- *Corresponding Author:
- Najat Agiel
Department of Biology
Faculty of Education
Tripoli University
Tripoli 13275, Libya
E-mail: najataghil@yahoo.com
| Date of Received | 17 July 2020 |
| Date of Revision | 26 August 2021 |
| Date of Acceptance | 08 April 2022 |
| Indian J Pharm Sci 2022;84(2):444-456 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The use of Crataegus species for the treatment of cardiovascular ailments is widely distributed. Even then only a few species are included in the Pharmacopoeias. In this study bioactive flavonoids of Crataegus azarolus and Crataegus pallasii were identified and quantified in comparison with the well-known pharmaceutical product of Crataegus; Crataegutt® Tropfen using reverse phase-high performance liquid chromatography. The method developed is simple, fast, reliable and sensitive. In an attempt to reduce matrix effect prior to high performance liquid chromatography analysis, a novel approach is proposed as an efficient simple clean-up technique termed “indirect-dispersive liquid-liquid microextraction”. Validation parameters of the method were calculated as follows: Limit of detection ranged from 0.4 to 3.4 mg/g and limit of quantitation from 1.3 to 11.3 mg/g, intraday and interday precision expressed as percentage relative standard deviation ranged from 1.0 to 2.8 and 1.5 to 4.3, respectively and r2 values were above 0.9950 for all analytes. The relative recovery of all analytes was more than 98 %. Four predominate peaks were identified using certified standards as Vitexin 2''-O-rhamnoside, rutin, vitexin and hyperoside, with mass concentration (% w/w) in Crataegus azarolus as 4.4 %, 2.9 %, 1.7 % and 4.4 %, in Crataegus pallasii as 4.4 %, 2.6 %, 1.4 % and 4.8 % and in Crataegutt® Tropfen as 1.6 %, 1.0 %, 0.6 % and 0.4 % respectively. Thus, the values met the criteria of the United States Pharmacopeia and the European Pharmacopeia monographs. Our investigations postulate that Crataegus azarolus and Crataegus pallasii could be a good source for the production of Crataegus phytomediciness.
Keywords
Crataegus azarolus, Crataegus pallasii, reverse phase-high performance liquid chromatography, indirect dispersive liquid-liquid microextraction, Vitexin 2''-O-rhamnoside, rutin, vitexin, hyperoside
Crataegus species, known as "Hawthorn", are specially used for mild heart diseases. Flavonoids are the main constituents responsible for biological activities. The most important feature of Crataegus extracts is their positive inotropic effect. They increase the activation of the heart muscle cells, provide them a good feeding, regulate the blood flow and are coronary dilatators[1,2]. The main flavonoids in these extracts are hyperoside, vitexin, rutin and vitexin 2''-O-rhamnoside, in addition to Oligomeric Procyanidins (OPC)[3,4]. Therapeutic preparations from the leaves and flowering tops of Crataegus species have been used for the treatment of cardiovascular disorders since the first century A.D[5]. Crataegus products have been in use for atleast 30 y and their safety and efficacy have been studied extensively[6,7]. Extracts prepared from some Crataegus species are documented in pharmacopeias as commercial extracts of standardized composition[8-11]. The chemistry and activities of the extracts obtained from Crataegus species, growing in Turkey have been investigated by one of us (AHM) significantly[12–24]. The herbal drug Crataegutt® Tropfen manufactured by Dr. W. Schwabe Pharmaceuticals GmbH and Co. KG, which is the standardized extract of the leaves and flowers of Crataegus monogyna (C. monogyna) and Crataegus laevigata (C. leavigata)[25,26] which is one of the most studied Crataegus products and used in the pharmaceutical market[6,27].
Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) using C18 column with gradient elution of the mobile phase composition is the technique of choice for the identification and quantitation of phenolic compounds extracted from plant materials[28]. However, sample preparation prior to High Performance Liquid Chromatography (HPLC) analysis is an important step to avoid matrix interference and facilitate preconcentration of analytes present in low concentrations. Dispersive Liquid- Liquid Micro Extraction (DLLME) is a microextraction technique proposed for the first time by Rezaee[29] is primarily used for this purpose[30]. Since its introduction in 2006, DLLME has found wide prominence among scientists as an efficient sample preparation method prior to instrumental analysis, including HPLC, for minimizing matrix effect and preconcentrating of analytes[31]. In conventional DLLME, the analytes are extracted into a micro volume of a water-immiscible organic solvent, where they are preconcentrated. The analyte-rich extraction phase is separated from the mixture, evaporated to dryness and the analytes are reconstituted into a water-miscible solvent prior to RP-HPLC. To achieve an efficient sample clean-up of hydrophobic matrix components which might pose a potential interference on analytic peaks, Indirect Dispersive Liquid-Liquid Micro Extraction (IDLLME) was thought to be possible. In this method, hydrophobic interfering compounds were extracted into the organic solvent, whereas the analytes remain in the aqueous solution, which could be directly injected into RP- HPLC.
