saline before use and incubated in a 37° cell incubator for 74 d to be an oligomerization state. Bicinchoninic Acid (BCA) protein kit, Enhanced Chemiluminescence (ECL) kit and P-Akt and P-GSK-3β antibodies were bought from Shanghai Renjie Biotechnology Co., Ltd. (China).
Model establishment:
Adult male Sprague-Dawley (SD) rats (SPF grade) weighing 200-220 g were fasted for 12 h before receiving operation. After anesthetization with 1 % pentobarbital sodium (65 mg/kg), their heads were fixed with a stereotaxic apparatus. We shaved the hair on their heads, wiped the heads with povidone iodine, made a 2 cm long longitudinal incision of the skin of the heads and separated the periosteum to allow the exposure of the skull. The hippocampal CA1 region could be found in the position 3.3 mm posterior to the anterior fontanelle, 1.7 mm off the midline on left and right sides and 3.5 mm beneath the endocranium. We opened the skull above the hippocampal CA1 region with a bone drill, inserted the micro syringe vertically with the depth of 2.8 mm and injected 2 μl incubated Aβ1-42 solution slowly with duration of 5 min. The needle was retained for 5 min in order to allow the sufficient diffusion of the solution and then the incision was sutured. Afterwards, 80 000 units of penicillin were given daily by intraperitoneal injection for 7 d after the operation to prevent postoperative infection and the vital signs of rats were also observed. For the sham operation group, the rats were injected with 2 μl normal saline after the endocranium was cut open and the other steps were the same with those mentioned above.
Animal grouping:
After 7 d of adaptive feeding, 50 SD rats were randomly divided into a sham operation group, a model group, an Rg1 (50 mg/kg), a P13K inhibitor LY294002 (LY) (10 mg/kg, LY) group and an Rg1 (50 mg/kg) plus LY294002 (10 mg/kg) group (n=10). On the 1st d after modeling, the treatment groups were intraperitoneally injected with corresponding drugs, once daily for 28 consecutive d. The sham operation group and model group were given the same amounts of normal saline.
Water maze test:
After 23 d of continuous drug administration, each group was subjected to the Morris water maze test for 5 consecutive d. Place navigation test was performed twice a day in the first 4 d. In details, after 30 min of administration every morning, each SD rat was placed alone in the water maze. The pool was divided into 4 quadrants, with a platform placed in the center of the northeast quadrant; the rat was dropped into the water daily from the east-west south quadrant and the southeast quadrant and the duration of finding the platform within 90 s was recorded and set as the rat’s escape latency. Escape latency was recorded as 90 s if the rat failed to find the platform, and then the rat was guided to the platform and rested for 10 s before being put back into the cage. Spatial probe test was performed on the 5th d. Detailed 30 min after drug administration in the morning; the rat was dropped into the water from the southeast quadrant with the platform being removed. Swimming trajectory and the times of crossing the site of the original platform of each group were recorded.
Measurement of SOD, GSH-Px and MDA levels in brain tissue:
The rats were sacrificed and their brains were taken out when the behavioral experiment finished. The tissues of hippocampal CA1 region were quickly separated on the ice-cold platform. Parts of these tissues were weighed and put into precooled Phosphate-Buffered Saline (PBS) buffer to make 10 % homogenate followed by the centrifugation at 4° at 4000 rpm for 10 min. The supernatant was analyzed according to the instructions of SOD, GSH-Px and MDA assay kits so as to calculate their activities or contents in each gram of brain tissue.
Observation of morphological changes of neurons in hippocampal tissue by HE staining:
The rats were sacrificed and their brains were taken out when the behavioral experiment finished. After fixed in 4 % formaldehyde for 24 h, their brains were routinely dehydrated and embedded in paraffin. The paraffin blocks embedding the hippocampal CA1 tissues were cut into 5 μm-thick slices followed by Hematoxylin and Eosin staining (HE staining) to allow the observation of the morphological changes of the hippocampal neurons in AD rats.
