- Corresponding Author:
- Sumitra Chanda
DPhytochemical, Pharmacological and Microbiological Laboratory
Department of Biosciences (UGC−CAS)
Saurashtra University, Rajkot−360 005, India
E−mail: svchanda@gmail.com
| Date of Submission | 28 May 2013 |
| Date of Revision | 31 January 2014 |
| Date of Acceptance | 05 February 2014 |
| Indian J Pharm Sci 2014;76(2):148-156 |
Abstract
The antimicrobial effect of 24 different hydroalcoholic extracts (100, 75, 50 and 25% methanol and water) obtained from four parts (leaf+stem (aerial), peel, pulp and seed) of Momordica charantia L. were investigated against five Gram-positive, six Gram-negative and four fungal strains. The extraction was done by individual cold percolation method using hexane, different hydroalcoholic solvent (100, 75, 50 and 25% methanol) and water. The antimicrobial activity was done by agar well diffusion assay. The extracts, which showed >15 mm zone of inhibition, were further screened to determine minimum inhibitory concentration and minimum bactericidal concentration using a broth dilution method performed in 96-well microtitre plate. The extractive yield was highest in aqueous extracts of all the four parts closely followed by 25% methanol. Micrococcus flavus was the most susceptible Gram-positive bacteria and Pseudomonas testosteroni was the most susceptible Gram-negative bacteria. The highest antibacterial activity was shown by 100% methanol. The Gram-negative Pseudomonas spp. was more susceptible towards all the extracts than the Gram-positive bacteria or fungal strains investigated. One hundred percent and 50% methanol extracts of seed showed lowest minimum inhibitory concentration and minimum bactericidal concentration values, that is <39 and 625 μg/ml, respectively, against Pseudomonas pictorum. Therefore, these extracts would be of interest in the control of Pseudomonas spp. in food industry as well as used for therapeutic purposes.
Keywords
Momordica charantia, MIC, MBC, food spoilage, antipseudomonal activity, peel, hydroalcoholic extract
Foodborne illness resulting from consumption of food contaminated with pathogenic bacteria has been of vital concern to public health. Consumers today are increasingly concerned about chemical preservatives in food and tend to choose food products that are natural, safe and with multi−health benefits[1−3]. Foodborne illness is a major problem associated with enormous costs. Foodborne pathogens occur widely in nature and it is difficult to prevent them from entering raw foods. Salmonella sp., Listeria monocytogenes, Bacillus subtilis and Escherichia coli account for the largest number of outbreaks, cases and deaths, and are capable of attaching to inert surfaces and subsequently forming bio films on food processing equipment and environment[4,5]. Staphylococcus aureus causes a range of illnesses and was found to be the most resistant organism[6]. Salmonella mutants survive and are able to persist in the food chain[7]. Many Pseudomonas spp. can cause food spoilage. Novel antipseudomonal activity is of particular interest as it is the leading cause of nosocomial infections and has developed mechanisms of resistance to common classes of antibiotics[8,9]. The resistance of bacteria and other microorganisms to antimicrobial agents has become a wide−spread medical problem especially as nosocomial pathogens. To reduce health hazards and economic losses due to foodborne microorganisms, the use of natural products as antibacterial compounds is gaining importance. However, it is necessary to establish the scientific basis for the therapeutic actions of traditional plant medicines. Several plants have been reported to be used in treating and managing the complicated diseases.
The food antimicrobials are classified into natural and synthetic substances depending on their origin. Although, many synthetic antimicrobials are found naturally (benzoic acid in cranberries, sorbic acid in rowanberries, citric acid in lemons, malic acid in apples and tartaric acid in grapes), the perception of natural has become important for many consumers[10]. The problems mentioned introduced new research directions in the field of bioactive principles from natural sources and their application as food additives or dietary supplements.
Momordica charantia L. (Cucurbitaceae) commonly known as ‘bitter gourd’ and ‘bitter melon’, ‘karela’ is a multipurpose herb widely cultivated in many tropical and subtropical regions of the world. The fruits are used as medicinal vegetable in different parts of the world. Apart from their role in food consumption, a wide array of pharmacological activities such as antidiabetic[11], antioxidant[12], anticancer activities[13] and antiulcer[14] are reported for this plant.
Materials and Methods
Collection of the plant material
Different parts (aerial, peel, pulp and seed) of Momordica charantia L. were collected in September 2011 from Chotila, Surendranagar, Gujarat, India and identified by comparison with specimens (PSN333) available at the Herbarium of the Department of Biosciences, Saurashtra University, Rajkot, Gujarat, India. The parts were separated, washed thoroughly with tap water, shade dried, homogenised to fine powder and stored in airtight bottle.
Hydroalcoholic extraction method
The dried powders of all the four parts were extracted individually by cold percolation method[15−17]. The hydroalchoholic extraction was done using methanol and water[18]. The dried powder was first defatted by hexane and then extracted in 100% methanol (MeOH), 75% MeOH, 50% MeOH, 25% MeOH and 100% water (aqueous). Ten grams of dried powder was taken in 100 ml of hexane in a conical flask, plugged with cotton wool and then kept on a rotary shaker at 120 rpm for 24 h. After 24 h, the extract was filtered with eight layers of muslin cloth; centrifuged at 5000 rpm for 10 min. Supernatant was collected and the solvent was evaporated. The residue was then added to 100 ml of each solvent, that is 100% MeOH, 75% MeOH, 50% MeOH, 25% MeOH and water in a conical flask, plugged with cotton wool and then kept on a rotary shaker at 120 rpm for 24 h. After 24 h, the extract was filtered with eight layers of muslin cloth; centrifuged at 5000 rpm for 10 min, the supernatant was collected and the solvents were partially evaporated using rotary vacuum evaporator (Equitron, India) then kept in petri plates to dry. The extract was stored at 4° in air tight bottles. The residues were weighed to obtain the extractive yield.
