- *Corresponding Author:
- Dengke Yang
Department of Dermatology, Kunming Hospital of Traditional Chinese Medicine Kunming, Kunming 650011, China
E-mail: 523687402@qq.com
| Date of Received | 25 March 2023 |
| Date of Revision | 26 November 2023 |
| Date of Accepted | 13 February 2024 |
| Indian J Pharm Sci 2024;86(1):246-252 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Psoriasis is a chronic immune-mediated inflammatory skin disease in which gamma delta T cells play an important role. Chinese herbal medicine Jia Wei Jing Xie Yin has its origin in Jing Xie Yin, a medicine created by the traditional Chinese medicine doctor Wu Jun. Previous studies have shown that Jia Wei Jing Xie Yin has good clinical efficacy for patients with blood-heat type psoriasis by inhibiting the interleukin-23/T-helper 17 immune inflammation axis. This study aims to determine the immunoregulatory effects and the underlying mechanisms of Jia Wei Jing Xie Yin in psoriasis-related inflammatory responses. Bagg albino mice with imiquimod-induced psoriasis-like dermatitis were treated with saline (Model), methotrexate that serves as a positive control, or low, medium and high doses of Jia Wei Jing Xie Yin intragastrically. The pathological changes, inflammatory cell infiltration, the expression of inflammatory cytokines, and Janus tyrosine kinase 3/signal transducers and activators of transcription pathway related proteins in the skin lesion were examined. The abundance of Vγ4+ T cells in lymph nodes and spleen was determined by flow cytometry. Jia Wei Jing Xie Yin ameliorated psoriasis area and severity index scores, reduced the infiltration of inflammatory cells and interleukin-17 producing gamma delta T cells, inhibited the messenger ribonucleic acid expression of interleukin-17, interleukin-22, interleukin-6, tumour necrosis factor alpha and matrix metalloproteinase-9, reduced inflammatory cell infiltration and pathological damage. Nevertheless, the expression of the Janus tyrosine kinase 3/signal transducers and activators of transcription signaling pathway was not significantly changed in the MTX and Jia Wei Jing Xie Yin groups. Our work demonstrates that Chinese herbal medicine Jia Wei Jing Xie Yin alleviates Imiquimod-induced inflammation in psoriasis. We have found a good regulatory effect of Jia Wei Jing Xie Yin on gamma delta T cells.
Keywords
Jia Wei Jing Xie Yin, psoriasis, gamma delta T cells
Psoriasis is one of the most common immunemediated chronic inflammatory skin disorders, which affects 3 % of the population worldwide, with 6 million people in China[1,2]. Psoriasis is not only manifested as skin damage, it is also associated with several important medical conditions, including depression, psoriatic arthritis and cardio metabolic syndrome[3]. Most psoriasis patients have some extent to their quality of life and mental health attributable to the disease[4]. Although the etiology of psoriasis is not fully understood, increasing evidence has demonstrated that the Interleukin (IL)-23/T helper 17 pathway is a critical axis in its pathogenesis[5]. Gamma Delta T (γδT) cells that have a distinctive T-Cell Receptor (TCR) on their surface. They are significantly enriched in mucosal and epithelial sites in the dermis of humans and mice, such as the skin, and respiratory, digestive, reproductive tracts and produce cytokines such as IL-17/ Interferon Gamma (IFN-γ)/IL-22. Recent studies have shown that γδT cells that produce IL-17 are a key component of the pathogenesis of psoriasis[6]. Traditional Chinese Medicine (TCM) believes that the occurrence of psoriasis is mostly related to two blood stasis and blood dryness, “blood stasis heat” and “dampness evil” are the key causes of psoriasis and cure, and its core pathogenesis is “blood stasis heat” and “rheumatism”. Preliminary clinical and basic studies have confirmed that Jia Wei Jing Xie Yin (JWJXY) has a significant effect on patients with psoriasis with blood fever, which can reduce the levels serum inflammatory factors (IL-17, IL- 6, Tumour Necrosis Factor Alpha (TNF-α), IL-2) and down-regulate the expression of Th17 cell related genes[5]. Based on the above relationship between γδT cells and psoriasis, this study intends to construct a mouse model of psoriasis induced by imiquimote. From the aspects of γδT cytokine secretion, signaling pathway expression, transcription factor expression and chemokine expression, the immunological mechanism of JWJXY regulating γδT cell-mediated antipsoriasis was elucidated, so as to clarify the target of JWJXY in the treatment of psoriasis to provide the basis for the clinical application of JWJXY in the treatment of psoriasis.
