- *Corresponding Author:
- Lei Zheng
Department of Pharmacy, Shandong Provincial Third Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong 250031, China
E-mail: zhenglei8501@163.com
| Date of Received | 18 February 2020 |
| Date of Revision | 26 November 2021 |
| Date of Acceptance | 05 May 2022 |
| Indian J Pharm Sci 2022;84(3):543-551 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to standardize the clinical application of adjuvant drugs and control the unreason-able increase in drug costs. Management measures were formulated for adjuvant drug utilization by promoting the rational use of adjuvant drugs. The efficacy and economics of the same types of drugs were compared to provide a scientific reference for clinical drug selection. In 2018, the total cost of the use of adjuvant drugs decreased by 7.4117 million renminbi compared with that in 2015. In the first half of 2019, the proportion of revenue from adjuvant drugs decreased by 10.77 % from that in the same period in 2015. From 2015 to 2018, the per capita drug cost of adjuvant drugs gradually decreased and in 2018, this resulted in savings of 568.41 renminbi. Through administrative intervention, prescription review, information management and pharmaco-economic evaluation, a scientific management system of adjuvant drugs was established to standardize the use of adjuvant drugs and to reduce their cost. The scientific management of adjuvant drugs can avoid unnecessary drug expenses for patients and provide effective reference values for the rational use of adjuvant drugs in hospitals.
Keywords
Adjuvant drugs, drug cost, evidence-based medicine, information management, rational drug use
With the development of disease rejuvenation and modern medical treatment, medical expenses have increased annually. Drug expenses account for a large proportion of this increase. However, the use of adjuvant drugs is widespread and is growing rapidly because of their wide indications and commercial promotions. Prescription analysis revealed that severe unreasonable drug-use problems exist, resulting in an economic burden on the patients and considerable pressure on the medical reimbursement establishments[1]. Therefore, promoting the rational use of adjuvant drugs is the key to reduce drug costs. Furthermore, many drugs of the same type exist and the criteria to use scientific methods for the comprehensive evaluation of the drugs of the same type should be set so that doctors can scientifically select drugs[2]. Since 2015, we have promulgated the scientific management of the adjuvant drugs based on evidence-based medicine and achieved considerable progress in this field. This study aimed to assess the outcomes of the clinical pharmacist intervention in the irrational use of adjuvant drugs and construct a sustainable and improved model of adjuvant drug management.
Materials and Methods
Establishing management organisation for adjuvant drugs and formulating principles for their use:
The medical and pharmaceutical departments in our hospital jointly established a working group for the supervision and management of the use of the drugs and formulated measures for their rational management. The responsibilities of the joint team included the division of responsibilities for each department, determination of the scope of the adjuvant drugs for catalogue, regular analysis of the dosage, reasonable comments and formulation of specific reward and punishment measures. The key point was to determine the basic principles of the clinical application of the adjuvant drugs and define the use of key drugs according to evidence-based medical practice.
According to PubMed’s Medical Subject Headings (MeSH) thesaurus of the National Medical Library of the United States, adjuvant drugs are defined as “the drugs that help to increase the effect of the main therapeutic drugs or increase their efficacy by influencing the absorption, mechanism of action and metabolism of the main therapeutic drugs or drugs that help to prevent and treat diseases or functional disorders”[3]. In accordance with this definition, we included the following drugs in our study: Drugs enhancing the tissue metabolism, vitamins, electrolytes, enteral and parenteral nutrition, neurotrophic drugs; free radical scavenging drugs; traditional Chinese medicine for promoting blood circulation and removing stasis; auxiliary treatment drugs for liver disease; auxiliary treatment drugs for tumours, and other auxiliary treatment drugs.
Determination of the criteria for the evidence-based evaluation of the adjuvant drugs:
For adjuvant drugs, the drug instructions provided by the manufacturer formed the main evaluation standard. For medication beyond the instructions, we referred to the treatment guide of each specialised disease and used a database to search the literature to determine any available evidence. The decision-making analysis was used to obtain opinions and decisions from clinical experts regarding usage with objections or clinical application effects but without the support of high-level evidence. Finally, according to the aforementioned evidence, we preliminarily defined the indication, dosage, solvent selection, course of treatment, contraindications and combination of drugs. We also focused on the evaluation and classification of the evidence-based quality and established an evaluation criteria based on the evidence level and did not refer to unqualified documentary evidence. Currently, clinical studies that can be retrieved through the database mainly include randomised controlled trials, cohort studies, case-control studies, series of case studies, case reports, traditional reviews and expert opinions or experiences. The widely accepted evidence classification standards are mainly the evidence classification standards of the evidence-based medicine centre at the Oxford University and the Grade standard formed by combining various classification standards (the standards for evidencebased assessment are detailed in Table 1)[4].