Antimicrobial activity
The microorganisms used in this investigation were obtained from National Chemical Laboratory, Pune, India. The microorganisms were maintained at 4°. The Gram−positive bacteria studied were Staphylococcus aureus ATCC29737 (SA), Staphylococcus albus NCIM 2178 (SAL), Corynebacterium rubrum ATCC14898 (CR), Listeria monocytogenes ATCC19112 (LM), Micrococcus flavus ATCC10240 (MF); Gram−negative bacteria used were Pseudomonas aeruginosa ATCC27853, (PA) Pseudomonas stutzeri NCIM5136 (PSt), Pseudomonas pictorum NCIB9152 (PPi), Pseudomonas putida NCIM2872 (PP), Pseudomonas testosteroni NCIM5098 (PT), Pseudomonas syrigae NCIM5102 (PS); and fungi were Candida albicans ATCC2091 (CA), Candida neoformans NCIM3542 (CN), Candida glabrata NCIM3448 (CG), Candida epicola NCIM3367 (CE). The organisms were maintained on nutrient agar and MGYP medium (Hi−Media, India) for bacteria and fungi respectively, at 4° and subcultured before use. The microorganisms studied are clinically important ones causing several infections and food spoilage. Ampicillin (AMP 10 μg/disc), chloramphenicol (CH 30 μg/disc), tetracycline (T 30 μg/disc), amphotericin B (AP 100 units/disc) and nystatin (NS 100 units/disc) were used as standard to determine antimicrobial susceptibility. Chloramphenicol and ceftazidime (CF) were used during minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determination. All antibiotics were purchased from Hi−Media Laboratory Pvt. Ltd., (Mumbai, India).
Agar well diffusion method
In vitro antimicrobial activity of the different solvent extracts was studied against pathogenic microbial strains by the agar well diffusion method[19−22]. Mueller−Hinton No. 2/Sabouraud dextrose agar (Hi−Media) was used for the antibacterial and antifungal susceptibility test, respectively. The different solvent extracts were diluted in 100% dimethyl sulfoxide (DMSO) to give a concentration of 20 mg/ml. The Mueller−Hinton agar/Sabouraud dextrose agar was melted and cooled to 48–50° and a standardised inoculum (1.5×108 CFU/ml, 0.5 McFarland) was then added aseptically to the molten agar and poured into sterile Petri dishes; wells (8.5 mm) were prepared in the seeded agar plates. The test compound (100 μl) was introduced into the well. The plates were incubated overnight at 37° and 28° for 24 and 48 h, respectively, for bacteria and fungi. DMSO was used as negative control. The microbial growth was determined by measuring the diameter of the zone of inhibition and the mean values are presented with ±SEM (standard error of mean).
Preparation of bacterial inocula and extracts or antibiotics for MIC and MBC study
The inoculum of the test organisms were prepared using the colony suspension method[23]. Colonies picked from 24 h old cultures, grown on nutrient agar, were used to make suspension of the test organisms in saline solution to give an optical density of approximately 0.1 at 600 nm. The suspension was then diluted 1:100 by transfer of 0.1 ml of the bacterial suspension to 9.9 ml of sterile nutrient broth before use to yield 6×105 CFU/ml. Twofold serial dilutions using 100% DMSO were carried out from the 1250 μg/ml stock plant extract to make six test concentrations ranging from 39 to 1250 μg/ml for each solvent extracts. Twofold dilutions of chloramphenicol and ceftazidime (1–32 μg/ml) were used as a positive control.
Determination of minimum inhibitory concentration
The MICs were determined only for the test organisms that had shown >15 mm zone of inhibition of the crude extracts. Micro broth dilution method performed in sterile flat bottom 96 well micro test plates (Tarsons Products Pvt. Ltd.) was performed to evaluate MIC of the plant extracts[24]. One hundred and fifty microlitres of Mueller−Hinton broth was introduced into all the 96 wells and 20 μl of varying concentrations of the extract was added in decreasing order along with 30 μl of the test organism suspension. A final volume of 200 μl was achieved in each well (150 μl Mueller−Hinton broth, 30 μl of the test organism suspension and 20 μl plant extract/antibiotic). Three control wells were maintained for each test batch. The positive control (antibiotic, Mueller−Hinton broth and test organism) and sterility control (Mueller−Hinton broth and DMSO) and organism control (Mueller−Hinton broth, test organism and DMSO). Plates were then incubated at 37° for 24 h overnight. Experiments were performed in triplicate. After incubation, 40 μl of 2−(4−iodophenyl)−3−(4−nitrophenyl) 5−phenyltetrazolium chloride (INT, Himedia, India) solution (0.2 mg/ml) dissolved in sterile distilled water was added to each well[25]. The plates were incubated for further 30 min, and estimated visually for any change in colour to pink indicating reduction of the dye due to bacterial growth. The highest dilution (lowest concentration) that remained clear corresponded to the MIC.
Determination of minimum bactericidal concentration
MBC was determined from all wells showing no growth as well as from the lowest concentration showing growth in the MIC assay for all the samples. Bacterial cells from the MIC test plate were sub−cultured on freshly prepared solid nutrient agar by making streaks on the surface of the agar. The plates were incubated at 37° for 24 h overnight. Plates that did not show growth were considered to be the MBC for the extract or drug used[26]. The experiment was carried out in triplicate.
Determination of MIC index and statistical analyses
The MIC index (MBC/MIC) was calculated for each extract and positive control drug to determine whether an extract had bactericidal (MBC/MIC ≤4) or bacteriostatic (>4 MBC/MIC <32) effect on growth of bacteria[27]. All experiments were repeated at least three times. Results are reported as mean±SEM.