Crataegus azarolus L. is considered a medicinal and edible wild plant in Cyprus[32,33] and is one of the Crataegus species inscribed in the European Pharmacopeia. Numerous research has been carried out to determine phenolic compounds in C. azarolus by different HPLC methods[34-38]. In Libya, Crataegus pallasii grows in Bata area of El-merj city and is the only Crataegus species in Libya that has survived diverse environmental conditions. It is traditionally used by Libyans to improve cardiac functions[39].
In this study, RP-HPLC-Diode Array Detector (RP-HPLC-DAD) method is reported for the first time on two non-investigated species of Crataegus; C. azarolus growing in Cyprus and C. pallasii growing in Libya. The analytical method was developed for the identification and determination of the most important bioactive flavonoids in C.azarolus and C. pallasii in comparison with the phytopharmaceutical product of Crataegus, Crataegutt® Tropfen drops to evaluate the possibility of other species for the production of Crataegus derived pharmaceutical products that meet the requirements of the pharmacopeias. Experimental parameters influencing the chromatographic efficiency of the method were optimized, which included the mode of elution, type and composition of the mobile phase, column temperature and flow rate. Validation parameters of the method were expressed in terms of linearity, accuracy, sensitivity and precision.
Materials and Methods
Plant materials and phytopharmaceutical product:
C. azarolus L. samples were collected on 26.3.2018 at Cengizköy, North Cyprus and deposited at the Near East University Herbarium, voucher number NEUN 6899. Samples of C. pallasii Griseb were collected on 23.3.2018 in El-merj, Bata area, authenticated by Dr. Mohammed Nuri Abuhadra and placed at the Herbarium of the Faculty of Science, Botany Department, University of Tripoli, Libya, voucher number: D6831131. Crataegutt® Tropfen, a Crataegus derived phytopharmaceutical drug, was obtained in 2018 from Marien Apotheke in Saarlouis, Germany.
Chemicals and apparatus:
For the extraction of the plant material, leaves and flowers were ground using commercial blender WARING® CB15V (USA) and extracted with ethanol which was purchased from Merck chemicals (Germany). Ethyl acetate, n-hexane and toluene were obtained from Sigma-Aldrich GmbH. E102 from Bor- Kim (Turkey). The rotatory evaporator was purchased from Buchi Heating mantle MTOPS Labortechnik AG (Switzerland). Lyophilization was carried out with Martin Christ Gefriertrocknungsanlagen GmbH. For the HPLC analysis, all reagents were of analytical grade. HPLC grade Acetonitrile (ACN) was purchased from VWR Prolabo Chemicals (USA). Absolute ethanol 99.9% was purchased from Tekkim Kimya San (Turkey). Phosphoric acid was obtained from Millipore (USA) and chloroform was from BDH Prolabo® VWR European economic community. Deionized (DI) water (18.2 MΩ- cm) was obtained using Purelab Ultra Analytic (ELGA Lab Water, UK). The Standard compounds Hyperoside and vitexin were obtained from HWI Pharma Services GmbH. Rutin were ordered from sigma life science. The authentic standard vitexin 2''-O-rhamnoside was purchased from Sigma-Aldrich (France). A digital ultrasonic bath from Bandelin Sonrex (Germany) was used for sonication. Centrifugation was performed with Hettich Eba 20 centrifuge and vortex was performed on a Heidolph Reax top vortex (Germany). Eppendorf micropipette from Sigma-Aldrich (USA). Filtration of all solutions and samples was carried out before use via vacuum filtration through 0.20 μm regenerated cellulose membrane filters obtained from Whatman (Germany) and 0.22 μm sterile nylon syringe filters from Chromfil (China). Degassing was performed through sonication.
Preparation of samples and standards:
An extraction protocol procedure was carried out to prepare the ethyl acetate extract of C. azarolus and C. pallasii with some modifications[23]. Leaves and flowering tops of both species were shade dried. After drying, samples were ground and 25.0 g were weighed and extracted by soxhlet apparatus with 96.0 % ethanol for 6 h. After washing the total ethanolic extract with n-hexane and toluene, the ethyl acetate fraction was evaporated to dryness in a rotary evaporator, freeze- dried and stored in a dry place until analyzed by RP- HPLC-DAD. To prepare the sample solution, 25.0 mg of ethyl acetate extract was weighed and the IDLLME procedure was carried out to reduce the matrix effect by adding 4.5 ml of DI water to the extract and sonicating the mixture for 5 min. Next, 500 µl ACN (as a disperser solvent), 100 µl of chloroform (as an extractant for interferences) and 100 µl of phosphoric acid were added. The solution was vortexed for 1 min and centrifuged for 1 min at 6000 rpm. The organic phase containing the interferences was discarded and the aqueous phase was directly injected into HPLC for analysis.
To prepare the appropriate concentrations for calibration curves, 1.0 (±0.01) mg of each standard was weighed and dissolved in ethanol in an HPLC vial to obtain a 1000 mg/l stock solution of individual standards, which were stored at a temperature of 4° until use. Intermediate stock solutions containing 100 mg/l were freshly prepared in ethanol.
The Crataegutt® Tropfen sample was prepared from 1.0 ml of the crude syrup and diluted to 10 ml with ethanol to make a 10 % (v/v) solution. The solution was filtered and injected into HPLC after appropriate dilution.