Detection of neuronal apoptosis in brain tissue by Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay:
Slices of hippocampal CA1 tissues were stained with TUNEL assay kit. After being deparaffinized and hydrated, the slices were treated with protease K (10 mmol/l) for 20 min and then washed by PBS buffer 3 times for 5 min each time. The slices were immersed and incubated in the TUNEL reaction mixture at 37° for 60 min in dark, followed by being washed by PBS buffer 3 times for 5 min each time. Then they were incubated with peroxidases reaction solution at 37° for 30 min in dark and then washed by PBS buffer 3 times for 5 min each time. Subsequently, the slices were incubated at room temperature for 10 min after 3,3'-Diaminobenzidine (DAB) substrate solution was added for color development, dehydrated with ethanol solutions of gradient concentrations, cleared with xylene and finally mounted with neutral resin. Neuronal apoptosis was observed under light microscope and the nucleus stained blue indicated normal cells while the brown granules in the nuclei showed the positive cells (apoptotic cells). The determination of the percentage of apoptotic neurons (apoptotic rate): 4 slices were taken from each rat and 3 non-overlapping fields were randomly selected for each slice under a 200-fold field of view and then calculate the proportion of positive cells in total cells of all chosen fields and the mean was the apoptotic rate.
Detection of P-Akt and P-GSK-3β expressions in brain tissue by Western blot:
Tissues from the hippocampal CA1 region were mixed with Radio-Immunoprecipitation Assay (RIPA) lysis buffer and ground with a homogenizer. After ice bath for 5 min, the homogenate was centrifuged at 13 000 rpm at 4° for 10 min in order to obtain the supernatant. The protein concentrations of the supernatant from different tissues were quantified by BCA protein quantification assay kit and the proteins were diluted with 2×electrophoresis buffer to the same concentration. The proteins were isolated by 10 % Sodium Dodecyl- Sulfate Polyacrylamide Gel Electrophoresis (SDSPAGE), transferred to Hydrophilic Polyvinylidene Fluoride (PVDF) membrane with Tris/glycine buffer and blocked in 5 % Tris-Buffered Saline with 0.1 % Tween® 20 detergent (TBST) solution at room temperature for 2 h. Then the PVDF membrane was incubated in the primary antibody diluent (1:1000) at 4° overnight. On the next day, the membrane was taken out, washed in TBST solution for 10 min and incubated in secondary antibody diluent of goat antirabbit Immunoglobulin G (IgG) labeled by Horseradish Peroxidase (HRP) (1:10 000) at room temperature for 2 h. The electrophoresis bands were visualized with the application of chemiluminescent solution and their gray values were obtained with Gel Imaging System. The relative expression levels of target proteins were calculated with Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) as internal reference.
Statistical analysis:
All data were statistically analyzed by SPSS16.0 software. The categorical data conforming to normal distribution were expressed as mean±standard deviation. Multi-group comparisons were conducted by one-way analysis of variance and pairwise comparisons were carried out with the independent t test, p<0.05 was considered statistically significant.
Results and Discussion
In the 4 consecutive d of water maze test, the escape latency of each group was gradually shortened with extended time. Compared with the sham operation group, the escape latency of the model group was significantly prolonged (p<0.05). After treatment with ginsenoside Rg1, the escape latency decreased significantly compared with that of the model group (p<0.05). There was no significant difference between LY (LY294002) group, LY+Rg1 group and model group (p>0.05) (Table 1).