Results
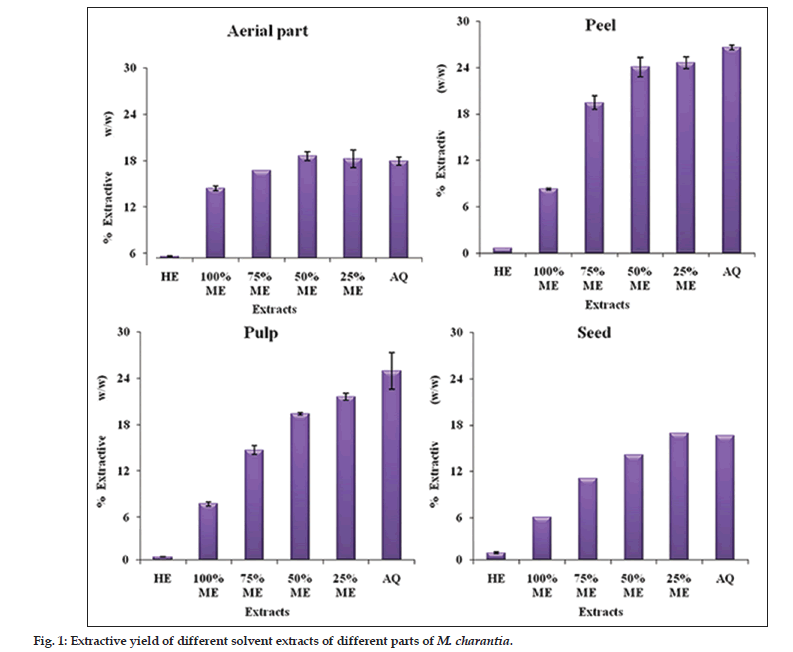
The extractive yield varied among different parts of M. charantia and also among different hydroalcoholic extracts (hexane, 100, 75 and 25% methanol and water) as shown in (fig. 1). The hexane extract had very negligible yield in all the four parts of M. charantia. The areal part aqueous extract had slightly more extractive yield that 100% MeOH. As the concentration of methanol decreased, there was a slight increase in extractive yield (fig. 1a). The peel aqueous extract had considerably more extractive yield than 100% MeOH. As the concentration of methanol decreased, the extractive yield increased almost reaching to that of pure aqueous extract (fig. 1b). The extractive yield of hydroalcoholic extracts of pulp showed a trend similar to that of peel (fig. 1c). Both these parts, that is peel and pulp had maximum extractive yield. In seed also, pure methanol had considerable less extractive yield than aqueous extract; the extractive yield of other hydroalcoholic extracts was similar to that of aerial parts (fig. 1d).
Antimicrobial activity of Momordica charantia aerial part
The antimicrobial activity of different hydroalcoholic extracts of aerial part of M. charantia is shown in Table 1 and 2. All the extracts showed activity against M. flavus and S. aureus. The highest activity was in 50% MeOH followed by hexane extract against M. flavus. In Gram−negative bacteria, all the extracts showed activity against P. syrigae and P. testosterone, except P. stutzeri. The hexane extract showed maximum activity against Pseudomonas spp. All extracts showed moderate activity against fungi.
Antimicrobial activity of Momordica charantia peel
The antimicrobial activity of different hydroalcoholic extracts of peel part of M. charantia is shown in Table 1 and 2. In Gram−positive bacteria, all extracts showed activity against M. flavus and the highest activity was in 50% MeOH extract. S. albus was resistant to all the extracts. In Gram−negative bacteria, all extracts showed activity against P. syrigae, P. testosteroni and P. putida; while remaining extracts showed different levels of activity against P. aeruginosa, P. stutzeri and P. pictorum. The 100 and 50% MeOH extracts showed activity against all Pseudomonas spp. screened. All extracts showed moderate activity against the fungi.
Antimicrobial activity of Momordica charantia pulp
Antimicrobial activity of different hydroalcoholic extracts of the pulp of Momordica charantia is shown in Table 1 and 2. In Gram−positive bacteria, all extracts showed activity against M. flavus and the highest activity was in 75% MeOH extract followed by the hexane extract. C. rubrum and S. albus were resistant to all extracts. L. monocytogenes was slightly susceptible to only 50% MeOH extract. In Gram−negative bacteria, all extracts showed activity against P. aeruginosa, P. testosteroni and P. putida; while remaining extracts showed different levels of activity against P. syrigae and P. pictorum. The hexane extract showed maximum activity against P. stutzeri. All extracts showed moderate activity against the fungi.
| Part name | Extracts | Zone of Inhibition (mm)* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria (Pseudomonas Spp.) | |||||||||||
| CR | SAL | SA | LM | MF | PA | PSt | PPi | PP | PT | PS | ||
| Aerial part | HE | 0±0 | 0±0 | 11±0 | 0±0 | 23±0 | 0±0 | 0±0 | 0±0 | 21.5±0.29 | 15.5±0.29 | 21.5±0.29 |
| MeOH | 0±0 | 11±0 | 14±0 | 0±0 | 20.5±0 | 14±0 | 0±0 | 15±0 | 15±0 | 14±0 | 20±0 | |
| 75% MeOH | 10±0 | 0±0 | 15±0 | 13.5±0.29 | 21±0 | 14±0 | 0±0 | 16±0 | 0±0 | 15±0 | 20±0 | |