Instrumentation and chromatographic conditions:
Chromatographic separations were performed using an HPLC instrument (Agilent Technologies 1200 series, USA) equipped with a quaternary pump, a solvent degasser system, an automatic injector, a column oven and a Diode Array Detector (DAD). Chemstation software (Rev. B.03.01, Agilent Technologies, USA) was used for evaluating the chromatograms. An ACE-C8 column (4.6 mm internal diameter (ID)×25 cm, 5 µm) was used for separation of the analytes by maintaining a column temperature of 20°. The mobile phase consisted of water (A) and ACN (B) using a gradient elution program of 20 % B at 0 min to 60 % B at 12 min at a flow rate of 0.8 ml/min. The wavelength was selected and monitored at 264 nm for hyperoside and 342 nm for the other analytes, which corresponded to their maximum absorption wavelengths and the injection volume was set at 20 µl.
Method validation:
The HPLC-DAD validation method was based on Association of Official Analytical Collaboration (AOAC) guidelines[42]. Analytical figures of merit were used to determine the linearity, sensitivity, precision and accuracy of the method. The linearity of the method was evaluated by performing standard-addition calibration graphs by spiking known concentrations of the standards into samples of C. azarolus, C. pallasii and Crataegutt® Tropfen drops and plotting the peak area vs. concentrations of standard solutions within the concentration range of 0-25.0 mg/l, with each measurement repeated three times (n=3).
Standard addition calibration curves were also used to evaluate the sensitivity of the method by calculating the Limits of Detection (LOD), using the equation 3.3 Sb/m and Limits of Quantification (LOQ) using the equation 10 Sb/m, where Sb is the standard deviation of the Y-intercept of the regression lines and m is the slope of the regression equation curve.
The method precision or reproducibility was expressed as Percentage Relative Standard Deviation (% RSD) at all concentrations of the analytes with intraday and interday precision and expressed as RSD of a series of measurements within the same day and different days respectively. To evaluate the accuracy of the method, addition-recovery tests were carried out by spiking samples of C. azarolus, C. pallasii and Crataegutt® Tropfen drops, with known concentrations of the standard of the analytes at three concentration levels (5.0, 10.0 and 15.0 mgl-1). The Percentage Relative Recoveries (% RR) were calculated. All analyses were carried out in triplicates.
Using single variable Analysis Of Variance (ANOVA), the p-values of the concentration of each analyte between pairs of selected samples of C. azarolus, C. pallasii and Crataegutt® Tropfen drops were compared.
Results and Discussion
Crataegus (hawthorn) is one of the most outstanding medicinal plants in phytotherapy and has been recently a topic of concern in the treatment of disease-related mainly to the cardiovascular system. Pharmacologically active metabolites are reported to be present in Crataegus extracts which are responsible for its activity namely flavonoids; hyperoside, vitexin, rutin and vitexin 2''-O-rhamnoside[12–24]. Therefore, phytopharmaceuticals intended to be produced from Crataegus species are standardized for the concentrations of these active metabolites according to that mentioned by the official pharmacopeias[8–11]. Since the interest in herbal medicine has been growing worldwide which has raised the international trade of herbal medicine and attracted pharmaceutical companies in commercializing herbal drugs, many international companies have taken over the production of drugs from Crataegus species. Various pharmaceutical dosage forms have been introduced into the international market from Crataegus, most well-known is Crataegutt® Tropfen the standardized extract of the leaves and flowers of C. monogyna and C. leavigata, manufactured by Dr. W. Schwabe Pharmaceuticals GmbH and Co. KG[25,26]. Therefore, this study aimed to develop a simple, fast, sensitive, accurate and reliable RP-HPLC-DAD analytical method for the identification and determination of the most important bioactive flavonoids from C. azarolus and C. pallasii in comparison with Crataegutt® Tropfen drops in a step to evaluate the possibility of other Crataegus species to produce Crataegus-derived pharmaceutical products that will meet the requirements of the pharmacopeias.
