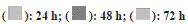- *Corresponding Author:
- N. Wisidsri
Faculty of Integrative Medicine, Rajamangala University of Technology Thanyaburi, Thanyaburi, Pathum Thani 12130, Thailand
E-mail: nakuntwalai_w@rmutt.ac.th
| Date of Received | 26 September 2021 |
| Date of Revision | 27 December 2022 |
| Date of Acceptance | 30 March 2023 |
| Indian J Pharm Sci 2023;85(2):463-471 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cissampelos pareira and Heliotropium indicum have been used as traditional medicine for treating several disorders. Specifically, Cissampelos pareira simply forms a gel without additional compounds which result from mainly pectin sources. In this study, the pectin from Cissampelos pareira was used to develop a hydrogel patch by adding Heliotropium indicum extract. The characterization of pectin-based hydrogel patch and pectin-based hydrogel containing Heliotropium indicum patch was evaluated. The results revealed suitable characterization of pectin-based hydrogel patches in physical and mechanical properties. The pectinbased hydrogel patches also presented biological activities through antioxidant and anti-inflammatory activities and these effects were synergized in a pectin-based hydrogel containing Heliotropium indicum patches. Furthermore, the pectin-based hydrogel patches presented biocompatibility by non-cytotoxicity to fibroblast and macrophage cells. Fibroblasts can proliferate in the pectin-based hydrogel patches. According to the results, the pectin-based hydrogel patches are of great benefit in pharmaceutical and cosmeceutical applications.
Keywords
Cissampelos pareira, Heliotropium indicum, pectin, cytocompatibility, antioxidant, anti-inflammation
Cissampelos pareira (C. pareira) (family Menispermaceae) is a tropical climbing plant omnipresent in Asia, South America and East Africa. This plant is consumed as food and traditionally used to treat disorders of skin, inflammation and gastrointestinal diseases such as diarrhea, dyspepsia, and anorexia[1]. It has been reported the pharmacological properties of various parts of C. pareira. The root extract has been revealed antioxidant[2], anti-inflammatory[3], anti-nociceptive, and anti-arthritic[4] activities both of in vitro and in vivo studies. Furthermore, the whole plant extract of C. pareira has been reported analgesic and anti-inflammatory[5] as well as cytotoxic to the cancer cells[6]. Likewise,the leaf extract of C. pareira has also been possessed anti-arthritics activity[7]. The water extract of fresh leaves of C. pareira presents a specially property that can form a gel at room temperature in a short period time without the need for sucrose or calcium[8,9]. The main gelling composition of C. pariera leaves compose mainly of galacturonic acid and small amounts of neutral sugars which are defined as low methoxyl-pectin[10]. In addition, our previous study showed that the pectin from C. pariera leaves demonstrated the radical scavenging, and anti-inflammatory activities in Lipopolysaccharide (LPS) stimulated macrophage cells[11].
Heliotropium indicum (H. indicum) (family Boraginaceae)is distributed in the tropical and temperate parts of the world such as India, Africa, Bangladesh, Sri Lanka, Nepal, the Philippines, and Thailand. It has been commonly used in India in traditional medicine to treat fever, diarrhea, skin disorders, wound and ulcer[12]. In addition, various pharmacological properties of H. indicum Extracts (HIE) have been reported. Among these, many effects are valuable in wound healing promotion including antimicrobial and antioxidant activities[13,14]. In previous research, we have found that HIE given radical scavenging, anti-inflammatory activities in macrophages, and also growth factor promotion in fibroblasts[15]. The in vitro and cellular studies according to the previous studies which demonstrated that HIE promoted wound healing in animal models including excision (normal and infected), incision, dead space, and diabetic wound[16, 17].
Hydrogels are insoluble hydrophilic materials which do not dissolve in water but swells considerably in an aqueous solution. They are popular groups of a wound dressing. The swelling property of hydrogels helps granulation tissues and epithelium in a moist environment. In general, hydrogels are transparent, maintain a moist wound environment, and are appropriate for wounds with low to moderate exudate[18]. Therefore, hydrogels have been widely used as wound dressing materials for dry chronic wounds, necrotic wounds, skin tears, surgical wounds, burn wounds, and pressure ulcers[19,20]. The forms of hydrogels are in the shapes of sheet hydrogel, amorphous gel, and impregnated gauze. Hydrogel sheets can be cut to fit around the wound due to their flexible nature without secondary dressing[21]. There are many hydrogels in the market such as sheet dressings, impregnated gauze, and water-based gels, Purilon™, Intrasite™, Nu-gel™ and Aquaform™ polymers.