| Group | 1st d (s) | 2nd d (s) | 3rd d (s) | 4th d (s) |
|---|---|---|---|---|
| Sham operation | 57.35±4.86 | 41.32±3.28 | 36.53±2.83 | 16.28±1.96 |
| Model | 73.45±5.72* | 62.56±5.21* | 52.93±4.42* | 50.14±4.25* |
| Rg1 | 62.86±5.06# | 48.50±3.87# | 41.45±2.51# | 25.24±2.01# |
| LY | 75.05±5.26 | 65.48±5.12 | 55.85±4.96 | 52.45±4.72 |
| Rg1+LY | 70.14±5.58 | 59.62±4.76 | 48.85±3.52 | 45.71±4.78 |
Note: *Compared with sham operation group, p<0.05; #compared with model group, p<0.05
Table 1: Water Maze test Results (N=10, X±S)
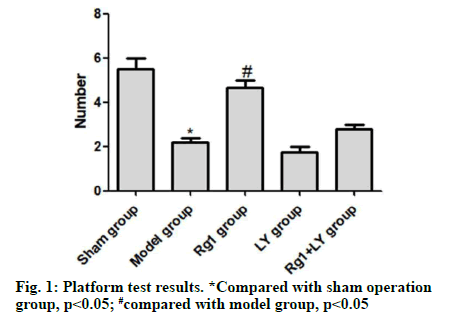
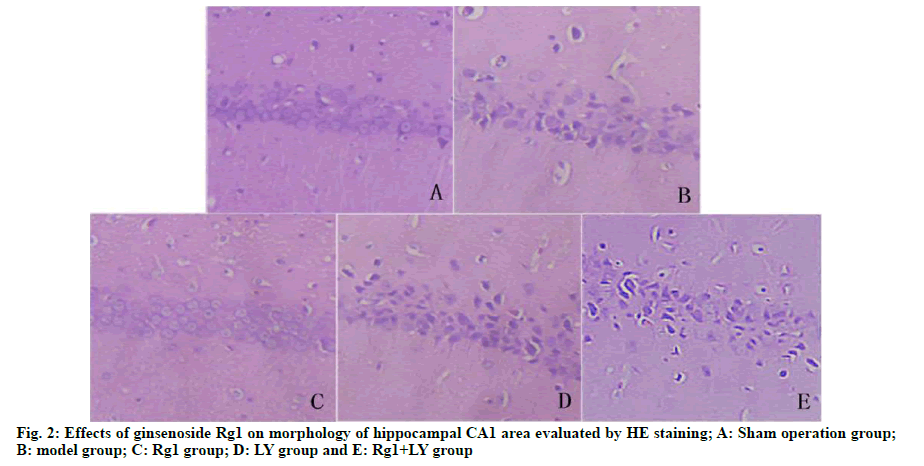
On the 5th d, compared with the sham operation group, the model group had significantly lower number of times crossing the quadrant of the original platform (p<0.05). Compared with the model group, the Rg1 group had significantly higher number of times crossing the platform (p<0.05). There was no significant difference between LY group, LY+Rg1 group and model group (p>0.05) (Fig. 1). HE staining showed that neurons in the hippocampal CA1 area of the sham operation group were intact and without necrosis. The neuronal structure in the hippocampal CA1 area of the model group was incomplete, the arrangement was not tight and the nuclear staining was unclear. Compared with the model group, the abnormal morphology of neurons and neuronal loss in the Rg1 group were significantly relieved (p<0.05). LY and Rg1+LY groups had similar neuronal structures to that of the model group (p>0.05) (Fig. 2).
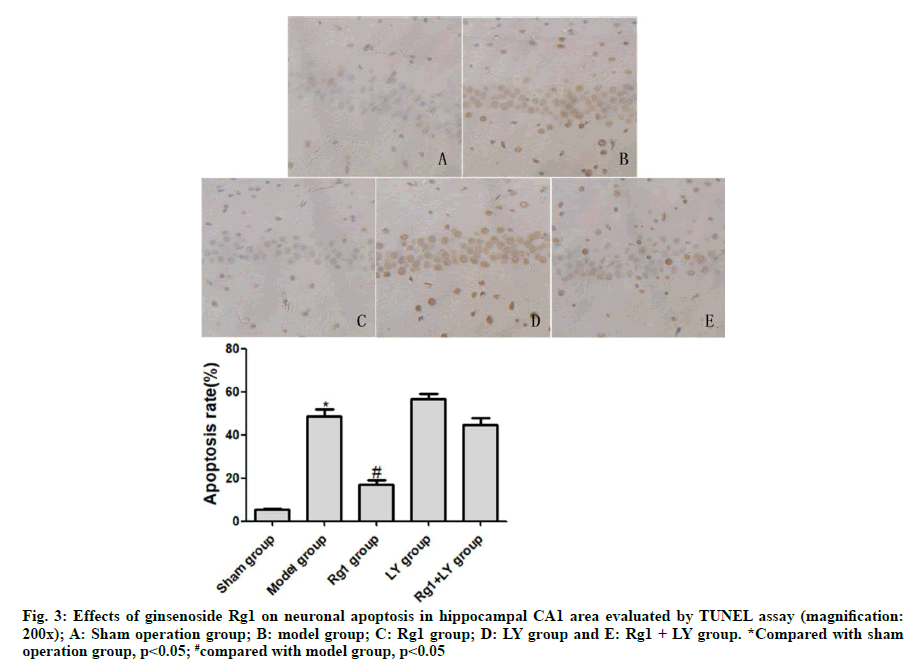
TUNEL assay exhibited that neurons in the sham operation group didn’t undergo apoptosis. Compared with the sham operation group, neurons in the model group underwent severe apoptosis, with a significant difference (p<0.05). Compared with the model group, neuronal apoptosis was significantly alleviated in the Rg1 group (p<0.05). LY and Rg1+LY groups had similar numbers of apoptotic neurons to that of the model group (p>0.05) (Fig. 3).