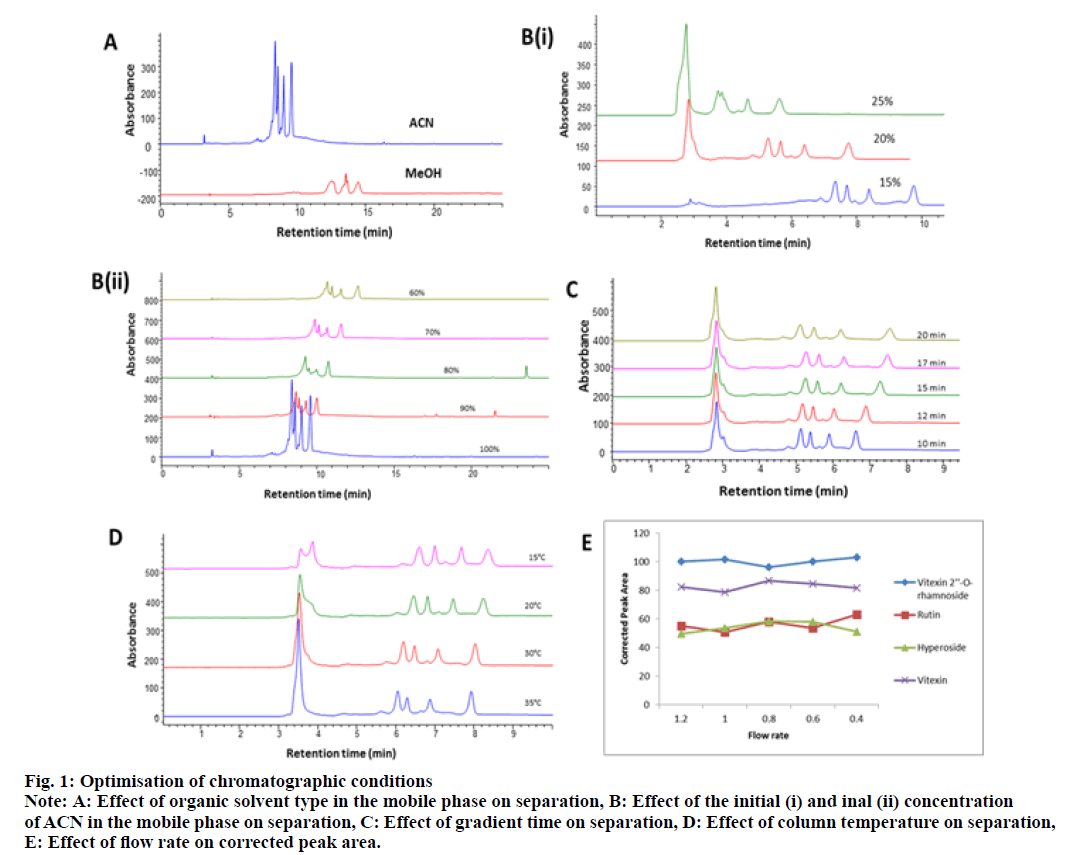
Optimisation of chromatographic conditions was carried out using a One-Factor-At-a-Time (OFAT) approach[40,41]. Short-chain aliphatic stationary phases (i.e., C4 and C8) were considered for preliminary experiments. A gradient scan using both columns with a mobile phase consisting of ACN and water over the range of 0 % to 100 % (v/v) ACN within 20 min revealed that C8 was more suitable for separation. Compared with methanol, ACN showed a better resolution and separation efficiency and hence, the latter was selected as the optimum mobile phase (fig. 1A). Preliminary experiments also revealed that isocratic elution was not possible. Optimum baseline resolution was obtained using a gradient composition starting from 20 % ACN to a final composition of 60 % ACN in water (fig. 1B). An optimum gradient time of 12 min was chosen based on peak efficiency and resolution (fig. 1C). The influence of column temperature was examined within the range of 15°-35°; optimum resolution and retention time were obtained at 20° for all analytical peaks (fig. 1D). Hence, this temperature was kept constant in subsequent experiments. The effect of flow rate was evaluated by plotting the corrected peak area against the flow rate. The effect was variable for the major peaks but there was a correlation between the analytical peaks having a maximum at 0.8 ml/min (fig. 1E). Optimum chromatographic conditions are summarized in Table 1.
| Variable | Optimum condition |
|---|---|
| Column | ACE5-C8, 4.6 mm ID×25 cm (5 µm) |
| Detector/wavelength | DAD, 264 nm for hyperoside and 342 nm for the other analytes, bandwidth: 16 nm |
| Injection volume (µl) | 20 |
| Mobile phase | (A) H2O: (B) ACN, 20 % B at 0 min to 60 % B at 12 min |
| Temperature | 20° |
| Flow Rate (ml/min) | 0.8 |
Table 1: Optimum Chromatographic Conditions
Fig. 1: Optimisation of chromatographic conditions
Note: A: Effect of organic solvent type in the mobile phase on separation, B: Effect of the initial (i) and final (ii) concentration
of ACN in the mobile phase on separation, C: Effect of gradient time on separation, D: Effect of column temperature on separation,
E: Effect of flow rate on corrected peak area.
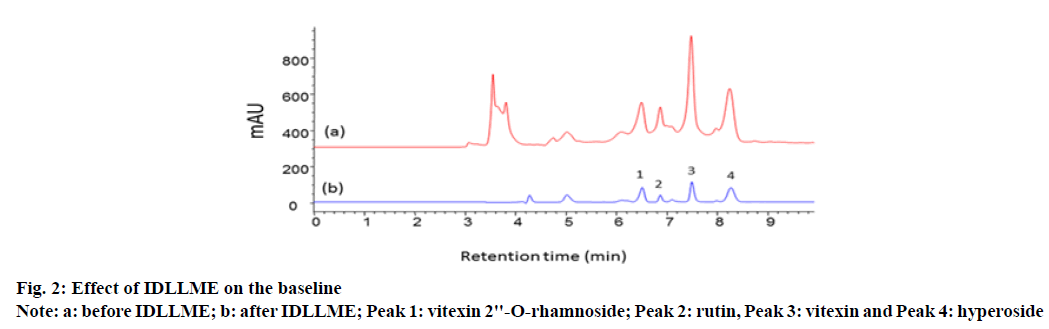
Initial chromatograms showed elevated baselines due to the matrix effect posing a risk for error in quantitation especially when the analytes were present at low concentrations near the LOQ. IDLLME was developed for the first time to overcome this problem. In this method, potential matrix constituents, which would interfere with the analytical peaks were extracted into the organic extraction phase, i.e., chloroform, while the analytes remained in the aqueous phase. The baselines obtained in both cases were compared. It was found that IDLLME was an efficient sample clean-up method, which significantly reduced the matrix effect as shown in fig. 2.