Due to their advantage in biological activities of C. pareira and H. indicum are promising natural alternatives that may extend to use in pharmaceutical and cosmeceutical applications. Therefore, this work aimed to evaluate the biological properties of C. pareira pectin-based hydrogel containing HIE through antioxidant and anti-inflammatory activity. Moreover, characterization and biocompatibility were also observed.
Materials and Methods
Materials:
C. pareira leaves were collected from Thailand’s Northeast province of Udonthani and H. indicum leaves were collected from Buri Rum province. Murine macrophage (RAW 264.7) and murine fibroblast (NIH 3T3) cell lines were procured from the American Type Culture Collection (ATCC, Manassas, VA). Dulbecco’s modified Eagle’s medium (DMEM), Fetal Bovine Serum (FBS), penicillin, streptomycin, and trypsin-Ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco, USA. Trypan blue, resazurin, sodium nitroprusside, and DPPH were purchased from Sigma-Aldrich, USA. Griess reagent kit was purchased from Promega, USA.
Preparation of C. pareira pectin and HIE:
In this research, the method for C. pareira pectin extraction was modified from Singthong et al.[9], whereby the fresh leaves of C. pareira were first cleaned with water and dried at 45° for 24 h. The dried leaves were ground and kept in a desiccator at room temperature. The dry powder of C. pareira was mixed with distilled water (1:50 w/v) and adjusted to pH 3.8-4.0 followed by heating at 60° in a water bath for 60 min. The crude extract was filtrated and centrifuged at 10 000 rpm for 15 min. The supernatant was collected to concentrate to half of its volume in a rotary evaporator followed by precipitation in 95 % ethanol (w/v) for the final concentration of 70 %. The precipitate was freeze-dried and ground for powder of C. pareira pectin. The powder was kept in a desiccator for subsequent development the hydrogel patch.
For HIE, the leaves of the plant were cleaned with water and dried at 50° for 72 h. The dried leaves were ground and kept in a desiccator at room temperature. The dry powder was macerated in 70 % ethanol and shook at 250 rpm with a shaker for 24 h. The macerated mixture was filtrated before collect the supernatant to evaporate using a rotary evaporator. The crude extract of H. indicum leaves was stored at -20° until used.
Preparation of the pectin-based hydrogel patch:
Preparation of C. pareira pectin solution: C. pareira pectin powder was dissolved in distilled water at a concentration 5 mg/ml. The solution was centrifuged at 8000 rpm for 10 min. The supernatant was collected and kept at 4°.
Preparation of H. indicum solution: The H. indicum solution of 1 % w/w was prepared by weighing 1 g of H. indicum crude extract and then added 100 ml of propylene glycol. The mixture was mixed continuously for 30 min.
Preparation of Pectin-Based hydrogel (PB) patch and Pectin-based Hydrogel containing HIE (PH) patch: The patches were prepared by mixing 50 g of C. pareira pectin solution, 20 g of 10 % polyvinyl alcohol, 10 g of 10 % gelatin, 5 g of 10 % carrageenan, and 15 g of propylene glycol (PB) or H. indicum solution (PH) using hot plate magnetic stirrers at 40° for 30 min. Two grams of the mixtures were poured into a petri dish (100×15 mm) and then dried in an oven at 60° for 12 h. After drying, PB and PH patches were stored away from moisture, heat and light.
Physical and mechanical characterization of the pectin-based hydrogel patch:
Color: The color of PB and PH patches was evaluated by organoleptic and spectrophotometer (ColorQuest XE, HunterLab, USA) based on CIEL*a*b* system. The color parameters were measured by the value of L* (black [0] to white [100]), a* (greenness [−] to redness [+]), and b* (blueness [−] to yellowness [+]).
Thickness: The thickness of PB and PH patches was measured using a digital vernier caliper at five locations (center, two points of edge, and two points within the patch) of three patches. The thickness of the PH was presented as mean±SD.
Weight: The weight of the PB and PH patches was determined three of patches on an electronic balance and mean weight was calculated. The weight of the patches was presented as mean±SD.
pH measurement: The 2 g PB or PH patches were dissolved with 25 g deionized water and stirred until homogenous solution at 25°. The pH of solutions was determined using a pH meter (SP-2100, Suntex, Taiwan).