| 50% MeOH | 11±0 | 0±0 | 15±0 | 13±0 | 24±0 | 15±0 | 0±0 | 15.5±0.29 | 0±0 | 14.5±0.29 14.5±0.29 | ||
| 25% MeOH | 0±0 | 0±0 | 12±0 | 0±0 | 13±0 | 11±0 | 0±0 | 11±0 | 0±0 | 14.5±0.29 15.5±0.29 | ||
| AQ | 0±0 | 0±0 | 10±0 | 0±0 | 10.5±0.29 | 0±0 | 0±0 | 0±0 | 16.5±0.29 | 15.5±0.29 | 17.5±0.29 | |
| Peel | HE | 0±0 | 0±0 | 11±0 | 0±0 | 25±0 | 0±0 | 0±0 | 0±0 | 15±0 | 13.5±0.29 | 14.5±0.29 |
| MeOH | 10±0 | 0±0 | 15±0 | 12±0 | 25±0 | 14.5±0.29 | 10.5±0.29 | 15±0 | 11.5±0.29 | 14.5±0.29 | 15.5±0.29 | |
| 75% MeOH | 9±0 | 0±0 | 13.5±0.29 | 11.5±0.29 | 25±0 | 13.5±0.29 | 11±0 | 13±0 | 11.5±0.29 | 14.5±0.29 | 11.5±0.29 | |
| 50% MeOH | 0±0 | 0±0 | 14.5±0.29 | 11±0 | 26.5±0.29 | 12±0 | 0±0 | 12.5±0.29 | 11.5±0.29 | 14.5±0.29 | 11.5±0.29 | |
| 25% MeOH | 0±0 | 0±0 | 12±0 | 0±0 | 14±0 | 11.5±0.29 | 11±0 | 11±0 | 15±0 | 14.5±0.29 | 10±0 | |
| AQ | 0±0 | 0±0 | 0±0 | 0±0 | 14.5±0.29 | 0±0 | 10.5±0.29 | 10±0 | 10.5±0.29 | 13±0 | 14.5±0.29 | |
| Pulp | HE | 0±0 | 0±0 | 0±0 | 0±0 | 21±0 | 11±0 | 0±0 | 0±0 | 14.5±0.29 | 16±0 | 19.5±0.29 |
| MeOH | 0±0 | 0±0 | 10.5±0.29 | 0±0 | 20.5±0.29 | 12.5±0.29 | 10±0 | 16±0 | 17.5±0.29 | 15±0 | 14.5±0.29 | |
| 75% MeOH | 0±0 | 0±0 | 11±0 | 0±0 | 23±0 | 13±0 | 10.5±0.29 | 13.5±0.29 | 14.5±0.29 | 16±0 | 16±0 | |
| 50% MeOH | 0±0 | 0±0 | 11.5±0.29 | 10±0 | 20.5±0.29 | 12±0 | 0±0 | 12.5±0.29 | 13.5±0.29 | 15.5±0.29 | 0±0 | |
| 25% MeOH | 0±0 | 0±0 | 0±0 | 0±0 | 20±0 | 10.5±0 | 0±0 | 0±0 | 12±0 | 14.5±0.29 | 0±0 | |
| AQ | 0±0 | 0±0 | 0±0 | 0±0 | 16±0 | 11.5±0 | 11.5±0.29 | 0±0 | 15±0 | 15±0 | 0±0 | |
| Seed | HE | 10±0 | 0±0 | 9±0 | 0±0 | 25±0 | 10.5±0 | 11±0 | 10±0 | 13.5±0.29 | 10±0 | 14.5±0.29 |
| MeOH | 10±0 | 0±0 | 10±0 | 11.5±0.29 | 16±0 | 12±0 | 10.5±0.29 | 11±0 | 17.5±0.29 | 10±0 | 20.5±0.29 | |
| 75% MeOH | 9±0 | 12±0 | 10.5±0.29 | 11±0 | 18.5±0.29 | 10.5±0 | 11±0 | 11±0 | 13±0 | 10.5±0.29 | 16.5±0.29 | |
| 50% MeOH | 10±0 | 11.5±0.29 | 11±0 | 12±0 | 18.5±0.29 | 11.5±0 | 11±0 | 11±0 | 15±0 | 10.5±0.29 | 15±0.29 | |
| 25% MeOH | 0±0 | 12±0 | 11.5±0.29 | 0±0 | 19±0 | 0±0 | 11.5±0.29 | 0±0 | 16.5±0 | 10.5±0.58 | 14.5±0.29 | |
| AQ | 0±0 | 10.5±0.29 | 17±0 | 0±0 | 18±0 | 0±0 | 11±0 | 0±0 | 15±0 | 10±0.29 | 15.5±0.29 | |
Table 1: Antibacterial activity of different parts of m. Charantia.
| Part name | Extracts | Zone of Inhibition (mm)* | |||
|---|---|---|---|---|---|
| Fungal Strains | |||||
| CE | CA | CG | CN | ||
| Aerial | HE | 11±0 | 15±0 | 13±0 | 11±0 |
| part | MeOH | 12±0 | 14±0 | 11.5±0.29 | 12±0 |
| 75% MeOH | 10.5±0.29 | 13.5±0.29 | 11.5±0.29 | 10.5±0.29 | |
| 50% MeOH | 9.5±0.29 | 11.5±0.29 | 10±0 | 9.5±0.29 | |
| 25% MeOH | 0±0 | 14±0 | 11±0 | 0±0 | |
| AQ | 11.5±0.29 | 12.5±0.29 | 10.5±0.29 | 11.5±0.29 | |
| Peel | HE | 10.5±0.29 | 11.5±0.29 | 11±0 | 10.5±0.29 |
| MeOH | 12±0 | 12±0 | 10.5±0.29 | 12±0 | |
| 75% MeOH | 10.5±0.29 | 12.5±0.29 | 10±0 | 10.5±0.29 | |
| 50% MeOH | 10±0 | 13.5±0.29 | 11.5±0.29 | 10±0 | |
| 25% MeOH | 11±0 | 13.5±0.29 | 12±0 | 11±0 | |
| AQ | 10±0 | 13±0 | 10.5±0.29 | 10±0 | |
| Pulp | HE | 14±0 | 12.5±0.29 | 11±0 | 14±0 |
| MeOH | 14±0 | 14±0 | 10±0 | 14±0 | |
| 75% MeOH | 11.5±0.29 | 14±0 | 10.5±0.29 | 11.5±0.29 | |
| 50% MeOH | 14.5±0.29 | 13.5±0.29 | 11±0 | 14.5±0.29 | |
| 25% MeOH | 13±0 | 13±0 | 10.5±0.29 | 13±0 | |
| AQ | 10±0 | 13±0 | 10±0 | 10±0 | |
| Seed | HE | 11.5±0.29 | 11.5±0.29 | 10±0 | 11.5±0.29 |
| MeOH | 10.5±0.29 | 14±0 | 10.5±0.29 | 10.5±0.29 | |
| 75% MeOH | 10±0 | 13±0 | 10.5±0.29 | 10±0 | |
| 50% MeOH | 11±0 | 12±0 | 12±0 | 11±0 | |
| 25% MeOH | 10±0 | 12.5±0.29 | 10±0 | 10±0 | |
| AQ | 0±0 | 0±0 | 9.5±0.29 | 0±0 | |
Table 2: Antifungal activity of different parts of m. Charantia.