Method validation was based on AOAC guidelines[42]. Standard-addition calibration graphs were plotted by spiking known concentrations of the standards, vitexin 2''-O-rhamnoside, rutin, vitexin and hyperoside into the samples of C. azarolus, C. pallasii and Crataegutt® Tropfen drops and plotting the peak area in the y-axis vs. concentrations of standard solutions within the range of 0-25.0 mgl-1 in the x-axis, with each measurement repeated three times. The method showed good linearity with coefficients of determination (r2) more than 0.9950 in the range of the concentrations studied. The method also revealed good sensitivity by using standard addition calibration curves to calculate the limits of detection LOD and LOQ which ranged from 0.4 to 3.4 mgg-1 and from 1.3 to 11.3 mgg-1, respectively. The precision of the method was expressed as % RSD at all concentrations of the analytes with intraday and interday precisions ranging from 1.0 to 2.8 and from 1.5 to 4.3 respectively, which indicate good precision of the method. The method showed high accuracy based on the relative recovery of the analytes shown to be more than 98 % by spiking known concentrations of the standards (5.0, 10.0 and 15.0 mgl-1 into a fixed amount of samples of C. azarolus, C. pallasii and Crataegutt® Tropfen drops. Method validation parameters are indicated in Table 2.
| Analytes | ||||
|---|---|---|---|---|
| Paramater | Vitexin2''-O-rhamnoside | Rutin | Vitexin | Hyperoside |
| Regressiona equation | y=49.5 (±0.66) x+611.21(±9.93) | y=26.9 (±0.13) x+636.26 (±2.0) | y=26.5 (±0.24) x+552.8 (±3.59) | y=43.04 (±0.41) x+476.3 (±6.20) |
| r2 | 0.9970 | 0.9970 | 0.9990 | 0.9980 |
| LODb | 1.0 | 0.4 | 2.2 | 3.4 |
| LOQc | 3.3 | 1.3 | 7.3 | 11.3 |
| % RSDd (intraday) | 2.8 | 1.0 | 2.5 | 1.9 |
| % RSDd (interday) | 3.2 | 1.5 | 4.3 | 3.0 |
| Relative Recovery % RR Added (mgl−1) 5 10 15 |
100.73±1.02 98.21±2.55 98.67±1.90 |
100.09±0.13 99.53±0.67 99.75±0.35 |
98.95±1.50 98.91±1.55 99.48±0.74 |
99.40±0.85 99.95±0.07 101.62±2.28 |
Note: aPeak area=slope (±SD)×(Analyte (mgl-1))+intercept (±SD): bLimit of detection (mgg-1): cLimit of quantitation (mgg-1): dPercentage relative standard deviation, n
Table 2: Validation Parameters of RF-HPLC-DAD Method for Analyte Determination
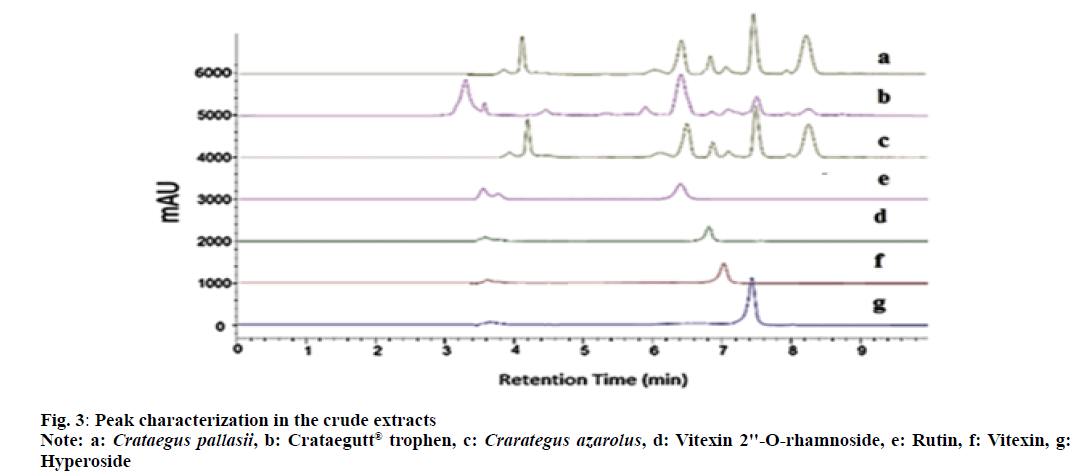
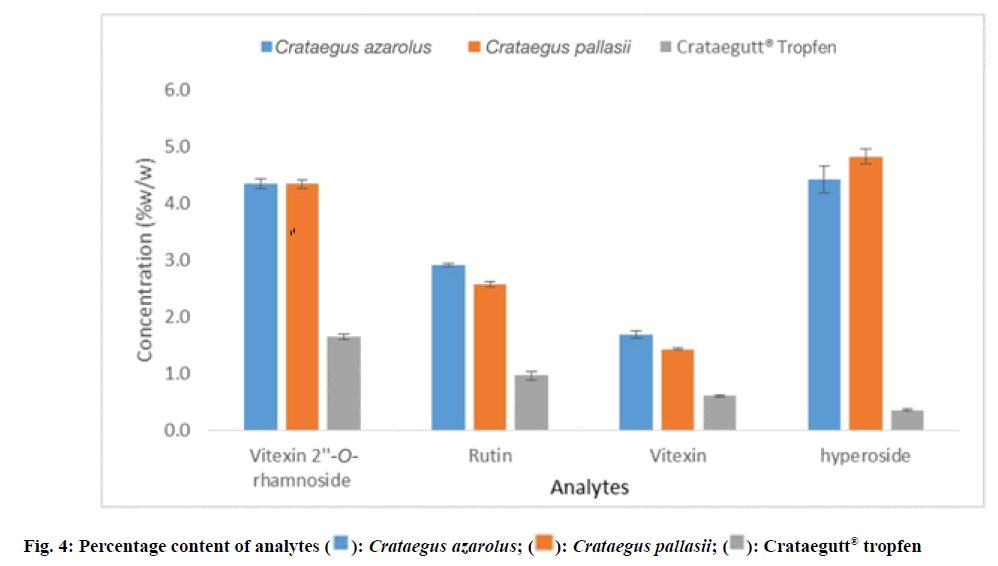
The chromatograms showed four peaks which were identified as vitexin 2''-O-rhamnoside, rutin, vitexin and hyperoside by comparing their retention times and the Ultraviolet (UV) spectra with those obtained with the standard compounds (fig. 2 and fig. 3). C. azarolus showed the highest concentration for rutin (2.9 %) and vitexin (1.7 %), C. pallasii with a concentration of (2.6 %) and (1.4 %) respectively. Whereas C. pallasii revealed the highest concentration of hyperoside (4.8 %) to C. azarolus (4.4 %). In consideration with the Crataegus phytomedicine Crataegutt® Tropfen, all analytes were in a lower concentration as compared with C. azarolus and C. pallasii (1.6 %, 1.0 %, 0.6 % and 0.4 %). These results are consistent with that of other researchers on Crataegus species in that hyperoside was the main