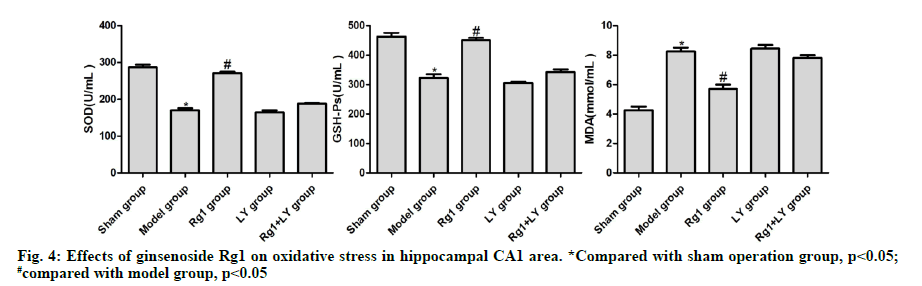
Compared with the sham operation group, the levels of SOD and GSH-Px in the model group significantly decreased, but that of MDA significantly increased (p<0.05). Compared with the model group, the levels of SOD and GSH-Px in the Rg1 group increased significantly, whereas that of MDA decreased significantly (p<0.05). The levels of SOD, GSH-Px and MDA in the hippocampal CA1 area of LY and Rg1+LY groups were not significantly different from those of the model group (p>0.05) (Fig. 4).
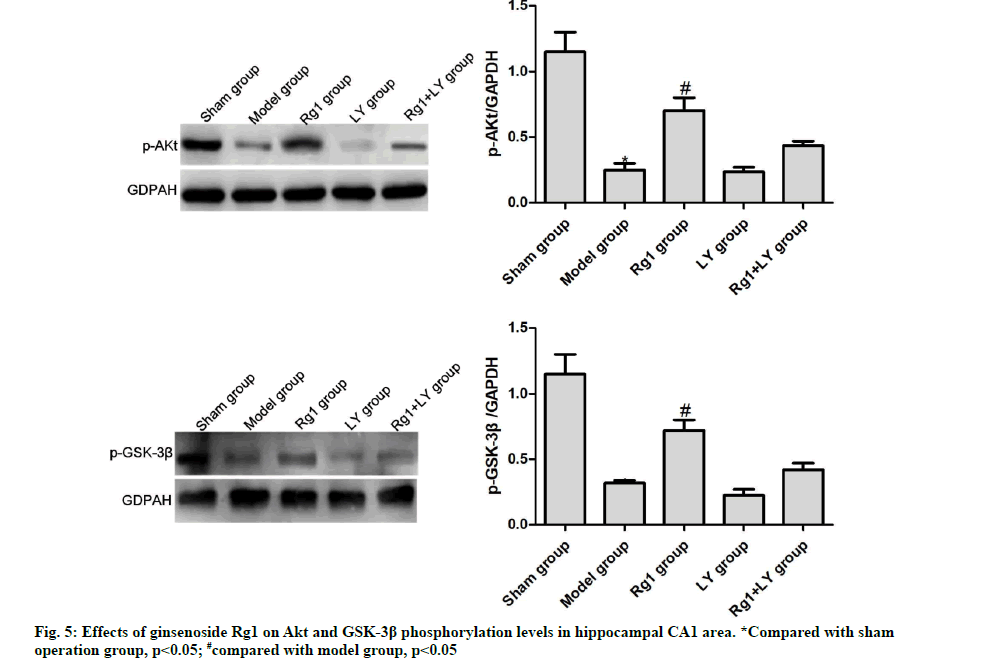
Effects of ginsenoside Rg1 on Akt and GSK-3β phosphorylation levels in hippocampal CA1 area
Compared with the sham operation group, the phosphorylation levels of Akt and GSK-3β in the model group significantly decreased (p<0.05). Compared with the model group, such levels of the Rg1 group significantly increased (p<0.05), but those of LY and Rg1+LY groups were similar (p>0.05) (Fig. 5). AD is a chronic degenerative disease of central nervous system.