Antimicrobial activity of Momordica charantia seed
Antimicrobial activity of different hydroalcoholic extracts of seed part of M. charantia is shown in Table 1 and 2. In Gram−positive bacteria, all the extracts showed activity against M. flavus and S. aureus but susceptibility of M. flavus was considerably more than that of S. aureus. The highest activity was shown by the hexane extract against M. flavus. In Gram−negative bacteria, all the extracts showed activity against P. testosteroni, P. putida, P. stutzeri and P. syrigae; while P. aeruginosa and P. pictorum were not susceptible to any of the hydroalcoholic extracts. The highest activity was shown by 100% MeOH extract against P. aeruginosa. All extracts showed moderate activity against fungi. All 24 extracts were compared with 5 standard antibiotics. These antibiotics were tested against 15 medically important microbial strains, the results of which were presented in Table 3. The antimicrobial activity of some of the hydroalcoholic extracts was comparable with that of standard antibiotics.
Determination of MIC, MBC and MIC index
The MIC and MBC values of different hydroalcoholic extracts of different parts of M. charantia and standard antibiotics are shown in Tables 4−7. Inhibitory effects of bacterial growth by the extracts from different parts were in the range from <39 to >1250 μg/ml expressed as MIC values and in the range from 625 to >1250 μg/ml expressed as MBC values. Inhibitory effects of bacterial growth by the standard antibiotics were in the range from 4 to >32 μg/ml expressed as MIC values and in the range from 16 to >32μg/ml expressed as MBC values.
MIC and MBC values of different hydroalcoholic extracts of aerial part of M. charantia and standard antibiotics are shown in Table 4. Extract of 75% MeOH showed least MIC value and MBC values, that is 156 and 1250 μg/ ml, respectively against M. flavus. However 100 and 75% MeOH extracts showed MIC index of 4; therefore both extracts showed bactericidal effect; it is comparable to standard chloramphenicol. Extract of 75% MeOH showed bactericidal effect against P. pictorum.
MIC and MBC values of different hydroalcoholic extracts of M. charantia pulp and standard antibiotics are shown in Table 5. Extracts of 75 and 100% MeOH showed least MIC values 312 and 156 μg/ml, respectively, and MBC was 1250 μg/ml against M. flavus. Extracts of 100% and 75% MeOH showed MIC index of 8 and 4, respectively; therefore, 100 and 75% MeOH showed bactericidal and bacteriostatic effect against M. flavus. Extracts of 100% MeOH showed MIC value of 625 μg/ml while MBC was >1250 μg/ml against P. putida.
| Antibiotic name | Concentration | Gram positive | Gram negative | Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LM | SAL | MF | SA | CR | PA | CE | CA | CG | CN | ||
| Ampicilin | 10 µg | 0 | 0 | 0 | 23 | 25 | 14 | NT | NT | NT | NT |
| Tetracycline | 30 µg | 0 | 21 | 0 | 22 | 0 | 0 | NT | NT | NT | NT |
| Chloramphenicol | 30 µg | 0 | 26 | 0 | 18 | 15 | 0 | NT | NT | NT | NT |
| Nystatin | 100 units | NT | NT | NT | NT | NT | NT | 22 | 18 | 18 | 22 |
| Amphotericin | 100 units | NT | NT | NT | NT | NT | NT | 16 | 10 | 10 | 11 |
Table 3: Antimicrobial Activity Using Standard Antibiotics.
| Extracts | MF1 | PPi2 | PP2 | PT2 | PS2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | |
| HE | >1250 >1250 | ND | - | - | - | >1250 | >1250 | ND | >1250 | >1250 | ND | >1250 | >1250 | ND | |
| 100% MeOH | 312 | 1250 | 4.0 | - | - | - | - | - | - | - | - | - | >1250 | >1250 | ND |
| 75% MeOH | 156 | 625 | 4.0 | 312 | 1250 | 4.0 | - | - | - | - | - | - | >1250 | >1250 | ND |
| 50% MeOH | 625 | >1250 | ND | >1250 | >1250 | ND | - | - | - | - | - | - | - | - | - |
| 25% MeOH | - | - | - | - | - | - | - | - | - | -- | - | - | - | - | - |
| AQ | - | - | - | - | - | - | >1250 | >1250 | ND | >1250 | >1250 | ND | >1250 | >1250 | ND |
| CH | 4 | 16 | 4 | 32 | >32 | ND | 16 | >32 | ND | 32 | >32 | ND | 16 | >32 | ND |
| CF | 16 | 32 | 2 | 8 | >32 | ND | 32 | >32 | ND | 8 | 32 | 4.0 | >32 | >32 | ND |
Table 4: mic and mbc of different solvent extracts of m. Charantia aerial parts
| Extracts | MF1 | PP2 | PT2 | PS2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | ||
| HE | 625 | >1250 | ND | - | - | >1250 | >1250 | ND | >1250 | >1250 | ND | ||
| 100% MeOH | 156 | 1250 | 8.0 | 625 | >1250 | ND | - | - | ND | - | - | ND | |
| 75% MeOH | 312 | 1250 | 4.0 | - | - | ND | >1250 | >1250 | ND | - | - | - | |
| 50% MeOH | 625 | >1250 | ND | - | - | - | >1250 | >1250 | ND | - | - | - | |
| 25% MeOH | 1250 | >1250 | ND | - | - | - | - | - | - | - | - | - | |
| AQ | >1250 | >1250 | ND | - | - | - | - | - | - | - | - | - | |
| CH | 4 | 16 | 4 | 16 | >32 | ND | 32 | >32 | ND | 16 | >32 | ND | |
| CF | 16 | 32 | 2 | 32 | >32 | ND | 8 | 32 | ND | >32 | >32 | ND | |
Table 5: Mic and Mbc of different solvent extracts of m. Charantia pulp.
