- Corresponding Author:
- J. C. Rivera−leyva Departamento de Farmacia, División de Ciencias Naturales y Exactas, Universidad de Guanajuato, Guanajuato (Gto), CP 36050, México
E-mail: juliorivera@hotmail.com
| Date of Submission | 09 October 2011 |
| Date of Revision | 08 August 2012 |
| Date of Acceptance | 16 August 2012 |
| Indian J Pharm Sci, 2012, 74 (4): 312-318 |
Abstract
In vitro dissolution studies for solid oral dosage forms have recently widened the scope to a variety of special dosage forms such as suspensions. For class II drugs, like Ibuprofen, it is very important to have discriminative methods for different formulations in physiological conditions of the gastrointestinal tract, which will identify different problems that compromise the drug bioavailability. In the present work, two agitation speeds have been performed in order to study ibuprofen suspension dissolution. The suspensions have been characterised relatively to particle size, density and solubility. The dissolution study was conducted using the following media: buffer pH 7.2, pH 6.8, 4.5 and 0.1 M HCl. For quantitative analysis, the UV/Vis spectrophotometry was used because this methodology had been adequately validated. The results show that 50 rpm was the adequate condition to discriminate the dissolution profile. The suspension kinetic release was found to be dependent on pH and was different compared to tablet release profile at the same experimental conditions. The ibuprofen release at pH 1.0 was the slowest.
Keywords
Dissolution, ibuprofen, particle size, suspension, ultraviolet spectrophotometer, validation
Ibuprofen (IBP) is a nonsteroidal antiinflammatory drug (NSAID) derived from propionic acid and used widely as an analgesic and antipyretic; although it is also used for relief from symptoms of rheumatoid arthritis and osteoarthritis in addition to treatment of dysmenorrhoea, amongst other indications. It has a pKa value of 4.5 [1,2] and is poorly soluble in water (0.078 μg/ml). According to the Biopharmaceutical Classification System (BCS), IBP had been classified as a class II drug (low solubility and high permeability); therefore, drug dissolution may be a rate limiting step in the drug absorption process [3]. For in vitro dissolution of poorly soluble drugs, it is difficult to find adequate hydrodynamic conditions as agitation rate, medium composition and suitable volume, as well as a good discriminating power. In these conditions adequate dissolution cannot be achieved with aqueous solutions within physiologic pH ranges (1.2−6.8).
The dissolution test for immediate or controlled release in solid oral dosage forms has recently widened to a variety of novel or special dosage forms such as suspensions, chewing gums, transdermal patches, implants and others [4,5]. Because of the different characteristics of early dosage form, the site absorption and dosing routes and applications, it is essential to give appropriate consideration to the following factors in the development of the test method: Apparatus selection, dissolution medium, agitation and temperature.
The dissolution studies are used to simulate in vitro behaviour of the pharmaceutical dosage form therefore, the method validation is required to asses reproducibility test. These characteristics will help to predict the in vitro performance [6]. To date, there are very few reports about dissolution test for suspensions, showing major information related to pharmaceutical dosage form is needed [5,7]. The suspensions are disperse systems, and although there are numerous factors that influence the drug dissolution rate, including the physicochemical properties (particle size) and formulation characteristics. They are preferred by paediatric and geriatric population because of their ease of administration and acceptability [8]. In Mexico there are various marketed ibuprofen products, and whose consumption is not regulated. Some of these products are generic and have not demonstrated their efficacy and safety [9,10].
The aim of this study was to verify the discriminative power of a dissolution method for IBP suspension with BCS criteria [11,12] using UV/Vis spectrophotometry for quantitative drug analysis, and comparing the release behaviour with commercial tablet forms.
Materials and Methods
Advil IBP suspension, 100 mg/5 ml, Lot 6N529A, from Wyeth Co., MA, USA and Ibuflam IBP tablets, 400 mg, Lot 71710 from 3M Pharmaceutical Inc., México City, México were purchased from a Mexican drugstore.