Swelling study: The PB and PH patches were cut in 2×2 cm size and dried at 60° for 48 h. The dried patches were kept in a beaker with 20 ml of pH 7.4 phosphate buffer at room temperature. The swollen patches were weighted in grams. The swelling ratio was calculated using the following formula:
Percentage swelling=[(WS-WD)/WD]×100
Where WS=Weight of the swollen patches in gram and WD=Weight of the dry patches in gram
Morphological study by scanning electron microscope: The morphology of the patches was examined by scanning electron microscopy (JEOL JSM-IT300, Oxford X-Max 20, USA). The PB and PH patches were cut into 10×10 mm pieces. Samples were gold coated and observed using an accelerating voltage of 2.0 kV.
Mechanical properties: The tensile strength and elongation of PB and PH patches were measured using a universal tester with sample size 1×5 cm, with a load cell of 50 newtons and 10 mm/min testing speed. The strength and elongation were presented as tensile strength and percentage elongation of mean±SD.
Heating-cooling cycle test: PB and PH patches were kept in the refrigerator at 4±2° for 24 h and in the hot air oven at 45±2° for 24 h per one cycle. The test was carried out for 7 cycles. Physical properties of PB and PH patches in terms of color and pH were evaluated at the end of the test.
Antioxidant activities of pectin-based hydrogel:
DPPH radical scavenging activity: The radical scavenging activity of PB and PH patches was modified from Norajit et al.[22]. The patches were cut into 1 g and dissolved in pH 7.4 phosphate buffer saline 5 ml. The dissolved patches were centrifuged, and the 75 µl of the supernatants were collected to mixed with 150 µl of 0.2 mmol/l 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (in methanol) and allowed to stand for 30 min without direct exposure to light. The absorbance was determined at 520 nm using a microplate reader. In addition, PBS and L-ascorbic acid were used as the negative and positive controls, respectively. The DPPH scavenging capacity of the experimental pectin is expressed as a percentage of DPPH radical inhibition as below, where OD is the optical density;
Percentage DPPH radical inhibition=[(ODwithout extract–ODwith extract)/ODwithout extract)×100
Nitric Oxide (NO) radical scavenging activity: The supernatants 1 ml were incubated with the NO donor (sodium nitroprusside) 10 mmol/l in a pH 7.4 phosphate buffer saline for 180 min. Approximately 100 μl of the resulting solution was withdrawn to react with a Griess Reagent kit (Promega, USA), whereby the solution was reacted with 20 μl sulfanilamide for 10 min and then 20 μl N-(1-napthyl)ethylenediamine dihydrochloride for another 10 min. The reaction mixture absorbance was measured at 560 nm and the NO concentrations were determined as the nitrite (NO2–) concentrations from the standard curve of a standard nitrite solution. PBS and L-ascorbic acid were used as the negative and positive controls, respectively. The NO scavenging capacity of the experimental pectin was expressed as a percentage of nitrite production inhibition using the following formula;
Percentage NO2– production inhibition=[(NO2–without extract–NO2–with extract)/NO2–without extract]×100
Cell culture:
Murine macrophage (RAW264.7) and murine fibroblast (NIH3T3) cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % FBS and 100 U/ml penicillin and 100 µg/ml streptomycin, and incubated at 37° in a humidified atmosphere of 5 % CO2. The macrophages were passaged twice a week using cell scraper. Fibroblasts were passaged twice a week using trypsinization with 0.25 % trypsin-EDTA. The cell viability was determined with 0.4 % trypan blue. Cells with more than 85 % viability were used in the experiments.
In vitro cytotoxicity test: The cytotoxicity test of PB and PH patches was modified from Li et al.[23]. The patches were cut into 5×5 mm size and were UV irradiated in 12 well plates for 30 min. The irradiated patches were incubated with 1 ml of DMEM containing 10 % FBS and 100 U/ml penicillin and 100 µg/ml streptomycin and incubated at 37° in a humidified atmosphere of 5 % CO2 for 24 h. The conditioned medium was then removed for testing cytotoxicity in fibroblast cells. NIH3T3 fibroblast cells were plated in 96 well plate (2×105 cell/ml). The cells were treated with condition medium for 24, 48, and 72 h. The treated cells were determined cell viability using resazurin reduction assay, whereby the treated cells were incubated for 4 h at 37° in 100 µl fresh DMEM containing 50 μg/ml resazurin. The reaction mixture absorbance was determined at 560 against 600 nm. The percentage of cell viability was calculated using the formula:
Percentage cell viability=[(OD560–OD600)with extract–(OD560–OD600)without extract]×100
In vitro cytocompatibility test: To evaluate the cytocompatibility of PB and PH patches, the method was modified from Li et al.[23]. The PH patch was cut into 5×5 mm size and was UV irradiated in 12 well plates for 30 min. NIH3T3 fibroblast cells (4×105 cell/ml) were plated together with the patch for 24, 48, and 72 h. Finally, the morphology and proliferation of the cells were observed with an optical microscope.