MIC and MBC values of different hydroalcoholic extracts of M. charantia peel and standard antibiotics are shown in Table 6. Extracts with 50% and 100% MeOH showed least MIC values, 312 and 156 μg/ml, respectively, and MBC was 1250 and 625 μg/ml, respectively, against M. flavus. Both extracts showed bactericidal effect.
MIC and MBC values of different hydroalcoholic extracts of M. charantia seed and standard antibiotics are shown in Table 7. MeOH extracts (50 and 100%) showed least MIC values <39 μg/ml and MBC was 625 μg/ml against P. putida. Remaining extracts showed >1250 μg/ml MIC and MBC values. In this study, bactericidal effect was shown by 100, 75 and 50% MeOH extracts against M. flavus and P. pictorum while remaining extracts showed bacteriostatic effects.
| Extracts | MF1 | PS2 | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC index | MIC | MBC | MIC index | |
| HE | >1250 | >1250 | ND | - | - | - |
| 100% MeOH | 156 | 625 | 4 | >1250 | >1250 | ND |
| 75% MeOH | - | - | - | - | - | - |
| 50% MeOH | 312 | 1250 | 4 | - | - | - |
| 25% MeOH | - | - | - | - | - | - |
| AQ | - | - | - | >1250 | >1250 | |
| CH | 4 | 16 | 4 | 16 | >32 | ND |
| CF | 16 | 32 | 2 | >32 | >32 | ND |
Table 6: Mic and mbc of different solvent extracts of m. Charantia peel.
| Extracts | MF1 | PPi2 | PP2 | PT2 | PS2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | MIC | MBC | MIC index | ||
| HE | >1250 | >1250 | ND | - | - | - | - | - | - | - | - | - | - | |||
| 100% MeOH | - | - | - | - | - | - | <39 | 625 | ND | - | - | >1250 | >1250 | ND | ||
| 75% MeOH | >1250 | >1250 | ND | - | - | - | - | - | - | - | - | >1250 | >1250 | ND | ||
| 50% MeOH | >1250 | >1250 | ND | - | - | - | <39 | 625 | ND | - | - | - | - | |||
| 25% MeOH | >1250 | >1250 | ND | - | - | - | >1250 | >1250 | ND | - | - | - | - | |||
| AQ | >1250 | >1250 | ND | - | - | - | - | - | - | - | - | >1250 | >1250 | ND | ||
| CH | 4 | 16 | 4 | 32 | >32 | ND | 16 | >32 | ND | 32 | >32 | ND | 16 | >32 | ND | |
| CF | 16 | 32 | 2 | 8 | >32 | ND | 32 | >32 | ND | 8 | 32 | 4 | >32 | >32 | ND | |
Table 7: Mic and mbc of different solvent extracts of seeds of m. Charantia.
Discussion
Normally, a high extraction yield is required for an efficient process; although it is not necessary that high concentration of bioactive components are present in them. Since some bioactive components are very sensitive to oxygen and heat[28], care should be taken to prevent their oxidation and thermal degradation. Therefore, the extraction yield and the bioactive component characteristics should also be considered when an extraction method is selected. The traditional healers or practitioners make use of water primarily as a solvent but there are many reports where organic solvents showed better activity as compared with aqueous extracts[29−31]. In the present study, extractive yield was considerably more in water than in organic solvent methanol and as the concentration of methanol decreased, extractive yield increased clearly indicating that in these plant parts, water soluble phytoconstituents were more.
The results of antimicrobial activity clearly indicated that M. flavus was the most susceptible Gram−positive bacteria and P. testosterone was the most susceptible Gram−negative bacteria. Aqueous extract showed poor activity as compared with pure methanol or hydroalchoholic extracts; best antibacterial activity was shown by 100% MeOH extract, which is an extract with a pure organic solvent. Almost all the extracts showed antifungal activity against all the four fungi studied, though the activity was moderate.
Gram−negative bacteria were more susceptible towards all the extracts than Gram−positive bacteria. This is very good report since there is a general consensus that plant extracts are more active against Gram−positive bacteria than Gram−negative bacteria[32−40]. Therefore, the search is always to find plant extracts that are capable of inhibiting Gram−negative bacteria, which are equally dangerous in causing infectious diseases like Gram−positive bacteria. The Gram−negative cell wall (made up of lipopolysaccharide) is complex and multilayered structure, which makes access to membrane more restricted and barrier to many environmental substances including synthetic and natural antibiotics. The results of the present study indicate that extracts of M. charantia contain some secondary metabolites, which are able to cross this tough barrier.
The MIC is defined as the lowest concentration of the antimicrobial agent that will inhibit the visible growth of a microorganism after overnight incubation[41,42], whereas the MBC is interpreted as the lowest concentration that can completely remove the microorganisms. A pinkish coloration is indicative of microbial growth because of their ability to convert INT to red formazan[43]. The concentrations of MIC and MBC for plant extracts and standard antibiotics were 1250–39 μg/ml and 32–1 μg/ml, respectively. MIC and MBC were expressed in terms of μg/ml. Braca et al.[44], Coutinho et al.[45] and Roopashree et al.[46] used in their studies M. charantia extracts/essential oils, which possessed potential activity against Staphylococcus aureus. Castilho et al.[47] reported that antimicrobial activity of Origanum essential oils and all the studied extracts showed MIC values >200 μg/ml against P. aerugenosa. In the present study, better results were found in seed (100% methanol and 50% methanol) and MIC value was <39 μg/ml, which was near to ceftazidime against P. pictorum.