The reagents were obtained from different local distributors: Monobasic potassium phosphate, monobasic sodium phosphate monohydrated, monobasic sodium phosphate, glacial acetic acid and methanol HPLC were purchased from J. T. Baker, PA, USA; sodium hydroxide and hydrochloric acid (HCl) were purchased from Merck, MA, USA.
Viscosity measurement
The dynamic viscosity of the suspensions was measured using a Brookfield dial reading viscometer and spindles model LV with at least three replicates. The results were expressed in centipoises (cPs).
Particle size distribution
An aliquot of pharmaceutical suspension was added to deionised water adjusted to a pH of 4 with HCl. The sample was sonicated for 1 h. Particle size was determined by a Horiba LA−300 laser−scattering particle size analyser [13].
Ibuprofen assay
IBP present in the different dissolution media tested was measured in an UV/Vis spectrophotometer Shimadzu UV 2401 PC, (Shimadzu Company, Japan) at 221 nm after appropriate dilution and treatment of samples. A standard curve of IBP was prepared in the range from 5 to 30 μg/ml, after making up the volume with the dissolution medium tested. The methodology was validated previously to quantification as per International Conference of Harmonization, ICH and United States Pharmacopoeia, USP. A blank formulation was included by preparing a solution of sucrose (1%), glycerine (1%) and sorbitol (1%) in water to evaluate interferences.
Validation
The IBP analysis through UV spectrophotometry for the dissolution test was validated including the following parameters: Specificity, linearity, accuracy and reproducible results, quantification and detection limits, as well as possible drug adsorption on the filters [12,14,15].
Linearity
Linearity was evaluated by analysing six different concentrations of IBP in the range 5−30 μg/ml. A calibration curve was prepared in triplicate by each dissolution medium for interday evaluation. The correlation coefficient was obtained.
Precision
Precision was determined as repeatability. From the linearity data, the response factor (absorbance/concentration) and the relative standard deviation (RSD) were determined.
Accuracy
The accuracy was assessed by the concentration recovered from each point on the linearity curve. The method is considered accurate if the percentage of recovery compared to the nominal concentration was between 97.0 and 103.0%.
Specificity
Specificity in every medium was evaluated using the standard addition assay as follows: A homogenised solution of the commercial suspension (100 μg/ml) and tablets (50 μg/ml) were prepared separately in 900 ml of each media. A quantitation curve was prepared transferring an aliquot of the homogenised suspension and tablets (containing 1 μg/ml) to each 10 ml flask, adding respective standard solution to each point and the remaining volume was completed with the dissolution medium. The resulting curves were compared with a quantitation curve prepared only in dissolution medium.
Limit of detection and limit of quantification
Limit of detection (LOD) and limit of quantification (LOQ) were estimated based on the analytical curve parameters [15]. They were calculated for all dissolution media investigated in this work.
Filter adsorption
For filter adsorption, a standard IBP solution (5, 15 and 30 μg/ml) was prepared in every dissolution medium tested. Three samples of 5 ml each from the solutions were filtered using polyethylene filters (1.0 μm) and acrodisc syringe filters with nylon membrane (0.2 μm). Both filtered and nonfiltered samples were analysed at the UV/Vis spectrophotometer in order to evaluate the possible waste of the drug adsorbed on the filters.
Solubility test in dissolution medium
100 mg of IBP were introduced in the vessels with 900 ml of all media tested. The liquid mixtures were then stirred in dissolution system with 25 rpm for 48 h in a water bath kept at 37.0±0.05° to reach the equilibrium (this equilibrium time was established by quantifying the drug concentration up to obtain a constant value). After this time, the supernatant solutions were filtered through a 0.2 μm membrane. Concentrations were determined by measuring absorbance after appropriate dilution and interpolated from respective calibration curves. All solubility analyses were repeated at least 3 times and the results were averaged.