Materials and Methods
Experimental animals:
Bagg Albino (BALB/c) mice used in this experiment were purchased from Sibeifu (Beijing) Biotechnology Co. Ltd. with an Animal license number: SCXK (Beijing) 2019-001. Before the experiment, all mice were acclimated in our facility for 1 w under specific pathogen-free conditions. Animal experiments are conducted in animal experimental barrier systems. The protocols used in these experiments were reviewed and approved by the Experimental Animal Ethics Committee of Yunnan University of TCM (review number: R-062021LH-065).
Drugs and reagents:
The composition of JWJXY (60 kg adult dose) is as follows; buffalo horn 40 g, Rehmanniae radix 30 g, Lithospermum 30 g, Scutellariae radix 15 g, cortex dictamni 15 g, Spatholobi caulis 15 g, Oldenlandia diffusa 30 g, radix rubiae 10 g, radix clematidis 10 g, rhizoma atractylodes 10 g, Sophora flavescens 15 g, radix rhapontici g, and tripterygium 30 g. The drug was produced at the pharmacy of Kunming Municipal Hospital of TCM. The procedure was as follows; add 10 times of water, soak for 30 min, boil for 30 min, decoct 3 times, combine the decocted liquid, filter with gauze, centrifuge, evaporate with a rotary evaporator to concentrate the extract, weigh the mass, and convert 9.17 g of raw medicine into 1 g of extract of the original medicine. Based on the conventional dose of JWJXY used in the clinical treatment of adult psoriasis and the validation of previous studies, the concentration and dose of JWJXY were determined according to the conversion formula of body surface area between adult and mouse and the conversion ratio of drug dose. Imiquimod (IMQ) cream was purchased from China Sichuan Mingxin Pharmaceutical Co. (batch No. H20030128, Sichuan, China). Methotrexate tablets were purchased from Beijing Solarbio Technology Co., Ltd (batch No. IM0160, Beijing, China). Tri quick reagent, total Ribonuclic Acid (RNA) extraction reagent was purchased from Beijing Solarbio Technology Co., Ltd (batch No. Cat#R1100, Beijing, China). CY3 labeled goat anti-rabbit Immunoglobulin G (IgG) (H+L) was purchased from Beyotime (batch No. A0516, shanghai, China). Anti-CD11b antibody (ab8878) and anti-IL-17A antibody (ab79056) were purchased from Abcam (Cambridge, United Kingdom). TCR gamma/delta monoclonal antibody was purchased from Invitrogen (batch No. MA5-44014, Waltham, Massachusetts, United States of America (USA)). Signal Transducers and Activators of Transcription (STAT3) monoclonal antibody (9D8) (MA5-15193), Phospho-STAT3 (Tyr705) monoclonal antibody (MA1-13042), Janus Tyrosine Kinase 3 (JAK3) monoclonal antibody (AHO1572) was purchased from Invitrogen. PE anti-mouse TCR γ/δ Antibody (118108) and Fluorescein Isothiocyanate (FITC) anti-mouse TCR Vγ4 antibody (141104) were purchased from Biolegend (San Diego, California, USA).
IMQ-induced psoriatic BALB/c mice:
Sixty female BALB/c mice, with an average body weight of (18-20) g, were randomly divided into 6 groups; a control group, a model group, a low, medium and high-dose JWJXY group (8, 16 and 32 g/kg respectively); and a methotrexate-positive control group (MTX group), with 10 mice per group. All mice were housed in clean animal feeding rooms. The hair from the central area of the ear of the mouse was carefully shaved, the surface back was removed with a mild depilatory cream, and moisturizer was applied to the surface for later use. If the hair grew out again during the test, hair removal was repeated 1-2 times. IMQ cream of 5 % was evenly applied to the alopecia area at a dose of 50 mg/cm2, once a day for 6 d. The model group was administered 0.4 ml of normal saline by i.g., and the dose group was administered 0.4 ml of the corresponding concentration of the drug solution by i.g., once a day for 6 d.
Hematoxylin and Eosin (HE) staining:
6 d after drug administration, the mice in each group were sampled, and the samples were fixed in a 4 % paraformaldehyde solution. Transparent tissue sections were made by embedding, sectioning, drying, dewaxing, hydrating and HE staining. The tissue structure and inflammatory cell infiltration of the skin of the mice in each group were observed under an inverted light microscope, and images were collected with a digital camera.
Immunofluorescence staining:
Skin samples from the back lesions of mice were collected in 10 % formalin and embedded in paraffin. Sections (5 μm) were stained with H&E and anti-Rabbit CY3, Anti-CD11b antibody and TCR gamma/delta Monoclonal Antibody and Anti- IL-17A antibody (Abcam, USA) diluted 1:500, and staining was assessed using light and fluorescence microscopes (Olympus, Japan).