The spread of multidrug−resistant strains of microorganisms and the reduced number of drugs available makes it necessary to discover new classes of antibacterial and antifungal agents that overcome these resistant mechanisms. This led to search for therapeutic alternatives, particularly among medicinal plants and compounds isolated from them used empirically for their antibacterial and antifungal properties.
Foodborne disease is one of the major concerns to food producers and consumers and spoilage of foods is still a major problem in different parts of the world. In an effort to meet this demand, the food industry has a great interest in using natural antimicrobial compounds. The hydroalcoholic extracts of M. charantia possessed significant antibacterial activity (MIC ≤39 μg/ml) against Pseudomonas spp. Therefore, the use of this plant as antimicrobial agent is validated by the results obtained in this work. Further studies are in progress to identify the chemical compounds present in these extracts with antimicrobial activity as well as to identify synergism between plant extracts and standard antibiotics. The results of the present investigation also provide an approach to develop promising natural antimicrobial agents with potential applications in the food and pharmaceutical industries. This fact is of paramount importance from the point of view of food safety.
References
- Chanda S, Rakholiya K. Indian Combination therapy: Synergismbetween natural plant extracts and antibiotics against infectious
- diseases. In: Mendez-Vilas A, editor. Science against MicrobialPathogens: Communicating Current Research and TechnologicalAdvances. Vol. 1. Spain: Formatex Research Center; 2011. p. 520-9.
- Chanda S, Amrutiya N, Rakholiya K. Evaluation of antioxidantproperties of some Indian vegetable and fruit peels by decoction extraction method. Am J Food Technol 2013;8:173-82.
- Rakholiya K, Kaneria M, Chanda S. Medicinal plants as alternativesources of therapeutics against multidrug-resistant pathogenicmicroorganisms based on their antimicrobial potential and synergisticproperties. In: Rai M, Kon K, editor. Fighting Multidrug Resistancewith Herbal Extracts, Essential Oils and their Components, Vol. 1. SanDiego, CA, USA: Elsevier; 2013. p. 165-79.
- Caillet S, Cote J, Sylvain J, Lacroix M. Antimicrobial effects offractions from cranberry products on the growth of seven pathogenicbacteria. Food Control 2012;23:419-28.
- Cruz CD, Fletcher GC. Assessing manufacturers’ recommendedconcentrations of commercial sanitizers on inactivation of Listeriamonocytogenes. Food Control 2012;26:194-9.
- Ivanovic J, Misic D, Zizovic I, Ristic M. In vitro control ofmultiplication of some food-associated bacteria by thyme, rosemary and sage isolates. Food Control 2012;25:110-6.
- Moretro T, Heir E, Nesse LL, Vestby LK, Langsrud S. Control ofSalmonella in food related environments by chemical disinfection. FoodRes Int 2012;45:532-44.
- Marzouk B, Marzouk Z, Decor R, Edziri H, Haloui E, Fenina N,et al. Antibacterial and anticandidal screening of Tunisian Citrulluscolocynthis Schrad. from Medenine. J Ethnopharmacol 2009;125:344-9.
- Yao X, Zhu X, Pan S, Fang Y, Jiang F, Phillips GO, et al.Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem 2012;132:1883-90.
- Negi PS. Plant extracts for the control of bacterial growth: Efficacy,stability and safety issues for food application. Int J Food Microbiol2012;156:7-17.
- Fuangchana A, Sonthisombata P, Seubnukarnb T, Chanouanc R,Chotchaisuwatd P, Sirigulsatien V, et al. Hypoglycemic effect of bittermelon compared with metformin in newly diagnosed type 2 diabetespatients. J Ethnopharmacol 2011;134:422-8.
- Rakholiya K, Kaneria M, Chanda S. Vegetable and fruit peels as anovel source of antioxidants. J Med Plants Res 2011;5:63-71.
- Pitchakarn P, Suzuki S, Ogawa K, Pompimon W, Takahashi S,Asamoto M, et al. A triterpeniod from Momordica charantia leaf,modulates the progression of androgen-independent human prostatecancer cell line, PC3. Food Chem Toxicol 2012;50:840–7.
- Grover JK, Yadav SP. Pharmacological actions and potential uses ofMomordica charantia: a review. J Ethnopharmacol 2004;93:123-32.
- Parekh J, Chanda S. In vitro antibacterial activity of the crude methanolextract of Woodfordia fruticosa kurz. flower (lythraceae). Braz JMicrobiol 2007;38:204-7.
- Rakholiya K, Chanda S. In vitro interaction of certain antimicrobialagents in combination with plant extracts against some pathogenicbacterial strains. Asian Pac J Trop Biomed 2012;2:S876-80.
- Rakholiya K, Chanda S. Pharmacognostic, physicochemical andphytochemical investigation of Mangifera indica L. var. Kesar leaf.Asian Pac J Trop Biomed 2012;2:680-4.
- Kaneria M, Kanani B, Chanda S. Assessment of effect ofhydroalcoholic and decoction methods on extraction of antioxidants
- from selected Indian medicinal plants. Asian Pac J Trop Biomed2012;2:188-95.
- Perez C, Paul M, Bazerque P. An antibiotic assay by the agar welldiffusion method. Acta Biol Med Exp1990;15:113-5.
- Chanda S, Baravalia Y, Kaneria M, Rakholiya, K. Fruit and vegetablepeels – strong natural source of antimicrobics. In: Mendez–Vilas A,editor. Current Research, Technology and Education Topics in AppliedMicrobiology and Microbial Biotechnology. Vol. 1. Spain: FormatexResearch Center; 2010. p. 444-50.
- Rakholiya K, Kaneria M, Chanda S. Mango Pulp: A potential sourceof natural antioxidant and antimicrobial agents. In: Gupta VK, editor.Medicinal Plants: Phytochemistry, Pharmacology and Therapeutics.Vol 3. New Delhi, India: Daya Publication House; 2013. p. 253-84.