Dissolution study
The dissolution media used were: Phosphate buffer, pH 7.2, which were prepared according to USP 30 [10]; phosphate buffer pH 6.8, phosphate buffer pH 4.5 and HCl 0.1 M.
The amount of suspension (containing about 100 mg/5 ml mg of IBP) introduced into the vessels was assessed by weighing a syringe before and after the sample introduction and based on the density, which was previously determined. The dissolution study was conducted using 900 ml of different media maintained at 37°, using the paddle apparatus and stirring rate of 25 and 50 rpm (VK7010 Vankel dissolution apparatus). Samples were taken at 5, 10, 20, 30, 45, 60, 90, 120, 150 and 180 min at 25 rpm, with variations according to the behaviour of IBP in every dissolution media; however, when employing 50 rpm, probing in collect was interrupted after 180 min.
The samples for dissolution procedure were filtered by 1.0 μm polyethylene filters, assembled into sampling tubes. For analytical determinations, samples were diluted 1:20 in the dissolution medium. The drug absorption intensity was measured in a UV/Vis spectrophotometer at the maximum wavelength of 221 nm.
For tablets, dissolution tests were performed under conditions similar to suspension formulation at 50 rpm, including the pharmacopoeial conditions.
Results and Discussion
Table 1 shows the characterisation of evaluated IBP suspension, the dose of which was 100 mg/5 ml. The content assay [10] was found to be 108.31% and the kinematic viscosity was found as 136.5 mPa s (cPs), which correlate with other similar suspensions [16]. The average particle size was found as 39±11 μm with distribution between 1 and 100 μm, common size to elaborate pharmaceutical suspension. The density obtained was 1.24 g/ml.
The apparent solubility of IBP in each dissolution media are shown in Table 2. It can be seen that the solubility directly decreases with pH, since the maximum value was reached at pH 7.2 (5.86 μg/ml) and the lowest value was at 0.1 M HCl (2.18 μg/ml). The molecular state at pH 1.0 causes a decrease in drug solubility, whereas the ionisation of the drug increases its solubility at pH above 4.5.
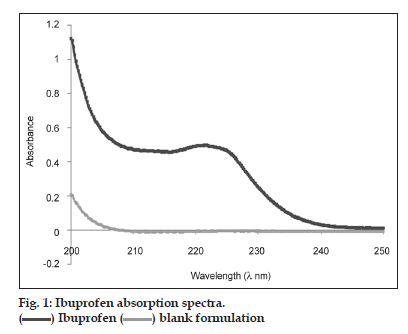
The analytical methods were validated for each dissolution media: Buffer pH 7.2, 6.8, 4.5 and 0.1 N HCl media. The last three corresponding to the BCS [11]. The maximum absorption wavelength of IBP was experimentally obtained between 220 and 223 nm (fig. 1). There are no differences for excipients between 221 and 230 nm. So, in this analytic method, 221 nm was used for all determinations.
The linear interval used was 5−30 μg/ml with six different concentrations; the correlation and determination coefficients were higher than 0.999 in all evaluated media (Table 3).
The intraday precision in each evaluated media was estimated as RSD of response factor, which corresponding directly to slope of quantitation curve was lesser than 1.56% to each evaluated media, as it is showed in Table 3. The interday precision showed an RSD lesser than 2.0%.
The accuracy in all media showed a recovery percent between 99 and 101%, and an absolute deviation lesser than 1%. The limits of detection (LOD) were between 0.1088 and 0.3385 μg/ml whereas limits of quantitation (LOQ) were between 0.3297 and 1.0258 μg/ml.
The filters used in dissolution samples did not retain a significant amount of drug, the differences between unfiltered and filtered solutions with polyethylene filters (1.0 μm) were not higher than 1.5% and the evaluation with acrodisc syringe filters with nylon membrane was lesser than 2.0%. It is recommended that the maximum loss of solute through adsorption on the filters is 2% according to Fortunato [17] and 5% according to Lindenberg et al. [18] Curves prepared with suspension as well as tablet homogenised by triplicate, were compared with curves prepared with drug solutions and no differences were observed between their respective slopes (Table 4, P>0.05).