Anti-inflammatory activity: Determination of anti-inflammatory activity of PB and PH patches was modified from Ninan et al.[24]. The RAW264.7 macrophage cells (4×105 cell/ml) were pretreated with the conditioned medium in 96 well plate and incubated at 37° for 24 h. The pre-treated cells were stimulated with 1 μg/ml of LPS and incubated for another 24 h. The NO concentrations were determined from NO2– in the stimulated-cell supernatant using a Griess reagent kit, whereby 100 μl of the supernatant was reacted with 20 μl sulfanilamide for 10 min and with 20 μl N-(1-napthyl)ethylenediamine dihydrochoride for another 10 min. The reaction mixture absorbance was measured at 560 nm and the NO concentrations were determined as the nitrite (NO2–) concentrations from the standard curve of a standard nitrite solution. DMEM and 100 μM of dexamethasone each with 1 μg/ml LPS were respectively used as the negative and positive controls.
Cell viability of the LPS-stimulated macrophage: The viability of the residual macrophage cells after the NO assay, given the conditioned medium was determined by resazurin reduction assay, whereby the residual cells were incubated for 2 h at 37° in 100 μl fresh DMEM containing 50 μg/ml resazurin. The reaction mixture absorbance was determined at 560 against 600 nm.
Statistical analysis:
Physical and mechanical characterization, antioxidant activities, and in vitro results were expressed as mean±Standard Error of the Mean (SEM) of triplicate experiments. Cell viability was analyzed by one-way Analysis of Variance (ANOVA) followed by Tukey’s (post hoc) using the SPSS 22.0 software and a value of p<0.05 was considered statistically significant. Descriptive statistics and mean±Standard Deviation (SD) were presented for physical and mechanical characterization.
Results and Discussion
The patches composed of low methoxyl pectin from C. pareira, gelatin, polyvinyl alcohol, gelatin, and carrageenan were formulated. Propylene glycol was used as a plasticizer and extracted solvent. A previous study suggested that pectin provided the same utilities of alginate which offers the possibility to control dissolution[25]. Moreover, pectin has been used as a texturizer or stabilizer in the food industry. Recently, pectin was interested in its use as an additive in the drug delivery systems. Pectin-based formulations have developed in pharmaceutical applications for improving the properties for many reasons including higher drug loading efficiency, less pre-mature drug release, greater efficiency for the controlled release of peptide and protein drugs, increased biocompatibility and controllable swellability and degradability[26].
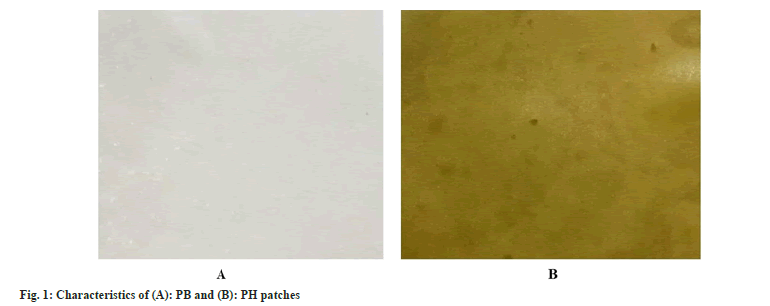
The characteristic of PB and PH patches is shown in fig. 1. For visual observation, the PB patch is translucent with white color while the PH patch is a dark-green color similar to HIE. The color of PB and PH patches were represented in L*, a*, and b* (Table 1). The color of the patch has a direct impact on the customer. PB patch showed a higher L* value than PH patch that represented lighter color. PH patch showed greenness with a higher a* value and quite a yellow color with higher b* value when compared with PB patch. The color is affected by the color of HIE. The thickness value affects the permeability and mechanical properties of the patch. PB and PH patches were not different in thickness and weight (Table 2). Thus, HIE was not affected by the thickness and weight of the patches. However, PH patches seem to be moist more than PB patches. That difference may be caused by HIE also. Both patches were smooth without pores and cracks on the surface. The pH of PB and PH patches are presented in Table 1.The pH of the PH patch was 6.13 which was more acidic than the PB patch significantly different due to the pH of HIE and propylene glycol, solvent for HIE. However, the pH value of both formulations was suitable and compatible for skin. Interestingly, the color and pH of the patches were not changed after stability testing. After stability testing, there was no visible change in the appearance of PB and PH patches. The color with L*, a*, and b* value and pH of the patches were not changed (Table 1).