Flow cytometry:
Prepare skin single cell suspension and spleen single cell suspension for each group, count cells, and adjust the cell count to 1×105 in each group, transfer it to 1.5 ml Eppendorf (EP) tubes. Add 5 μl FITC anti-mouse TCR Vγ4 Antibody and PE anti-mouse TCR γ/δ Antibody was labeled with fluorescence and incubated at 4° in dark for 30 min; after the completion of antibody incubation, use 500 μl of 1×Phosphate Buffer Solution (PBS) to wash the cells, then resuspend cells on 500 μl 1× PBS, analysis was performed by flow cytometry on the machine.
RNA extraction and real-time quantitative Polymerase Chain Reaction (PCR):
Mouse skin cells were collected and RNA was isolated using an RNeasy kit (Qiagen, Hilden, Germany). Complementary Deoxyribonucleic Acid (cDNA) was manufactured from 1 μg of total RNA and detected by reaction q-PCR using an SYBR Premix Ex Taq II kit. The expression of IL-17, IL-22, IL-6, TNF-α, Matrix Metalloproteinase-9 (MMP-9), messenger RNA (mRNA) in the mouse skin cells was determined, and the relative expression amount of β-actin in each sample was calculated by applying the 2−ΔΔCT approach. The primer sequences of specific genes are shown in Table 1.
| Gene name | Primer sequences (5'-3') |
|---|---|
| IL-17A | Forward: 5'-TACCTCAACCGTTCCACGTC-3' |
| Reverse: 5'-TTTCCCTCCGCATTGACACA-3' | |
| IL-22 | Forward: 5'-TCAGACAGGTTCCAGCCCTA-3' |
| Reverse: 5'-GATGTTCTGGTCGTCACCGC-3' | |
| IL-6 | Forward: 5′-CTGCAAGAGACTTCCATCCAG-3' |
| Reverse: 5′-AGTGGTATAGACAGGTCTGTTGG-3' | |
| TNF-α | Forward: 5'-CTGAACTTCGGGGTGATCGG-3' |
| Reverse: 5'-GGCTTGTCACTCGAATTTTGAGA-3' | |
| MMP-9 | Forward: 5'-GGACCCGAAGCGGACATTG-3' |
| Reverse: 5'-CGTCGTCGAAATGGGCATCT-3' | |
| GAPDH | Forward: 5'-AGGTCGGTGTGAACGGATTTG-3' |
| Reverse: 5'-TGTAGACCATGTAGTTGAGGTCA-3' |
Table 1: Sequences of Target Gene Specific Primer
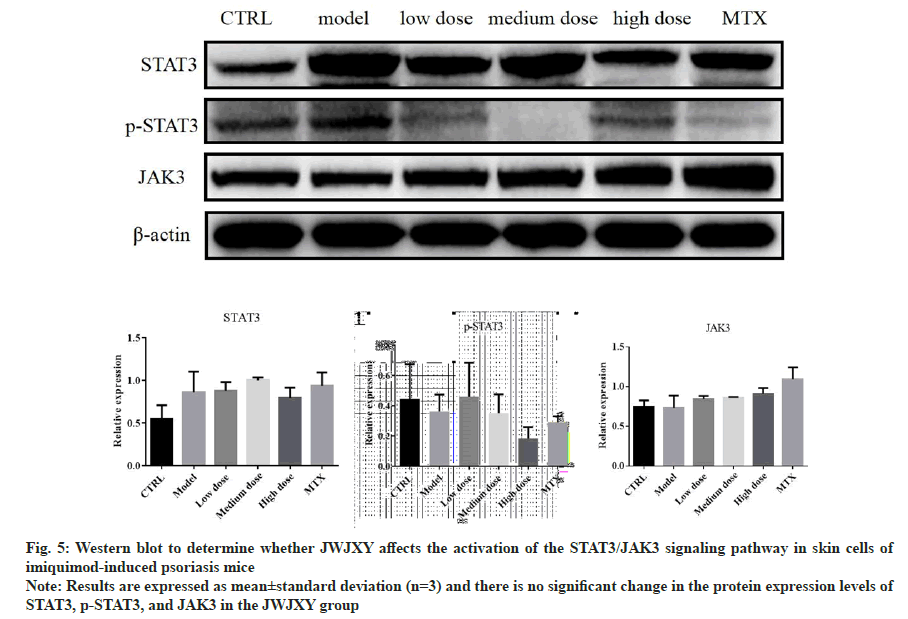
Western blotting:
Total cell and nuclear lysates were prepared as protocol using the following antibodies; p-STAT3, STAT3, JAK3 followed by incubation with a horseradish peroxidase-conjugated secondary antibody and visualized using a Bio-Rad ChemiDoc XRS Imaging System with an XRS camera (Bio- Rad, Hercules, California, USA).