The USP recommends evaluation of the suspension formulations using the apparatus II, 25 rpm if the viscosity is low or 50 rpm if the viscosity is relatively high, but does not mark a reference value for the viscosity [10].
In related articles [19], the suspension viscosity had a value close to 200 mPa.s when 50 rpm were used. In our study, in order to discern the better conditions to evaluate dissolution profiles and to establish if there are differences between kinetics release in all evaluated media, dissolution tests were made at 25 and 50 rpm to IBP suspension.
As it is observed in fig. 2, the IBP dissolved amount in pH 7.2 buffer was 106% in 60 min, in pH 6.8 and 4.5 media, the maximum concentrations were 66 and 68%, respectively, whereas at pH 1.0, the dissolved concentration was close to 10% and the higher percent dissolved at 180 min was 20%.
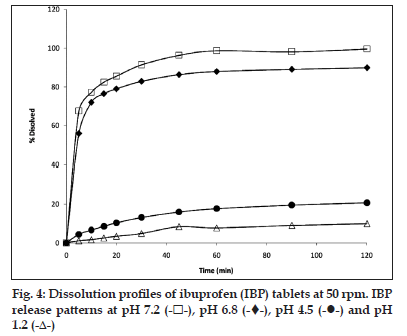
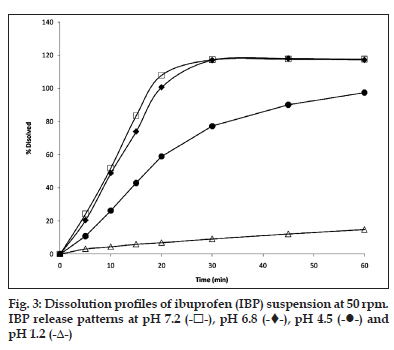
In the dissolution test at 50 rpm (fig. 3), it was observed that the higher amount of drug is released at pH 7.2 (more than 100%) during 30 min and the same behaviour was found at pH 6.8. The release at pH 4.5 is slow and incomplete in 60 min (90% approximately). The profile at pH 1.0 showed a very slow release (lesser than 10%) in 60 min and for 3 h does not show a significant increase (data not shown).
As per BCS, a drug is highly soluble if it has rapid dissolution (over 80% in less than 30 min) along the intestinal tract, so it is recommended to evaluate the dissolution of solid dosage forms in three different media, buffer pH 6.8, 4.5 and 1.2.
The IBP is a weak acid that dissolves quickly at pH 6.8, due to its pKa, its dissolution is expected to be slow at pH 4.5 and 1.0, as reflected in the dissolution profiles of the suspension, which consists of finely dispersed particles. The dissolution of these particles can be affected by the intrinsic properties of the drug, its size and components of formulation, especially the thickener agents. The results of the suspension profiles at 25 rpm and 50 rpm show that both the conditions adequately discriminated the kinetics of dissolution in all the media tested.
The evaluation of dissolution profiles of commercial tablets of IBP were made in the same dissolution media used for suspension at 50 rpm. The results are shown in fig. 4. Both, tablets and suspension have a similar behaviour at pH 6.8 and 7.2 by dissolving 80−100% of the drug in 60 min, respectively, whereas at pH 1.2, the maximum drug dissolved reaches only about 10%. The dissolution profiles of both dosage forms show similar behaviour, but the suspension seems to dissolve to a greater degree than the tablets in all the media tested. The biggest difference was found in the profiles corresponding to pH 4.5, where the suspension has a higher release reaching 90% released in 50 min, whereas the tablets reach only about 20% at the same time.