It is characterized by pathological changes including massive death and loss of hippocampal neurons, neurofibrillary tangles and Aβ deposition and clinical manifestations such as progressive deterioration of memory and intelligence[7]. It has become a heavy burden to families and society because of its high disability rate. Therefore, it has always been one of the research hotspots in the scientific community of seeking for effective drugs for the treatment of AD.
According to modern pathological theories, ginsenoside Rg1 has various biological functions such as improving immunity and inhibiting tumor cells[8]. The relationship between Rg1 and neuronal injuries has gradually become a hotspot with the development of researches. Li et al.[9] applied the TUNEL method and found that the pretreatment of ginsenoside Rg1 could significantly reduce the apoptosis rate of cortical neurons in rats with focal cerebral ischemia-reperfusion. Another study focusing on the effects of ginsenoside Rg1 on cortical neurons in CIR mice showed that Rg1 could inhibit the expression of caspase-9 protein in hippocampal neurons and that the apoptosis rate of neurons in this region was significantly reduced according to TUNEL staining results, thus indicating ginsenoside Rg1 could protect hippocampal neurons against apoptosis induced by ischemia[10]. In this study, we explored pharmacological effects of ginsenoside Rg1 in Aβ1-42-induced AD model. The results showed that for rats in the model group, their learning and memorizing abilities were obviously impaired and there was increased neuronal apoptosis in hippocampal CA1 region. The concentration of ginsenoside Rg1 was determined according to our preliminary experimental results. Compared with those in the model group, the administration of ginsenoside Rg1rescued rats’ learning and memorizing abilities dampened by AD and inhibited neuronal apoptosis, which were in accordance with previous studies.
A series of complex signal transmission pathways in cells realized the biological behavior. PI3K/Akt signaling pathway, which has drawn increasing attention in recent years, plays an important role in the regulation of cell survival such as cell migration, mobilization, differentiation and anti-apoptosis by intracellular signal transmission[11]. As an important target protein, PI3K is of vital importance to the mediation of various biological effects. PI3K phosphorylates Akt through PDK1, and as the phosphorylation level of Akt increases, it promotes the phosphorylation of GSK-3β in downstream pathways[12]. Phosphorylated GSK-3β can directly bind the mitochondrial membrane Permeability Transition Pore (mPTP) subunit and block the mPTP opening, thus contributing to the inhibition of apoptosis[13]. LY294002 is a recognized PI3K inhibitor which can specifically inhibit the activity of PI3K110 subunits and block PI3K-mediated signaling pathways[14]. In this study, we used LY294002 to verify the mechanism that ginsenoside Rg1 protects against Aβ1-42-induced AD through regulating PI3K/Akt/GSK3β signaling pathway. The results indicated that the phosphorylation levels of Akt and GSK-3β in Rg1 group were greatly increased compared with those in the model group, but their phosphorylation levels were significantly lower in Rg1+LY group than those in Rg1 group, which meant that LY294002 inhibited ginsenoside Rg1- mediated phosphorylation of Akt and GSK3β. These results indicated that ginsenoside Rg1 could allow the phosphorylation of Akt and GSK3β through PI3K. The PI3K/Akt/GSK-3β signaling pathway can inhibit cell apoptosis in cerebral cortex and hippocampal regions and participate in the repairing process in the later stage, thus protecting the physiological functions of the brain[15]. Rats in Rg1+LY group had significantly lower learning and memorizing abilities and more apoptotic neurocytes than Rg1 group, which indicated that the protective effect of Rg1 might be attributed to its regulation of PI3K/Akt/GSK-3β signaling pathway.