Statistical methods:
Statistical comparisons between the two groups were performed using a student’s test. Graph Pad Software Prism 6.0 was used for statistical analysis. p values <0.05 were considered significant.
Results and Discussion
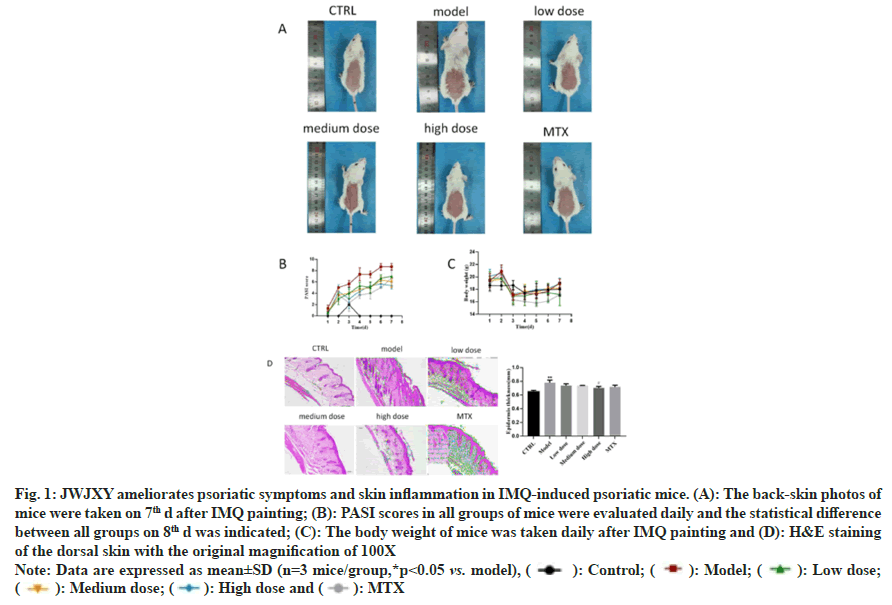
To confirm JWJXY ameliorated skin lesions in psoriasis, we established a mouse model of psoriasis induced via IMQ, in which control, MTX (2 mg/kg), and low, medium, and high doses of JWJXY (8, 16, and 32 g/kg, respectively) were administered for 6 d. Epidermis thickness was measured on 7th d. The epidermis thickness of the mice and Psoriasis Area and Severity Index (PASI) scores reflected the severity of the rash. On the 7th d, the severity of the skin rashes in the medium dose JWJXY group was significantly lower than that in the control group (fig. 1).
Fig. 1: JWJXY ameliorates psoriatic symptoms and skin inflammation in IMQ-induced psoriatic mice. (A): The back-skin photos of
mice were taken on 7th d after IMQ painting; (B): PASI scores in all groups of mice were evaluated daily and the statistical difference
between all groups on 8th d was indicated; (C): The body weight of mice was taken daily after IMQ painting and (D): H&E staining
of the dorsal skin with the original magnification of 100X
Note: Data are expressed as mean±SD (n=3 mice/group,*p<0.05 vs. model), ( ): Control; (
): Control; ( ): Model; (
): Model; ( ): Low dose;
(
): Low dose;
( ): Medium dose; (
): Medium dose; ( ): High dose and (
): High dose and ( ): MTX
): MTX
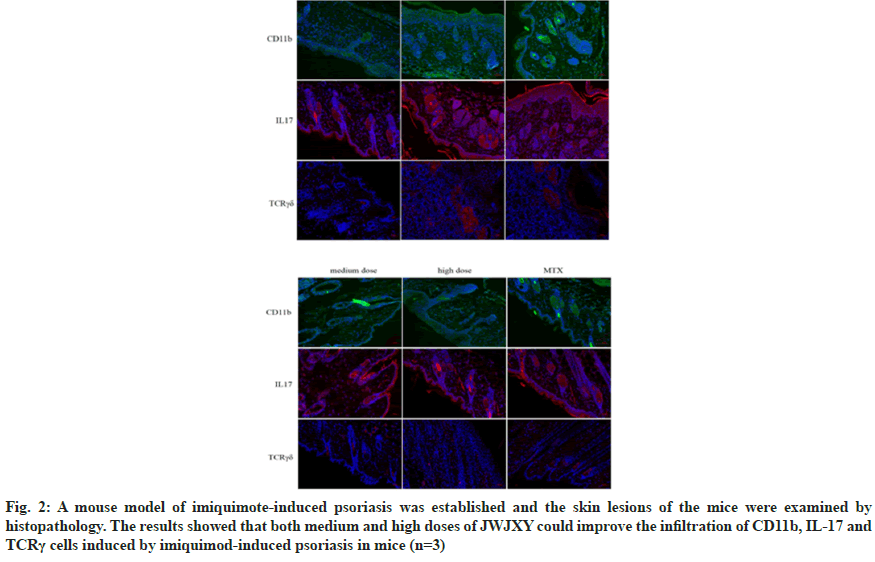
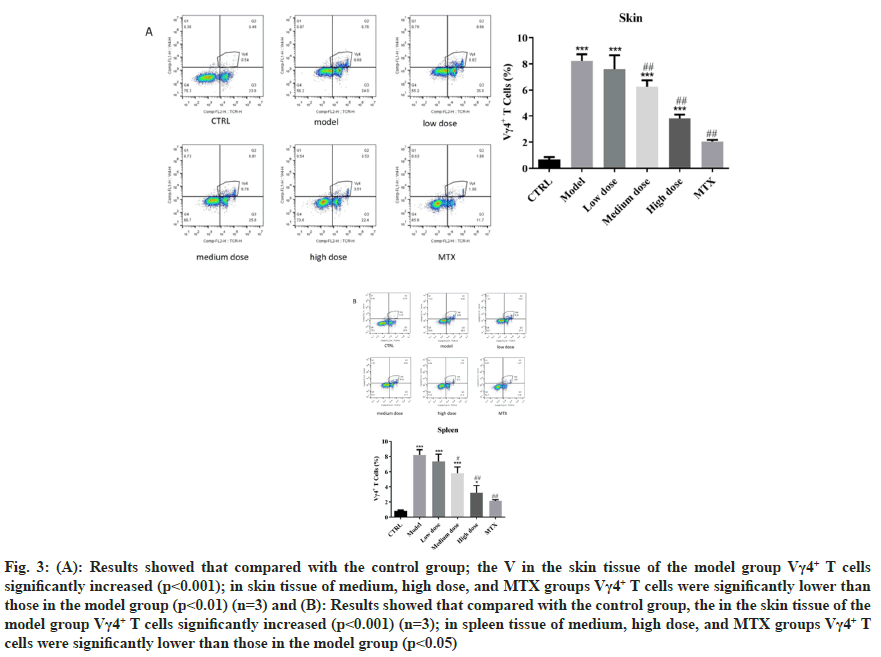