Food and Drug Administration (FDA) studies show the results of dissolution of ibuprofen suspension and paediatric drops, indicating that the condition for the dissolution of the suspension is 25 rpm and reaches 100% dissolved after 10 min [20]. In our work, we found that the evaluated suspension reaches 100% dissolved after 60 min at 25 rpm and after 20 min with 50 rpm. In the experiments carried out in various dissolution media, the sample placed in the bottom of the vessel formed a clump in acidic media. It was considered that this behaviour may be due to the presence of excipients with low compatibility in dissolution media.
Table 5 shows an adjustment of the kinetic release of both the products tested. The profiles of the suspension have first−order kinetics in the media of pH 7.2, 6.8 and 4.5, whereas the tablets show the same type only at pH 7.2 and 6.8. The profiles of pH 1.2 for both formulations have zero−order kinetics, since there is a slow and limited release. At pH 4.5, the adjustment is different for both formulations, first−order kinetic for the suspension and zero−order for the tablets. The difference could be due to effects of the suspension components, which in this case favour the release of the IBP [17].
Suspensions have many benefits, as seen in the case if IBP, which is widely used as antipyretic in children, it is accessible for the elderly who cannot swallow easily, as well as for administration to an unconscious person. The BCS suggests simulating the in vivo dissolution for dosage forms along the GIT. Currently, these recommendations are directed especially to solid dosage forms for immediate release, and can be used for oral delivery forms other than solid, of which is unknown their dissolution kinetics in the GIT; therefore, it is relevant to develop release studies under these conditions extensively in order to ensure proper drug dissolution.
Our study shows that the suspension dissolves more quickly than tablets in almost all conditions tested, including those of the pharmacopoeial test, which correlates well with the size of particles in suspension. However, the effect of the components of the formulation can vary the rate of dissolution, as observed in the profile at pH 4.5. The release of IBP from suspension and tablets was found to be similar in all media tested, but there are relevant differences between both formulations caused by the excipients, the process or characteristics of the active agent, that in principle, they can affect greatly the drug release and their bioavailability.
The dissolution profile of commercial presentations of IBP can be discriminated properly in the conditions recommended by USP and the BCS. The suspension of IBP has a relatively faster dissolution than the IBP tablets apparently due to the components of the formulation and processing technology. It is considered appropriate to follow the recommendations of the BCS to evaluate the dissolution of any oral dosage form, in order to ensure adequate bioavailability.
References
- Avdeef A, Box KJ, Comer JE, Gilges M, Hadley M, Hibbert C, et al. PH-metric log P 11. pKa determination of water-insoluble drugs in organic solvent–water mixtures. J Pharm Biomed Anal 1999;20:631-41.
- Herzfeldt CD, Kümmel R. Dissociation constants, solubilities and dissolution rates of some selected nonsteroidal antiinflammatories. Drug evInd Pharm 1983;9:767-93.
- Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitrodrug product dissolution and in vitro bioavailability. Pharm Res 1995;12:413-20.
- da Fonseca LB, Labastie M, de Sousa VP, Volpato NM. Development and validation of a discriminative dissolution test for nimesulide suspensions. AAPS PharmSciTech 2009;10:1145-52.
- Siewert M, Dressman J, Brown CK, Shah VP, Aiache J, Aoyagi N, et al. FIP/AAPS guidelines to dissolution/ in vitro release testing ofnovel/special dosage forms. AAPS PharmSciTech 2003;4:E7.
- Qureshi SA. Developing discriminatory drug dissolution tests and profiles: Some thoughts for consideration on the concept and its interpretation. Dissolution Technol 2006;13:18-23.
- Azam G, Haider SS. Evaluation of dissolution behavior of paracetamol suspensions. Dhaka Univ J Pharm Sci 2008;7:53-8.
- Gonzalez-Vidal NL, Zubata PD, Simionato LD, Pizzorno MT. Dissolution stability study of cefadroxil extemporaneous suspensions. Dissolution Technol 2008;15:29-36.
- Secretaría de Salud. <0291>Dissolution Profiles. Farmacopea de losEstadosUnidosMexicanos, 8ª. Edición. México: Comisión Permanente de la Farmacopea de los EstadosUnidosMexicanos; 2004. p. 2016-22.