constituent in addition to rutin, vitexin 2''-O-rhamnoside and vitexin[23]. While, following the USP Monographs 2009, the standardized extract of Crataegus leaf and flowers is required to contain not less than 0.6 % of C-glycosylated flavones, expressed as vitexin and not less than 0.45 % of O-glycosylated flavones expressed as hyperoside[8]. On the other hand, the European Pharmacopoeia 2008 states that the standardized hawthorn leaf and flower extract is required to contain at least 0.8 % to 3.0 % of flavonoids expressed as hyperoside[10]. Consonantly, with these requirements and comparing with the quantitative results obtained in this study related to the herbal product Crataegutt® Tropfen, the ethyl acetate extract of C. azarolus and C. pallasii met the criteria stated by the pharmacopeias. In addition, statistical analysis was carried out to compare the concentration levels of the analytes between the ethyl acetate extracts of C. azarolus, C. pallasii and phytomedicine Crataegutt® Tropfen. Using single variable ANOVA, the p-values of the concentration of each analyte between pairs of selected samples were compared as shown in Table 3. In general, p<0.05 for all comparisons indicates that there is a significant difference in the concentration of each analyte between the samples except for vitexin 2''-O-rhamnoside where p>0.005 indicates no significant difference between the pair of Crataegus species. The Percentage Mass concentration (% w/w) of the four analytes; vitexin 2''-O-rhamnoside, rutin, vitexin and hyperoside found in C. azarolus, C. pallasii and Crataegutt® Tropfen are given in Table 3 and fig. 4.
| Concentration (% w/w) | ||||
|---|---|---|---|---|
| Sample | Vitexin 2''-O-rhamoside | Rutin | Vitexin | Hyperoside |
| C. azarolus | 4.4±0.08 | 2.9±0.03 | 1.7±0.07 | 4.4±0.23 |
| C. pallasii | 4.4±0.08 | 2.6±0.05 | 1.4±0.02 | 4.8±0.12 |
| Crataegutt® Tropfen | 1.6±0.05 | 1.0±0.07 | 0.6±0.02 | 0.4±0.02 |
Table 3: Concentration of Analytes in the Real Samples
Recently, certain studies have also has been carried out to evaluate the effectiveness of herbal remedies in comparison to conventional drugs[43,44]. This study has been conducted in search of a new source of crude drug material to produce Crataegus-derived phytomedicine. A fast, simple, reliable and sensitive RP-HPLC-DAD method was proposed for the determination of the most important bioactive flavonoids in two non-investigated species of Crataegus, C. azarolus growing in Cyprus and C. pallasii growing in Libya in comparison with the phytomedicine Crataegutt® Tropfen. A novel, rapid and less solvent-consuming approach which could be applicable as a reference to other research including Crataegus extracts, based on DLLME with modifications termed ‘‘indirect-dispersive liquid- liquid microextraction’’ efficiently and significantly reduced matrix effect. The results obtained revealed that the percentage concentration of the flavonoids; vitexin 2''-O-rhamnoside, vitexin, rutin and hyperoside in C. azarolus and C. pallasii met the criteria set by the Pharmacopoeias recommended for preparations containing standardized extract of Crataegus flowers and leaves.