TCR is a heterodimer composed of two different peptide chains. According to the different peptide chains, TCR can be divided into αβTCR and γδTCR. The TCR of γδ T cells consists of a γ chain and a δ chain and γδ TCR has an innate ability to respond to inflammatory signals. The key role of γδTCR as the main source of cutaneous IL-17 in the pathogenesis of psoriasis γδT (γδT17) cells producing IL-17 and CD11b can be detected in the skin lesions of patients[7,8]. Previous studies have shown that JWJXY can regulate Th cell sub-populations and improve IMQ-induced CD11b cell infiltration in psoriatic skin, but whether JWJXY is involved in the regulation of IL-17 producing γδTCR cells is unclear[5]. We used immunofluorescence staining to observe the accumulation of skin inflammatory cells and γδT17 infiltration induced by IMQ in mice. By establishing a mouse model of imiquimote-induced psoriasis, the histopathological examination of the skin lesions was performed. γδT cells can be observed in inflammatory infiltration at the lesion, indicating their presence at the site of injury. The results showed that both medium and high doses of JWJXY could improve the infiltration of CD11b, IL17 and γδTCR in mice psoriasis induced by IMQ (n=3) as shown in fig. 2. Th17, a subtype of Clusters of Differentiation (CD) 4+T cells producing IL-17, was reported to play an important role in psoriasis. However recent studies revealed that other innate immune cells, such as IL-17 producing γδT cells, may be the primary cells that produce IL-17[9]. To clarify whether JWJXY can modulate γδT cells in psoriasis, flow cytometry was used to examine the expression of γδT (Vγ4+ T) in the spleen and skin of psoriatic mice. The ratio of γδT cells was notably lower in the medium dose group, high dose group and MTX group compared to the control group in skin (fig. 3A) and spleen (fig. 3B). The results showed that JWJXY significantly inhibited the expression of Vγ4+ T.