| Patch | Before | After | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | pH | L* | a* | b* | pH | |
| PB | 40.78±1.43 | 0.53±0.19 | 1.59±0.32 | 6.24±0.03 | 34.85±0.81 | 2.53±0.17 | 0.02±0.11 | 6.22±0.03 |
| PH | 31.56±0.66 | 1.24±0.04 | 7.31±0.55 | 6.13±0.01# | 21.54±0.83 | 5.82±0.18 | 12.46±1.49 | 6.16±0.02# |
Note: #Significantly difference at p<0.05 between sample.
Table1: Color and pH of PB and PH patches before and after heating-cooling cycle test.
| Patch | Thickness (mm) | Weight (mg) | Swelling ratio (%) |
|---|---|---|---|
| PB | 0.68±0.02 | 4.61±0.01 | 106.82±26.54 |
| PH | 0.66±0.01 | 4.43±0.18 | 93.87±2.51 |
Table 2: Thickness, Weight, and Swelling ratio of Pectin-Based Hydrogel Patches.
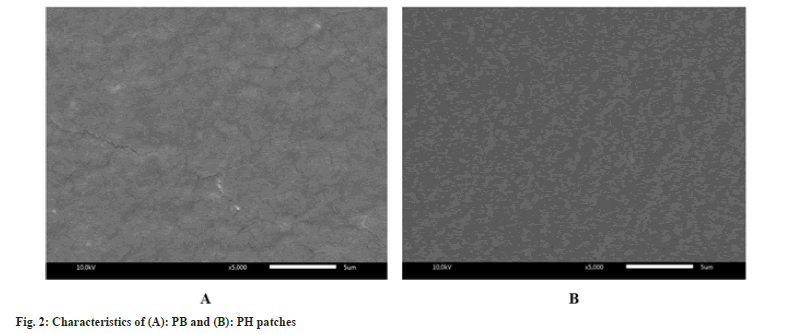
The capacity of the dressing patch to hold exudate or donate fluid is one of the factors needed to be taken into consideration when selecting dressings[20]. However, hydrogels are suitable for dry wounds or those with minimal exudates[27]. In this study, the results show a high swelling ratio more than 90 % in PB and PH patches (Table 2). This result in close to the effectiveness of low methoxyl pectin/gelatin/carboxymethyl cellulose hydrogel films with a high fluid absorption capacity of approximately 90 %[28]. However, the PH patch revealed slightly lower fluid handling properties than the PB patch. HIE may be attributed to the reduction in the number of intermolecular cross-links between polymeric networks within films. Scanning electron microscopy examinations were carried out to get a better surface image of the patches. Fig. 2 showed the scanning electron microscopy of PB and PH patches. It was observed the surface of the homogeneous without bubbles of PB and PH patches. A patch containing HIE showed more structural continuity with chemical composition interaction. Therefore, the PH patch displayed a smooth surface more than the PB patch.
Mechanical properties of dressing or patch are important to be durable, stress-resistant, flexible, pliable, and comfortable to use in different parts of the body[21]. The tensile strength is the maximum tensile stress maintained by the patches during the tension test while the elongation at break is an indication of flexibility and extensibility of patches before breakage[30]. High elongation at break and tensile strength are suitable for patch application and handling purposes. From the results, PH and PB patches showed no significant difference in tensile strength (Table 3). However, incorporation of HIE into the patch significantly increased the percentage of elongation at break. Therefore, the PH patch is flexible more than the PB patch. Conversely, the PB patch presented withstand the stress than the PH patch. This is mainly caused by the interaction between polymers and polyphenolic compounds from HIE as well as green tea extract increased percentage elongation of chitosan films[30].
| Patch | Tensile strength (MP) | Elongation (%) |
|---|---|---|
| PB | 74.27±11.28 | 34.90±3.08 |
| PH | 65.97±12.65 | 62.84±5.00* |
Note: *Significantly difference at p<0.05 between sample.