Acknowledgements:
The authors are thankful to Near East University for the financial support of this work (Project No: SAG-2017-01-017). Najat A. Agiel is thankful to the Higher Institute of Libyan Education for granting her the opportunity of a Ph.D. scholarship at Near East University. Special thanks to MSc. Fatma El-theera, for collecting the Crataegus pallasii samples from Libya.
Conflict of interests:
The authors have no conflict of interest to report.
References
- Ammon HP, Händel M. Crataegus, Toxikologie und Pharmakologie Teil II: Pharmakodynamik. Planta Med 198;43(11):209-39.
- Kaul R. Der Weissdorn:Wissenschaftliche Verlagsgesellschaft. Mbh Stuttgart; 1998.
- Ku SK, Kwak S, Kwon OJ, Bae JS. Hyperoside inhibits high-glucose-induced vascular inflammation in vitro and in vivo. Inflamma 2014;37(5):1389-400.
[Crossref] [Google Scholar] [PubMed]
- Koch E, Malek FA. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders-preclinical and clinical studies. Planta Med 2011;77(11):1123-8.
[Crossref] [Google Scholar] [PubMed]
- Weihmayr T, Ernst E. Therapeutic effectiveness of Crataegus. Fortschr Med 1996;114(1-2):27-9.
[Google Scholar] [PubMed]
- Holubarsch CJ, Colucci WS, Eha J. Benefit-risk assessment of Crataegus extracts WS 1442: An evidence-based review. Am J Cardiovasc Drugs 2018;18(1):25-36.
[Crossref] [Google Scholar] [PubMed]
- Tauchert M. Efficacy and safety of crataegus extract WS 1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am Heart J 2002;143(5):910-5.
[Crossref] [Google Scholar] [PubMed]
- United States Pharmacopoeia Offical Monographs. 1st ed; 2009. p.2771.
- Pharmacopeia G. Deutsches Arzneibuch (DAB). In: 2011th ed. Stuttgart: Deutscher Apotheker Verlag.
- European Pharmacopoeia 6.0. Ph Eur Monograph 1432: Hawthorn Leaf and Flower. In Strasbourg, Council of Europe, Strasbourg; 2008. p.2037.
- Commission CP. Pharmacopoeia of the People's Republic of China, vol. 1. PRC SPC of the editor. Bejing: China Medical Science Press; 2015; p 31-2.
- Meriçli AH, Ergezen K. Flavonoids of Crataegus tanacetifolia (Lam.) Pers.(Rosaceae), an endemic species from Turkey. Sci Pharma 1994;62:277-81.
- Tamer Ş, Birman H, Melikoğlu G, Meriçli AH. The comparative investigation of the leaf, flower and fruit extracts of Crataegus tanacetifolia and the medicinal species C. monogyna on their effects on the cardiovascular system. ACTA Pharma Sci 2000;42(1).
- Arslan R, Bor Z, Bektas N, Meriçli AH, Ozturk Y. Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thromb Rese 2011;127(3):210-3.
[Crossref] [Google Scholar] [PubMed]
- Özyürek M, Bener M, Güçlü K, Dönmez A, Süzgeç S, Pırıldar S, et al. Evaluation of antioxidant activity of Crataegus species collected from different regions of Turkey. Rec Nat Prod 2012;6(3):263-77.
- Topal G, Koç E, Karaca Ç, Altuğ T, Ergin B, Demirci C, et al. Effects of crataegus microphylla on vascular dysfunction in streptozotocin‐induced diabetic rats. Phytother Rese 2013;27(3):330-7.
[Crossref] [Google Scholar] [PubMed]
- Melikoglu G, Mericli F, Mericli AH. Flavonoids of Crataegus orientalis. Boll Chim Farmaceutico 1999;138:351-2.
- Melikoğlu G, Meriçli AH. Flavonoids of Crataegus stevenii. Die Pharmazie 2000;55(4):326-7.
[Google Scholar] [PubMed]
- Birman H, Tamer Ş, Melikoğlu G, Meriçli AH. Hypotensive activity of Crataegus tanacetifolia. J Pharm Istanbul Univ 2001;34(2):23-6.
- Birman H, Salmayenli N, Melikoğlu G, Meriçli AH. Effect of Crataegus tanacetifolia Extract on Total Body lon Concentration in Normal Rats. ACTA Pharma Sci 2003;45(3):215-9.
- Melikoğlu G, Bitiş L, MeriÇli AH. Flavonoids of Crataegus microphylla. Nat Prod Res 2004;18(3):211-3.
- Koçyõldõz ZÇ, Birman H, Olgaç V, Akgün‐Dar K, Melikoğlu G, Meriçli AH. Crataegus tanacetifolia leaf extract prevents L‐NAME‐induced hypertension in rats: A morphological study. Phytother Res 2006;20(1):66-70.