Fig. 2: A mouse model of imiquimote-induced psoriasis was established and the skin lesions of the mice were examined by histopathology. The results showed that both medium and high doses of JWJXY could improve the infiltration of CD11b, IL-17 and TCRγ cells induced by imiquimod-induced psoriasis in mice (n=3)
Fig. 3: (A): Results showed that compared with the control group; the V in the skin tissue of the model group Vγ4+ T cells significantly increased (p<0.001); in skin tissue of medium, high dose, and MTX groups Vγ4+ T cells were significantly lower than those in the model group (p<0.01) (n=3) and (B): Results showed that compared with the control group, the in the skin tissue of the model group Vγ4+ T cells significantly increased (p<0.001) (n=3); in spleen tissue of medium, high dose, and MTX groups Vγ4+ T cells were significantly lower than those in the model group (p<0.05)
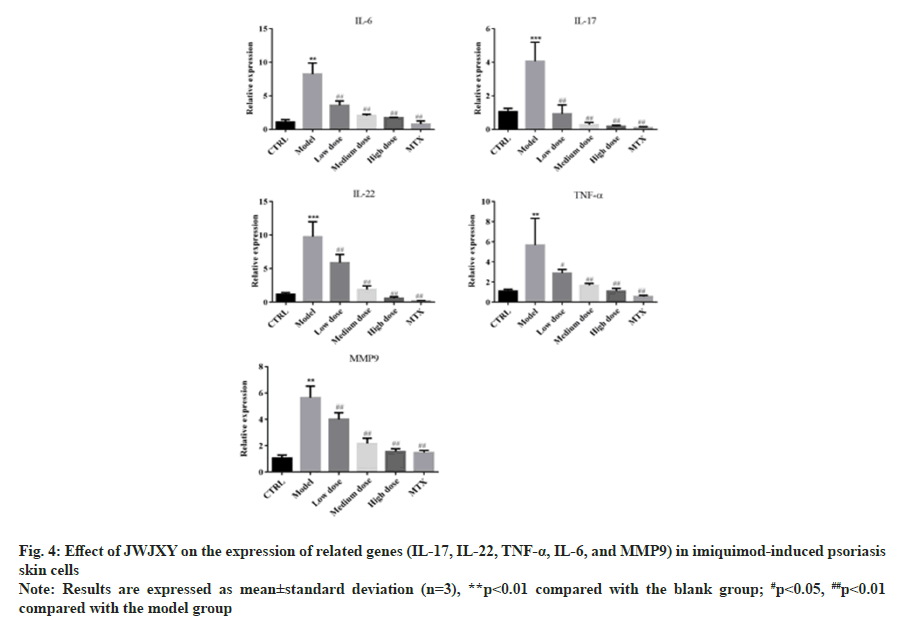
Research shows that γδ T cells expressing the Vγ4 TCR chain are amongst the highest contributors of IL-17A, which is a major cytokine that drives a psoriasis flare[10]. γδT17 cells can co-produce IL- 22 in a variety of environments[11]. TNF-α, IL-6, and MMP-9 have been shown to play important roles in the pathogenesis of psoriasis[12-14]. We used qRT-PCR to determine whether JWJXY affected the expression of inflammatory genes in skin cells of IMQ-induced psoriasis mice. The results showed that the transcription of IL-17, IL-22, TNF-α, IL-6 and MMP-9 was significantly reduced in the JWJXY group (fig. 4).
The involvement of JAK-STAT signaling pathway has been shown to play a key role in the pathogenesis of psoriasis. Genetic studies and TYK2 deficient mouse models have indicated JAK/STAT signaling pathway to participate in the pathogenesis of psoriasis via activation of the IL-23/IL-17 axis and induction of Keratinocytes (Kcs) and γδT cells proliferation[15]. We used Western blot to determine whether JWJXY affects the activation of the STAT3/JAK3 signaling pathway in skin cells of IMQ-induced psoriasis mice (fig. 5). The results showed that there was no significant change in the protein expression levels of STAT3, p-STAT3, and JAK3 in the JWJXY group.
Fig. 5: Western blot to determine whether JWJXY affects the activation of the STAT3/JAK3 signaling pathway in skin cells of
imiquimod-induced psoriasis mice
Note: Results are expressed as mean±standard deviation (n=3) and there is no significant change in the protein expression levels of
STAT3, p-STAT3, and JAK3 in the JWJXY group
JWJXY is a pure Chinese medicine compound that has been used in clinics for many years. This recipe originated from the famous old TCM doctor’s experience formula “Jing Xie Yin”. Previous studies have shown that JWJXY has a good clinical effect on psoriasis vulgaris with advanced blood-heat syndrome, and can inhibit IMQinduced psoriasis in mice by down-regulating Th17 response[5]. The composition of JWJXY includes buffalo horn, Rehmanniae radix, Lithospermum, Scutellariae radix, cortex dictamni, Spatholobi caulis, Oldenlandia diffusa, radix rubiae, radix clematidis, rhizoma atractylodes, Sophora flavescens, radix rhapontici and tripterygium. Accumulated evidence indicates that these Chinese herbs possess a broad range of pharmacological properties, including anti-inflammatory[16], anti-allergic[17], anticoagulation[18], antioxidant activity[19], antiangiogenesis[20]. These herbs play important role in inhibiting Th17 response and JAK2/STAT3 pathway[21].