Table 3: Mechanical Properties of Pectin-Based Hydrogel Patches.
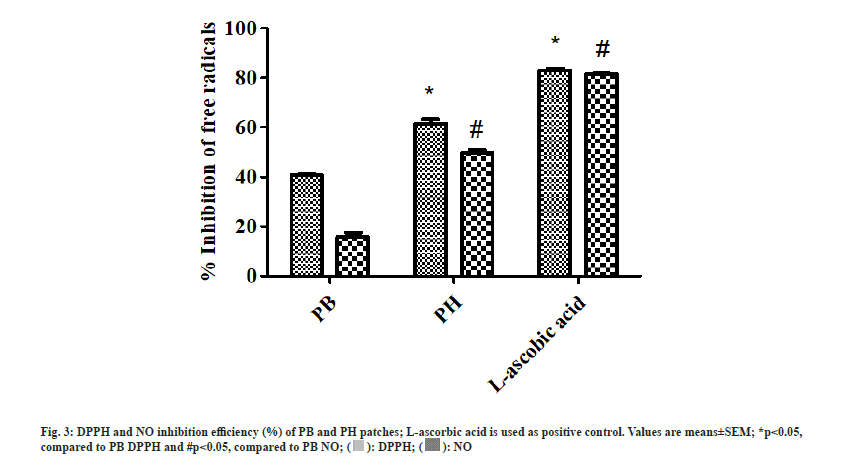
According to previous studies, C. pareira pectin and H. indicum ethanolic extract showed potent scavenging ability and anti-inflammatory property[11,15]. In this study, the antioxidant properties were evaluated through the scavenging performance of PB and PH patches. The results reveal that PB and PH patches significantly reduce DPPH and NO free radical, achieving the inhibition performance of 40.99±0.19 % and 13.24±0.30 %, and 61.57±3.05 % and 50.01±1.63 %, respectively (fig. 3). Interestingly, the addition of HIE significantly increased free radicals scavenging performance in pectin-based hydrogel patches. Likewise, Citrus unshiu (C. unshiu) peel pectin film alone showed lower antioxidant activity than C. unshiu peel pectin film containing Salvia officinalis leaf extract[31]. The antioxidant activities have also been found in alginate film containing ginseng extract[22] and gelatin-based containing Caesalpinia decapetal[32].
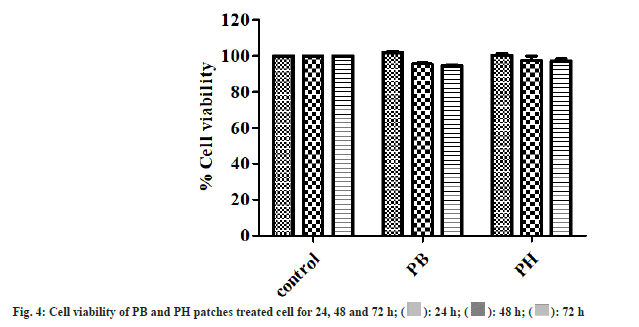
Hydrogel dressings for wound treatment are nonreactive with biological tissue, permeable to metabolites, and are non-irritant[18]. The cytocompatibility of PB and PH patches was observed in fibroblast cells. The results of cytocompatibility revealed that PB and PH patches were non-toxic to the fibroblast cells. The cell viability of PB and PH patches treated fibroblast cells was close to untreated control at 24, 48, and 72 h (fig. 4). The cells surrounding the gels adhered to, spread, and grew on the cell culture plate after 24, 48, and 72 h of plating. Furthermore, results also showed that fibroblast cells can proliferation in PB and PH patches (fig. 5). These results indicated that the pectin-based hydrogel patch was non-cytotoxic to the cells. Then it benefits in product development.