[Crossref] [Google Scholar] [PubMed]
- Sözer U, Dönmez AA, Meriçli AH. Constituents from the leaves of Crataegus davisii Browicz. Sci Pharma 2006;74(4):189-201.
- Koc E, Topal G, Dogan BS, Melikoglu G, Mericli AH, Karaca C, et al. Crataegus microphylla improves endothelial dysfunction in streptozotocin-induced diabetic rats. InFundamental Clin Pharmacol 2008;22:66.
- Elsadig KMG, Kuhnert N. UPLC-ESI-Q-TOF-MS/MS Characterization of phenolics from Crataegus monogyna and Crataegus laevigata (Hawthorn) leaves, fruits and their herbal derived drops (Crataegutt Tropfen). J Chem Biol Ther 2017;1(1):1-23.
- Veveris M, Koch E, Chatterjee SS. Crataegus special extract WS® 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci 2004;74(15):1945-55.
[Crossref] [Google Scholar] [PubMed]
- Venskutonis PR. Phytochemical composition and bioactivities of hawthorn (Crataegus spp.): Review of recent research advances. J Food Bioactives 2018;4:69-87.
- Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci 2007;30(18):3268-95.
[Crossref] [Google Scholar] [PubMed]
- Rezaee M, Assadi Y, Hosseini MR, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A 2006;1116(1-2):1-9.
[Crossref] [Google scholar] [PubMed]
- Ghalebi M, Tamizi E, Ahmadi S, Sheikhloo A, Nemati M. A Dispersive liquid-liquid micro-extraction technique for the pre-concentration and quantification of vitamin d3 in milk and yogurt samples using a non-aqueous HPLC method. Iran J Pharm Res 2019;18(2):677.
[Crossref] [Google Scholar] [PubMed]
- Lee YY, Shibamoto T, Ha SD, Ha J, Lee J, Jang HW. Determination of glyoxal, methylglyoxal, and diacetyl in red ginseng products using dispersive liquid-liquid microextraction coupled with GC-MS. J Sep Sci 2019;42(6):1230-9.
[Crossref] [Google Scholar] [PubMed]
- Ciftcioglu GC. Sustainable wild-collection of medicinal and edible plants in Lefke region of north cyprus. Agrofor Syst 2015;89(5):917-31.
- Viney DE. An Illustrated Flora of North Cyprus. Koeltz Scientific Books; 1994.p.240.
- Abu-Gharbieh E, Shehab NG. Therapeutic potentials of Crataegus azarolus var. eu-azarolus Maire leaves and its isolated compounds. BMC Complement Altern Med 2017;17(1):218.
[Crossref] [Google Scholar] [PubMed]
- Bahri-Sahloul R, Fredj RB, Boughalleb N, Shriaa J, Saguem S, Hilbert JL, et al. Phenolic composition and antioxidant and antimicrobial activities of extracts obtained from Crataegus azarolus L. var. Aronia (willd.) Batt. Ovaries calli. Journal of Botany 2014;623651.
- Mraihi F, Fadhil H, Trabelsi-Ayadi M, Cherif JK. Chemical characterization by HPLC-DAD-ESI/MS of flavonoids from hawthorn fruits and their inhibition of human tumor growth. J New Sci 2015;3:840–6.
- Mustapha N, Bzéouich IM, Ghedira K, Hennebelle T, Chekir-Ghedira L. Compounds isolated from the aerial part of Crataegus azarolus inhibit growth of B16F10 melanoma cells and exert a potent inhibition of the melanin synthesis. Biomed Pharmacother 2015;69:139-44.
[Crossref] [Google Scholar] [PubMed]
- Hamahameen BA, Jamal B. Determination of flavonoids in the leaves of hawthorn (Crataegus azarolus) of iraqi kurdistan region by HPLC analysis. Int J Biosci Biochem Bioinforma 2013;3(1):67-70.
- Jafri SMH and EL- Gadi A. Flora of Libya. 31st ed. Al Fatteh University, Faculty of Science, Department of Botany, Tripoli; 1977. p. 27-9.
- Sandford L, Shelver G. Using a design of experiments approach to develop fast LC methods for automated scale-up to preparative chromatography of sulfa drugs. Application Note, Fusion AE Method Develop 2009;95501:1-8.
- Sahu PK, Ramisetti NR, Cecchi T, Swain S, Patro CS, Panda J. An overview of experimental designs in HPLC method development and validation. J Pharma Biomed Anal 2018;147:590-611.
[Crossref] [Google Scholar] [PubMed]
- AOAC International. AOAC Official Methods of Analysis-Appendix K: Guidelines for Dietary Supplements and Botanicals; 2013:32.
- Afza S, Khan P, Rehman S, Ayub S, Singh R, Verma RS, et al. Comparative study of efficacy and safety of Unani coded drug UNIM-904 with allopathic drug amlodipine in the treatment of essential hypertension. IJUIM. 2021;5(1):01-08.
- Ahmad AS, Sharma R. Comparative analysis of herbal and allopathic treatment systems. Eur J Mol Clin Med 2020;7(7):2869-76.
 Crataegutt® tropfen
Crataegutt® tropfen