| Type of evidence | Evaluation essentials |
|---|---|
| Guide | Whether the literature has been reviewed comprehensively in the past 12 mo, whether the supporting evidence for each recommendation has been marked with the level and the source. When determining the clinical application according to the level of recommendation, if a treatment is recommended as a class, it can be used without contraindications, it can be used as a Class B recommendation, but it is noted that the evidence is not sufficient and it can be used or not when the reason is sufficient, we should pay attention to the publication of new evidence at any time. If it is a recommendation of Class C or D, it indicates that the evidence is more lacking, with greater uncertainty and it is clear that the auxiliary treatment drugs are unavailable. |
| Meta-review | Whether it is a systematic evaluation of randomised controlled trials, whether it has collected and included all relevant studies, whether it has evaluated the quality of a single trial, whether it has homogeneity among trials, whether it is meaningful, that is, how large and accurate the effect is, and the reliability and application value of its conclusions can be judged according to the evaluation of the authenticity and significance of the results of systematic evaluation. |
| Randomised controlled trial | High-quality evidence, but if there are limitations, inconsistent results, not providing direct evidence, inaccurate results and biased reports, the level of evidence will be decreased. The quality level of evidence will be improved if the observational study is designed rigorously, implemented well, found to be of significant efficacy or there is a dose-response relationship. |
| Expert consensus | To determine whether expert opinions are reliable, this is mainly based on whether their opinions have sufficient evidence basis. If there is no evidence, it can be questioned. In the absence of research evidence, the consensus reached by multiple experts is relatively more reliable than that of individuals. For rare or complex conditions without research evidence, expert opinions have more important reference value. |
Table 1: Evaluation Criteria
Using information technology to design an adjuvant drug management system:
We entered the drug use definition of each adjuvant drug into the information platform and preliminarily determined whether the adjuvant drugs in the medical order are reasonable according to this definition. Reminding the doctors immediately when they give orders, such as ‘contraindications reminder’, ‘overdose reminder’ and ‘over-treatment reminder’ is critical. By contrast, clinical pharmacists review them immediately. Special drug evaluation and rationality analysis should be conducted monthly, and the analysis and evaluation results should be publicised and linked with the performance evaluation.
Comprehensive evaluation of adjuvant drugs of the same type:
Clinical pharmacists select auxiliary drugs with similar indications or functions for comparison and evaluation and provide suggestions for clinical evaluation. In this study, a group of similar drugs (edaravone injection and monosialotetrahexosylganglioside sodium injection (GM1)) were used as examples for evaluation.
A systematic literature review adopts the general principles of Population Intervention Comparison Outcomes and Study (PICOS) to establish the inclusion criteria of effective literature search, including patient Population (P), Intervention measures (I), Control measures (C), Outcome indicators (O) and Study design (S)[5]. This study mainly includes drug specification, enterprise information, PubMed, China National Knowledge Infrastructure (CNKI), other literature retrieval databases, drug-related disease information network (www.cdidin.com), the International Network for Rational Use of Drugs (INRUD) clinical safety drug monitoring network (http://inrud.cdidin.com/), official notification information (such as information published on Food and Drug Administration (FDA) and Catalog of Federal Domestic Assistance (CFDA) websites), and other network information. The determination of the criteria for the evidence-based evaluation of the adjuvant drugs was used to evaluate the quality of the literature.
Drug efficacy evaluation:
By using literature analysis methods and target drugs as the keywords, we searched for relevant evidence-based diagnosis and treatment guidelines and system evaluation/meta-analysis, extracted the recommendation of target drugs and related research conclusions, tabulated the data and conducted descriptive evaluation and analyses.
Drug safety evaluation:
Based on the keywords ‘target drug’, ‘adverse reaction’, ‘adverse event’ and ‘safety’, the following information and data were retrieved: Safety information including toxicology, carcinogenesis, teratogenicity and reproductive toxicity, adverse reaction, contraindications, precautions, special population (pregnant and lactating women, children, the elderly and patients with liver or kidney function damage), drug interaction, drug overdose and safety differences among ethnic groups, was put in the market before the drug; adverse events and adverse reactions after listing; measures by the government, such as withdrawal, warning and modification of instructions.
Drug economic evaluation:
According to the commonly used amount of each drug, we calculated the daily average cost and the cost of each treatment course for each auxiliary drug by referring to the catalogues of the national basic drugs, national basic medical insurance, industrial injury insurance and maternity insurance drugs and the supplementary catalogue for the provincial/municipal medical insurance, and compared the situations for which the drugs are listed in the aforementioned catalogues before conducting the factor Analysis of Variance (ANOVA).
Evaluation of the management effect:
The cost of adjuvant drugs (total cost, per capita drug cost, proportion of auxiliary treatment drug cost in total drug cost) was statistically analysed and Statistical Package for the Social Sciences (SPSS) 21.0 software was used for single-factor analysis.