Psoriasis is an autoimmune disease that results in a red, scaly rash. The IL-23/IL-17 axis plays a crucial role in psoriasis and a variety of cytokines and enzymes, such as IL-17A, IL-22, IL-6, TNF-α, MMP-9, etc, are involved in the inflammatory response and abnormal epidermal proliferation of psoriasis[22]. Researches show that γδ T cells expressing the Vγ4 TCR chain are amongst the highest contributors of IL-17A, which is a major cytokine that drives a psoriasis flare, making Vγ4+ γδT cells a suitable target to restrict psoriasis progression[10]. The involvement of JAK-STAT signaling pathway has been shown to play a key role in the pathogenesis of psoriasis. Genetic studies and TYK2 deficient mouse models have indicated JAK/STAT signaling pathway to participate in the pathogenesis of psoriasis via activation of the IL- 23/IL-17 axis and induction of Kcs and γδT cells proliferation[15]. Dermal γδT-cells are characterized by intermediate TCRγδ expression and surface CCR6 expression, they are recruited to the skin in response to CCL20 produced during skin inflammation[23]. In this study, we demonstrated that JWJXY alleviated IMQ induced psoriasiform dermatitis in mice. Feeding with JWJXY greatly reduced thickening scores. These mice treated with JWJXY showed lower PASI scores. The inflammatory cell infiltration and pathological injury degree were significantly reduced in MTX group and JWJXY medium and high dose groups. Meanwhile, immunofluorescence results showed that JWJXY reduced inflammatory cell and γδT17 infiltration in skin tissue.
Further research showed that JWJXY decreased the proportion of Vγ4+ T cells in spleen and skin tissues, especially in medium and high dose groups. And JWJXY inhibited the expression of γδT cell-related gene in skin tissues in low, medium and high dose groups, including IL-17, IL-22, IL-6, TNF-α and MMP-9. Nevertheless, the expression of JAK/STAT3 signaling pathway was not significantly changed in MTX and JWJXY groups. In conclusion, our work demonstrates that Chinese herbal medicine JWJXY alleviates IMQ-induced inflammation in psoriasis. We have found a good regulatory effect of JWJXY on γδT cells. Our results presented a novel mechanism of Chinese herbal medicine in psoriasis and provided a potentially effective approach for psoriasis therapy.
Funding:
This research was funded by a grant from the Applied Basic Research Program of Yunnan Province (No.202101AZ070001-323). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical statement:
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiment was approved by Medical Ethics Committee of Yunnan Provincial Hospital of TCM (No. S2020-010), in compliance with institutional guidelines for the care and use of animals.
Conflict of interests:
The authors declared no conflict of interests.
References
- AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis–comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol 2020;59(5):566-71.
[Crossref] [Google Scholar] [PubMed]
- Ying L, Suyun J, Yanhua L, Yunsheng L, Li D, Lin D, et al. Safety and Efficacy of Ixekizumab in Chinese adults with moderate-to-severe Plaque psoriasis: A prospective, multicenter, observational study. Ad Ther 2023;40(12):5464-74.
[Crossref] [Google Scholar] [PubMed]
- Weigle N, McBane S. Psoriasis. Lancet 2013;87(9):626.
- Yamazaki F. Psoriasis: Comorbidities. J Dermatol 2021;48(6):732-40.
- Yang D, Guo Y, Wu J, Qin J, Wu J, Lu Y, et al. Chinese herbal medicine Jia Wei Jing Xie Yin (JWJXY) ameliorates psoriasis via suppressing the Th17 cell response. Ann Transl Med 2022;10(6):627139.
[Crossref] [Google Scholar] [PubMed]
- Qi C, Wang Y, Li P, Zhao J. Gamma delta T cells and their pathogenic role in psoriasis. Front Immunol 2021;12:627139.
- Wang S, Kozai M, Mita H, Cai Z, Masum MA, Ichii O, et al. REV-ERB agonist suppresses IL-17 production in γδT cells and improves psoriatic dermatitis in a mouse model. Biomed Pharmacother 2021;144:112283.
[Crossref] [Google Scholar] [PubMed]
- Zhou Y, Xu F, Chen XY, Yan BX, Wang ZY, Chen SQ, et al. The epidermal immune microenvironment plays a dominant role in psoriasis development, as revealed by mass cytometry. Cell Mol Immunol 2022;19(12):1400-13.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Zhang J, Yu J, Chen X, Zhang F, Wei W, et al. Hyperforin ameliorates imiquimod-induced psoriasis-like murine skin inflammation by modulating IL-17A–producing γδ T cells. Front Immunol 2021;12:635076.