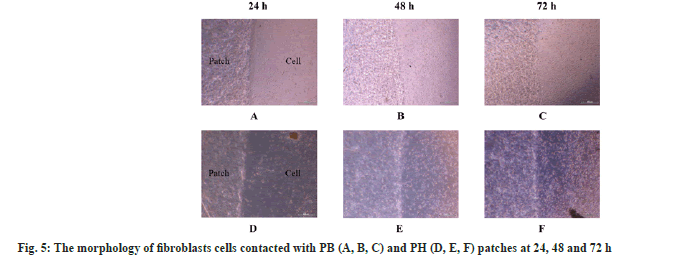
Inflammation is the host defense mechanism against pathogens, tissue damage, or abnormal cells. In process of inflammation, NO plays an important role which acts as a signaling molecule. Macrophages are a major source of NO production during the inflammatory process[33]. This study focuses on the investigation of the anti-inflammatory properties of pectin-based hydrogel patch through NO inhibitory activity. The macrophage cells were stimulated with LPS to become activated macrophages. The activated macrophages can produce a high amount of NO. The inhibition of NO production refers to the anti-inflammatory activity of the patches. The result showed the decreasing of NO production in PB and PH patches under LPS-stimulated macrophages when compared with the positive control, dexamethasone (fig. 6). These results suggested that PB and PH patches demonstrated anti-inflammatory properties by inhibiting NO production in activated macrophages. NO inhibition was more pronounced in the PH treatment. Some previous studies reported anti-inflammatory activity in agarose hydrogel containing tannic acid and its benefit for wound healing[23]. Given the non-cytotoxicity of PB and PH patches, the pectin patch-treated cells could achieve high NO table 1e results indicated that the cell viability of macrophages was close to nontreated control cells.
According to the results of this study, pectin-based hydrogel patches revealed suitable physical and mechanical characterization. The pectin-based hydrogel containing HIE patches increases in antioxidant and anti-inflammatory properties. Furthermore, the pectin-based hydrogel patches also presented cytocompatibility and non-cytotoxicity to fibroblast and macrophage cells. These results suggested the benefit of pectin-based hydrogel patches in pharmaceutical and cosmeceutical applications. However, in vivo toxicity and efficacy are further study.
Acknowledgements:
The authors acknowledge the financial support received from the National Research Council of Thailand. We are grateful to Faculty of Integrative Medicine, Rajamangala University of Technology Thanyaburi for providing the facilities and place to carry out the research.
Conflicts of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Samanta J, Bhattacharya S, Rayat R. Phytochemical investigation and pharmacognostic standardization of Cissampelos pareira root. Anc Sci Life 2012;31(4):181-4.
[Crossref] [Google Scholar] [PubMed]
- Bafna A, Mishra S. Antioxidant and immunomodulatory activity of the alkaloidal fraction of Cissampelos pareira linn. Sci Pharm 2010;78(1):21-32.
[Crossref] [Google Scholar] [PubMed]
- Amresh G, Reddy GD, Rao CV, Singh PN. Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol 2007;110(3):526-31.
[Crossref] [Google Scholar] [PubMed]
- Amresh G, Singh PN, Rao CV. Antinociceptive and antiarthritic activity of Cissampelos pareira roots. J Ethnopharmacol 2007;111(3):531-6.
[Crossref] [Google Scholar] [PubMed]
- Reza HM, Shohel M, Aziz SB, Pinaz FI, Uddin MF, Al-Amin M, et al. Phytochemical and pharmacological investigation of ethanol extract of Cissampelos pareira. Indian J Pharm Sci 2014;76(5):455-8.
[Google Scholar] [PubMed]
- Thavamani BS, Mathew M, Dhanabal SP. Anticancer activity of cissampelos pareira against dalton's lymphoma ascites bearing mice. Pharmacogn Mag 2014;10(39):200-6.
[Crossref] [Google Scholar] [PubMed]
- HemRaj V. Anti-arthritics activity of Cissampelos pareira leaves and Stephania glabra rhizome ethanolic extract on adjuvant and potassium oxonate treated rat. Open Rheumatol J 2019;13:45-52.
- Arkarapanthu A, Chavasit V, Sungpuag P, Phuphathanaphong L. Gel extracted from Khruea‐ma‐noi (Cyclea barbata Miers) leaves: Chemical composition and gelation properties. J Sci Food Agric 2005;85(10):1741-9.
- Singthong J, Ningsanond S, Cui SW, Goff HD. Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocoll 2005;19(5):793-801.
- Singthong J, Cui SW, Ningsanond S, Goff HD. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr Poly 2004;58(4):391-400.
- Wisidsri N, Thungmungmee S. Radical scavenging and anti-inflammatory properties of pectin from Cissampelos pareira Linn. Walailak J Sci Technol 2019;16(11):841-50.