It has been reported that activated GSK-3β could well inhibit cellular oxidative stress[16]. Feng et al.[17] found that with the elevation of phosphorylation level of GSK- 3β, SOD activity and the ratio of GSH/GSSG increased while the content of MDA decreased in serum; however, the antioxidant effect was greatly reversed by the administration of GSK-3β inhibitor TDZD-8. Oxidative stress plays a significant role in the development of AD. The oxidative process is normally in equilibrium with the antioxidative process in the body, but the balance can be disturbed by some factors which allow the oxidative process to overwhelm the antioxidative process, namely a state of oxidative stress. In this circumstance, the contents of antioxidant enzymes such as SOD and GSH-Px are reduced because of the large consumption while lots of free radicals produced by oxidative stress accumulate in the body and cause injuries to tissues and organs, especially the brain[18]. Lipid peroxidation occurs and MDA is produced in brain tissues because of free radicals, which can also cause DNA damage to brain cells in severe cases[19]. In our study, for rats in model group receiving microinjection of Aβ1-42 into the hippocampal CA1 region, SOD activity and the ratio of GSH/GSSG increased while the content of MDA decreased in serum. The results suggested that oxidative stress occurred and caused oxidative injuries to brain tissues in rats with AD induced by Aβ1-42. Rg1 treatment evidently recovered the levels of SOD, GSHPx and MDA changed by Aβ1-42, but there were no significant differences in oxidative stress parameters in the LY group and the Rg1+LY group compared with the model group (p>0.05). It revealed that with the possible involvement of GSK-3β signaling pathway, ginsenoside Rg1 could reduce the oxidative stress induced by Aβ1- 42 in AD rats and protect the brain with the possible involvement of GSK-3β signaling pathway.
In summary, ginsenoside Rg1 can activate the PI3K/ Akt/GSK-3β signaling pathway, thereby alleviating the injuries of learning and memorizing abilities, inhibiting neuronal apoptosis in the hippocampus, reducing oxidative stress and protecting the brains of AD rats induced by Aβ1-42. Nevertheless, further studies are needed to identify whether ginsenoside Rg1 can affect the progression of this disease through the regulation of other pathways.
Acknowledgements:
Lishui Wang and Song Shen have contributed same to this work.
Conflict of interests:
The authors declared no conflict of interest.
References
- Thomas J, Thomas CJ, Radcliffe J, Itsiopoulos C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: A focus on Alzheimer’s disease. Biomed Res Int 2015;2015:172801.
[Crossref] [Google Scholar] [Pub Med]
- Area-Gomez E, Schon EA. Mitochondria-associated ER membranes and Alzheimer disease. Curr Opin Genet Dev 2016;38:90-6.
[Crossref] [Google Scholar] [Pub Med]
- Gupta VK, Chitranshi N, Gupta VB, Golzan M, Dheer Y, Vander Wall R, et al. Amyloid β accumulation and inner retinal degenerative changes in Alzheimer’s disease transgenic mouse. Neurosci Lett 2016;623:52-6.
[Crossref] [Google Scholar] [Pub Med]
- Zhan X. Author response: Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurol 2017;88(24):2338.
[Crossref] [Google Scholar] [Pub Med]
- Dong G, Chen T, Ren X, Zhang Z, Huang W, Liu L, et al. Rg1 prevents myocardial hypoxia/reoxygenation injury by regulating mitochondrial dynamics imbalance via modulation of glutamate dehydrogenase and mitofusin 2. Mitochondrion 2016;26:7-18.
[Crossref] [Google Scholar] [Pub Med]
- Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, et al. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A comprehensive overview of experimental studies. Cells 2018;7(12):270.