- United States Pharmacopoeia, Ibuprofen oral suspension. United States Pharmacopoeia/National Formulary, 30th/25th ed. Rockville, MD: Pharmacopeial Convention; 2007. p. 2326.
- Food and Drug Administration. Guidance for Industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate-releasesolid oral dosage forms based on a biopharmaceutics classification system U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER), 2000. Available from: http://www.fda.gov/downloads/Drugs/.../ Guidances/ucm070246.pdf [Last accessed 2012 Mar 21].
- Secretaría de Salud. Proyecto de Norma NOM-177-SSA1-2009, queestablecelaspruebas y procedimientos para demostrarqueunmedicamentoesintercambiable. Requisitos a quedebensujetarse los tercerosautorizadosquerealicenlaspruebas. Secretaría de Salud. México. 2008: 1-52. Available from: http://www.salud.gob.mx/unidades/cofepris/cis/ tramites/regmed/pdf/NOM_177.pdf [Last accessed 2012 Mar 22].
- Vlasova M, Patiño GD, Kakazey N, Patiño MD, Romero DJ, Méndez YE. Structural-phase transformations in bentonite after acid treatment. Sci Sinter 2003;35:155-66.
- ICH. Q2A, Harmonized tripartite guideline, test on validation of analytical procedures, IFPMA. In: Proceedings of the International Conference on Harmonization Geneva, 1994. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073381.pdf [Last accessed 2012 Mar 22].
- ICH. Q2B, Harmonized tripartite guideline, validation of analytical procedure: Methodology, IFPMA. In: Proceedings of the International Conference on Harmonization, Geneva, 1996. Available from: http:// mx.search.yahoo.com/r/_ylt=A0oGkmfZXJBQTn8AMopzKRh.;_ylu=X 3oDMTByZWgwN285BHNlYwNzcgRwb3MDMQRjb2×vA3NrMQR2d GlkAw--/SIG=14709dbmd/EXP=1351667033/**http%3a//www.ikev.org/ haber/stabilite/kitap/36%25201.8%2520%2520Stability%2520Workshop %2520ICH%2520Q2B%2520C%2520.pdf [Last accessed 2012 Mar 23].
- Nep EI, Conway BR. Evaluation of grewia polysaccharide gum as a suspending agent. Int J Pharm PharmSci 2011;3:168-73.
- Fortunato D. Dissolution method development for immediate release solid oral dosage forms. Dissolution Technol 2005;12:12-5.
- Lindenberg M, Wiegand C, Dressman JB. Comparison of the adsorption of several drugs to typical filter materials. Dissolution Technol 2005;12:22-5.
- Devrim B, Bozkir A, Canefe K. Formulation and evaluation of reconstitutable suspensions containing ibuprofen-loaded Eudragit microspheres. Acta Pol Pharm 2011;68:593-9.
- Food and Drug Administration, Center for Drug Evaluation. Application number 20812. Clinical pharmacology/biopharmaceutical review, USA, 1997. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/ nda/98/020812_Pediactric_Advil_Clin_Pharm_biopharm.pdf [Last accessed 2012 July 25].
 Ibuprofen
Ibuprofen blank formulation
blank formulation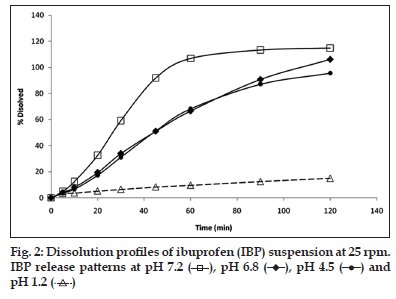
 , pH 6.8
, pH 6.8  , pH 4.5
, pH 4.5  and pH 1.2
and pH 1.2 
 ), pH 6.8 (
), pH 6.8 ( ), pH 4.5 (
), pH 4.5 ( ) and
) and