- Chanda S, Kaneria M. Indian nutraceutical plant leaves as a potentialsource of natural antimicrobial agents. In: Mendez-Vilas A, editor.Science against Microbial Pathogens: Communicating Current Researchand Technological Advances. Vol. 2. Spain: Formatex Research Center;2011. p. 1251-9.
- European Committee for Antimicrobial SusceptibilityTesting (EUCAST). Determination of minimum inhibitoryconcentrations (MICs) of antibacterial agents by broth dilution. Clin JMicrobiol Infect 2003;9:1-7.
- Sampaio FC, Pereira S, Dias CS, Costa VC, Conde NC, Buzalaf MA.In vitro antimicrobial activity of Caesalpinia ferrea martius fruitsagainst oral pathogens. J Ethnopharmacol 2009;124:289-94.
- Frey FM, Meyers R. Antibacterial activity of traditional medicinalplants used by Haudenosaunee peoples of New York State. BMCComplement Altern Med 2011;10:64.
- Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screeningof crude extracts of six medicinal plants used in South-West Nigerianunorthodox medicine for anti-methicillin resistant Staphylococcus aureusactivity. BMC Complement Altern Med 2005;5:6.
- Stefanovic O, Comic L. Inhibitory effect of Cytisus nigricans L. andCytisus capitatus scop on growth of bacteria. Afr J Microbiol Res2011;5:4725-30.
- Ishida Y, Kitagawa K, Goto K, Ohtani H. Solid sampling techniquefor direct detection of condensed tannins in bark by matrix-assistedlaser desorption/ionization mass spectrometry. Rapid Commun MassSpectrom 2005;19:706–10.
- de Boer HJ, Kool A, Broberg A, Mziray WR, Hedberg I, Levenfors JJ.Antifungal and antibacterial activity of some herbal remedies fromTanzania. J Ethnopharmacol 2005;96:461-9.
- Parekh J, Chanda S. In vitro antimicrobial activities of extracts ofLaunaea procumbens Roxb. (Labiateae), Vitis vinifera L. (Vitaceae) andCyperus rotundus L. (Cyperaceae). Afr J Biomed Res 2006;9:89-93.Kaneria M, Chanda S. Evaluation of antioxidant and antimicrobialcapacity of Syzygium cumini L. leaves extracted sequentially in differentsolvents. J Food Biochem 2013;37:168–76.
- Parekh J, Chanda SV. In vitro antimicrobial activity and phytochemicalanalysis of some Indian medicinal plants. Turk J Biol 2007;31:53-8.
- Parekh J, Chanda SV. Antibacterial activity of aqueous and alcoholicextracts of 34 Indian medicinal plants against some StaphylococcusSpecies. Turk J Biol 2008;32:63-71.
- Baravalia Y, Kaneria M, Vaghasiya Y, Parekh J, Chanda S. Antioxidantand antibacterial activity of Diospyros ebenum Roxb. leaf extracts. TurkJ Biol 2009;33:159-64.
- Chanda S, Dudhatra S, Kaneria M. Antioxidative and antibacterialeffects of seeds and fruit rind of nutraceutical plants belonging to thefamily Fabaceae. Food Funct 2010;1:308-15.
- Yagi S, Chretien F, Duval RE, Fontanay S, Maldini M, Piacente S,et al. Antibacterial activity, cytotoxicity and chemical constituents ofHydnora johannis roots. S Afr J Bot 2012;78:228-34.
- Chanda S, Rakholiya K, Dholakia K, Baravalia Y. Antimicrobial,antioxidant and synergistic property of two nutraceutical plants:Terminalia catappa L. and Colocasia esculenta L. Turk J Biol2013;37:81-91.
- Chanda S, Rakholiya K, Nair R. Antimicrobial Activity of Terminaliacatappa L. leaf extracts against some clinically important pathogenicmicrobial strains. Chin Med 2011;2:171-7.
- Chanda S, Rakholiya K, Parekh J. Indian medicinal herb: Antimicrobialefficacy of Mesua ferrea L. seed extracted in different solvents againstinfection causing pathogenic strains. J Acute Dis 2013;2:277-81.
- Rakholiya K, Kaneria M, Dishant D, Chanda S. Antimicrobialactivity of decoction extracts of residual parts (seed and peels) of Mangifera indica L. var. Kesar against pathogenic and food spoilagemicroorganism. In: Mendez-Vilas A, editor. Microbial Pathogens andStrategies for Combating them: Science, Technology and Education.Vol. 2. Spain: Formatex Research Center; 2013. p. 850-6.41. Andrews JM. Determination of minimum inhibitory concentration.J Antimicrob Chemother 2001;48:5-16.
- Thongson C, Davidson PM, Mahakarrchanakul W, Weiss J.Antimicrobial activity of ultrasound-associated solvent extracted species.Lett Appl Microbiol 2004;39:401-6.
- Iwalewa EO, Suleiman MM, Mdee LK, Eloff JN. Antifungaland antibacterial activities of different extracts of Harungana madagascariensis stem bark. Pharm Biol 2009;47:878-85.
- Braca A, Siciliano T, D’Arrigo M, Germano MP. Chemical compositionand antimicrobial activity of Momordica charantia seed essential oil.Fitoterapia 2008;79:123-5.
- Coutinho HD, Costa JG, Falcao-Silva VS, Siqueira-Junior JP, Lima EO.Effect of Momordica charantia L. in the resistance to aminoglycosidesin methicillin-resistant staphylococcus aureus. Comp ImmunolMicrobiol Infect Dis 2010;33:467-71.
- Roopashree TS, Dang R, Shobha Rani RH, Narendra C. Antibacterialactivity of antipsoriatic herbs: Cassia tora, Momordica charantia andCalendula officinalis. Int J Appl Res Nat Prod 2008;1:20-8.
- Castilho PC, Savluchinske-Feio S, Weinhold TS, Gouveia SC.Evaluation of the antimicrobial and antioxidant activities of essentialoils extracts and their main components from oregano from Madeira Island. Portugal. Food Control 2012;23:552-8.