[Crossref] [Google Scholar] [Pub Med]
- Yu ZH, Cai M, Xiang J, Zhang ZN, Zhang JS, Song XL, et al. PI3K/Akt pathway contributes to neuroprotective effect of Tongxinluo against focal cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol 2016;181:8-19.
[Crossref] [Google Scholar] [Pub Med]
- Zhen N, Jin L, Ma J, Zhu J, Gu S, Wang J, et al. Ginsenoside Rg1 impairs homologous recombination repair by targeting CtBP-interacting protein and sensitizes hepatoblastoma cells to DNA damage. Anticancer Drugs 2018;29(8):756-66.
[Crossref] [Google Scholar] [Pub Med]
- Li L, Deng WX, He JF, Zeng G, Liu WG, Li H, et al. [Neuroprotective Effect of Ginsenoside Rg1 on Focal Cerebral Ischemia-Reperfusion Rats]. Neur Injury Funct Reconstruct 2016;11:95-8.
- Qi Y, Dou DQ, Jiang H, Zhang BB, Qin WY, Kang K, et al. Arctigenin attenuates learning and memory deficits through PI3k/Akt/GSK-3β pathway reducing tau hyperphosphorylation in Aβ-induced AD mice. Planta Med 2017;83(01-02):51-6.
[Crossref] [Google Scholar] [Pub Med]
- Sun Y, Cheng XI, Wang H, Mu X, Liang Y, Luo Y, et al. Dl-3-n-butylphthalide promotes neuroplasticity and motor recovery in stroke rats. Behav Brain Res 2017;329:67-74.
[Crossref] [Google Scholar] [Pub Med]
- Tian N, Kanno T, Jin Y, Nishizaki T. Lithium potentiates GSK-3β activity by inhibiting phosphoinositide 3-kinase-mediated Akt phosphorylation. Biochem Biophys Res Commun 2014;450(1):746-9.
[Crossref] [Google Scholar] [Pub Med]
- Bai Q, Song D, Gu L, Verkhratsky A, Peng L. Bi-phasic regulation of glycogen content in astrocytes via Cav-1/PTEN/PI3K/AKT/GSK-3β pathway by fluoxetine. Psychopharmacol 2017;234(7):1069-77.
[Crossref] [Google Scholar] [Pub Med]
- Zhang C, Zhao S, Zang Y, Gu F, Mao S, Feng S, et al. The efficacy and safety of Dl-3n-butylphthalide on progressive cerebral infarction: A randomized controlled STROBE study. Med 2017;96(30).
[Crossref] [Google Scholar] [Pub Med]
- Jeong JH, Kang EB. Effects of treadmill exercise on PI3K/AKT/GSK-3β pathway and tau protein in high-fat diet-fed rats. J Exerc Nutr Biochem 2018;22(1):9.
[Crossref] [Google Scholar] [Pub Med]
- Wang H, Li Y, Wu Q, Xu C, Liu Q. Combination of butylphthalide with umbilical mesenchymal stem cells for the treatment of delayed encephalopathy after carbon monoxide poisoning. Med 2016;95(49):e5412.
[Crossref] [Google Scholar] [Pub Med]
- Chao FE, Ji-fang JI, Mei-xia TA, Meng CH. Protective effect of gastrodin on a rat model of amyloid-β1–42-induced Alzheimer’s disease. Nat Product Res Dev 2018;30(1):89.
- Wang CY, Wang ZY, Xie JW, Wang T, Wang X, Xu Y, et al. Dl-3-n-butylphthalide-induced up regulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer's disease mouse model. Neurobiol Aging 2016;38:32-46.
[Crossref] [Google Scholar] [Pub Med]
- Xu ZP, Gan GS, Liu YM, Xiao JS, Liu HX, Mei B, et al. Adiponectin attenuates streptozotocin-induced tau hyperphosphorylation and cognitive deficits by rescuing PI3K/Akt/GSK-3β pathway. Neurochem Res 2018;43(2):316-23.
[Crossref] [Google Scholar] [Pub Med]