- Dash GK, Abdullah MS. A review on Heliotropium indicum L.(Boraginaceae). Int J Pharm Sci Res 2013;4(4):1253.
- Wani PA, Tolu AM, Wahid S. Antioxidant, antimicrobial and antibiotic resistance modifying effect of Heliotropium indicum. Biocatal Agric Biotechnol 2018;15:113-8.
- Rao PR, Nammi S, Raju AD. Studies on the antimicrobial activity of Heliotropium indicum Linn. J Nat Remedies 2002;2(2):195-8.
- Wisidsri N, Thungmungmee S, Khobjai W. Fator promoting wound healing: Radical scavenging and anti-inflammatory activity and growth factor promotion of Heliotropium indicum. Int J Pharm 2019;11(5):44-8.
- Dash GK, Murthy PN. Studies on wound healing activity of Heliotropium indicum Linn. leaves on rats. ISRN Pharmacol 2011;2011:847980.
[Crossref] [Google Scholar] [PubMed]
- Ayisha S, Baskaran K. Wound healing potential of ethanolic leaf extract of Heliotropium indicum on excision wound model in diabetic rats. Int J Pharm Sci Res 2016;7:155-58.
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: Current advances and future directions. J Appl Poly Sci 2019;136(27):47738.
- Dhivya S, Padma VV, Santhini E. Wound dressings–a review. BioMedicine 2015;5(4):22.
[Google Scholar] [PubMed]
- Vowden K, Vowden P. Wound dressings: Principles and practice. Surgery (Oxford) 2017;35(9):489-94.
- Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: A review. J Pharm Sci 2008;97(8):2892-923.
[Crossref] [Google Scholar] [PubMed]
- Norajit K, Kim KM, Ryu GH. Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J Food Eng 2010;98(3):377-84.
- Li X, Kong X, Zhang Z, Nan K, Li L, Wang X, et al. Cytotoxicity and biocompatibility evaluation of N, O-carboxymethyl chitosan/oxidized alginate hydrogel for drug delivery application. Int J Biol Macromol 2012;50(5):1299-305.
[Crossref] [Google Scholar] [PubMed]
- Ninan N, Forget A, Shastri VP, Voelcker NH, Blencowe A. Antibacterial and anti-inflammatory pH-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl Mater Interfac 2016;8(42):28511-21.
- Munarin F, Petrini P, Tanzi MC, Barbosa MA, Granja PL. Biofunctional chemically modified pectin for cell delivery. Soft Matter 2012;8(17):4731-9.
- Sutar PB, Mishra RK, Pal K, Banthia AK. Development of pH sensitive polyacrylamide grafted pectin hydrogel for controlled drug delivery system. J Mater Sci Mater Med 2008;19(6):2247-53.
[Crossref] [Google Scholar] [PubMed]
- Ilenghoven D, Chan CY, Wan Ahmad Kamal WS, MohdYussof SJ, Ibrahim S. A review of wound dressing practices. Clin Dermatol J 2017;2(6):000133.
- Jantrawut P, Bunrueangtha J, Suerthong J, Kantrong N. Fabrication and characterization of low methoxyl pectin/gelatin/carboxymethyl cellulose absorbent hydrogel film for wound dressing applications. Materials 2019;12(10):1628.
[Crossref] [Google Scholar] [PubMed]
- Kavoosi G, Nateghpoor B, Dadfar SM, Dadfar SM. Antioxidant, antifungal, water binding, and mechanical properties of poly (vinyl alcohol) film incorporated with essential oil as a potential wound dressing material. J Appl Poly Sci 2014;131(20):1-8.
- Siripatrawan U, Harte BR. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll 2010;24(8):770-5.
- Han HS, Song KB. Antioxidant activities of mandarin (Citrus unshiu) peel pectin films containing sage (Salvia officinalis) leaf extract. Int J Food Sci Technol 2020;55(9):3173-81.
- Gallego MG, Gordon MH, Segovia F, Almajano Pablos MP. Gelatine-based antioxidant packaging containing Caesalpinia decapetala and Tara as a coating for ground beef patties. Antioxidants 2016;5(2):10.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Gray Z, Willette-Brown J, Zhu F, Shi G, Jiang Q, et al. Macrophage inducible nitric oxide synthase circulates inflammation and promotes lung carcinogenesis. Cell Death Discov 2018;4(1):642.
[Crossref] [Google Scholar] [PubMed]