[Crossref] [Google Scholar] [PubMed]
- Dhillon-LaBrooy A, Braband KL, Tantawy E, Rampoldi F, Kao YS, Boukhallouk F, et al. Inhibition of mitochondrial translation ameliorates imiquimod-induced psoriasis-like skin inflammation by targeting Vγ4+ γδ T cells. J Investig Dermatol 2023;S0022-202X(23)02924-X.
[Crossref] [Google Scholar] [PubMed]
- Yang T, Barros-Martins J, Wang Z, Wencker M, Zhang J, Smout J, et al. RORγt+ c-Maf+ Vγ4+ γδ T cells are generated in the adult thymus but do not reach the periphery. Cell Rep 2023;42(10):113230.
[Crossref] [Google Scholar] [PubMed]
- Springall R, Ortega-Springall MF, Guerrero-Ponce AE, Vega-Memije ME, Amezcua-Guerra LM. Interleukin-17 and tumor necrosis factor show a functional hierarchy to regulate the production of matrix metalloproteases by monocytes from patients with psoriasis. J Interferon Cytokine Res 2023;43(3):140-6.
[Crossref] [Google Scholar] [PubMed]
- Rohrbeck A, Bruhn VA, Hussein N, Hagemann S, Just I. Clostridium botulinum C3bot mediated effects on cytokine-induced psoriasis-like phenotype in full-thickness skin model. Naunyn Schmiedebergs Arch Pharmacol 2023:1-6.
[Crossref] [Google Scholar] [PubMed]
- Xing M, Yan X, Guo J, Li W, Li Z, Dong C, et al. Banzhilian formula alleviates psoriasis-like lesions via the LCN2/MMP-9 axis based on transcriptome analysis. Front Pharmacol 2023;14:1055363.
[Crossref] [Google Scholar] [PubMed]
- Szilveszter KP, Németh T, Mócsai A. Tyrosine kinases in autoimmune and inflammatory skin diseases. Front Immunol 2019;10:1862.
[Crossref] [Google Scholar] [PubMed]
- Liu FY, Wang MQ, Liu MM, Li T, Wang XH, Jiang F, et al. Therapeutic effects of shikonin on adjuvant-induced arthritis in rats and cellular inflammation, migration and invasion of rheumatoid fibroblast-like synoviocytes via blocking the activation of Wnt/β-catenin pathway. Phytomedicine 2023;116:154857.
[Crossref] [Google Scholar] [PubMed]
- Wang YS, Cho JG, Hwang ES, Yang JE, Gao W, Fang MZ, et al. Enhancement of protective effects of radix scutellariae on UVB-induced photo damage in human HaCaT keratinocytes. Appl Biochem Biotechnol 2018;184(4):1073-93.
[Crossref] [Google Scholar] [PubMed]
- Sun L, Li Q, Guo Y, Yang Q, Yin J, Ran Q, et al. Extract of Caulis spatholobi, a novel platelet inhibitor, efficiently suppresses metastasis of colorectal cancer by targeting tumor cell-induced platelet aggregation. Biomed Pharmacother 2020;123:109718.
[Crossref] [Google Scholar] [PubMed]
- Yadav S, Sharma A, Nayik GA, Cooper R, Bhardwaj G, Sohal HS, et al. Review of shikonin and derivatives: Isolation, chemistry, biosynthesis, pharmacology and toxicology. Front Pharmacol 2022;13:905755.
[Crossref] [Google Scholar] [PubMed]
- Zhou K, Chang Y, Han B, Li R, Wei Y. MicroRNAs as crucial mediators in the pharmacological activities of triptolide. Exp Ther Med 2021;21(5):499.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Lin M, Li Y, Shao J, Huang R, Qiu Y, et al. Total flavonoids of Sophora flavescens and kurarinone ameliorated ulcerative colitis by regulating Th17/Treg cell homeostasis. J Ethnopharmacol 2022;297:115500.
[Crossref] [Google Scholar] [PubMed]
- Liu T, Li S, Ying S, Tang S, Ding Y, Li Y, et al. The IL-23/IL-17 pathway in inflammatory skin diseases: From bench to bedside. Front Immunol 2020;11:594735.
[Crossref] [Google Scholar] [PubMed]
- Shin JW, Kwon MA, Hwang J, Lee SJ, Lee JH, Kim HJ, et al. Keratinocyte transglutaminase 2 promotes CCR6+ γδT-cell recruitment by upregulating CCL20 in psoriatic inflammation. Cell Death Dis 2020;11(4):301.
[Crossref] [Google Scholar] [PubMed]