Results and Discussion
Formulation of clinical application principles of adjuvant drugs was explained here. The management group should integrate all expert opinions and discuss with the pharmaceutical management professional committee to determine the principles of clinical application of adjuvant drugs. The general principle is as follows: When adjuvant drugs are used, the ratio of the drug cost and curative effect should be completely considered, the least and the most economical drugs should be used to achieve the expected treatment purpose, and special attention should be paid to the use of Chinese patent medicine injections (Table 2 show the specific clinical application principles).
| Content | Specific requirement |
|---|---|
| Indication | It is not allowed to approve the indications for drug use beyond the drug specification. The indication medication shall be approved in strict accordance with the drug instructions. The indications for approval of over-specification must be evaluated by evidence-based medicine and recorded in accordance with the relevant provisions of over-specification drug use. The compound preparation of the two components should be carried out when suffering from two main diseases at the same time, otherwise it should not be used as the first drug. |
| Usage and dosage | Do not overdose. The single dose and daily dose shall not be higher than the recommended dose in the manual. Strictly follow the recommended administration frequency, solvent and infusion concentration in the instructions. |
| Do not exceed the course of treatment. Do not exceed the minimum number of days of treatment specified in the instructions. If a course of treatment is not prescribed in the instructions or evidence-base, it shall not exceed 7 d. | |
| Combined medication | Adjuvant drugs of the same type cannot be used in combination. Under non-special circumstances, each patient can only use one type of adjuvant drug (classified by pharmacological action and indication, no matter whether oral or injection). Special cases only include those where there are professional guidelines or authoritative evidence to recommend joint application. |
| Contraindication | Do not use contraindications and do not use adjuvant drugs with drug risks. |
| Rational use of traditional Chinese medicine injection | Oral administration is not suitable for injection administration. It should be used in strict accordance with the functional indications specified in the drug manual. Traditional Chinese medicine used for promoting blood circulation and removing blood stasis has a wide range of pharmacological effects, but to avoid adverse drug reactions, it is stipulated that those with bleeding tendency shall not use this kind of medicine; this kind of medicine shall not be used in combination with non-steroidal anti-inflammatory drugs or platelet inhibition drugs. This kind of medicine belongs to the category of traditional Chinese medicine. While mastering its modern pharmacological action, it should be used according to the syndrome differentiation of patients tongue and pulse signs to play its best role and effect, so as to combine traditional Chinese medicine and Western medicine in an appropriate manner. |
Table 2: Clinical Application Principles of Adjuvant Drugs
We have defined the drugs included in the list of adjuvant drugs and provided supporting data and classification for the drugs beyond the instructions. Table 3 presents an example of drug definition for the adjuvant drugs [6,7].
| Drug name | Indications and limitations | Contraindication | Single maximum dose Maximum daily dose Maximum administration frequency Course of treatment Compatibility of solvents |
Interactions and attention |
|---|---|---|---|---|
| Tanshinone II: A sodium sulphonate injection | The use for the auxiliary treatment of coronary heart disease, angina pectoris and myocardial infarction. | Patients with a history of allergy to these drugs | 80 mg 80 mg 1 time only 7 d 5 % glucose injection or 0.9 % sodium chloride injection 250-500 ml |
Alprostadil can enhance the efficacy and the cardiac function should be closely monitored when the two drugs are combined. |
| Troxerutin cerebroprotein hydrolysate injection | The compound preparation of the two components should be selected when suffering from two main diseases at the same time, otherwise it should not be used as the first drug. | Severe renal insufficiency is forbidden. It is forbidden for patients with epileptic persistent state or grand mal. |
10 ml 10 ml 1 time only 20 d 250-500 ml 0.9 % sodium chloride injection or 5 % glucose injection |
It should not be used with balanced amino acid injection. Adverse interactions with antidepressants can lead to inappropriate stress. At this time, it is suggested to reduce the dosage of antidepressants[6]. |
| Vinpocetine injection | To improve the symptoms induced by cerebral infarction, cerebral haemorrhage and cerebral arteriosclerosis. | In the acute stage of intracranial haemorrhage, those who have not completely stopped bleeding after intracranial haemorrhage are forbidden. Those who have serious ischemic heart disease and serious arrhythmia are forbidden. |
30 mg 30 mg 1 time only 5 % glucose or 0.9 % sodium chloride injection Use no more than 14 d |
When vinpocetine and methyldopa are used together, there is a slight synergistic effect on the latter, so it is suggested to monitor blood pressure when they are used together. Although there is no interaction between vinpocetine and drugs acting on nervous system, or antiarrhythmic and anticoagulant drugs at the same time in clinical research, it is still recommended to pay attention to the observation when using vinpocetine in combination[7]. |
Table 3: Definition of Adjuvant Drugs
Clinical pharmacists use information technology to establish the use of adjuvant drugs, such as limiting the drug prescription of departments and diseases. When setting the course of treatment beyond the norm, realtime reminders can be provided at the doctor’s office, critical information reminders can be provided regarding patient’s specific medication and irrational medication requirements frequently in special circumstances and irrational medication can be controlled in real-time when prescribing.
By referring to the aforementioned definitions and evidence base, clinical pharmacists take the top ten adjuvant drugs based on consumption over each month for special prescription review, and the review results will be fed back into the system. Severe unreasonable drug use is assessed according to the management methods prescribed by the committee. The main problems are over-indication, overdose, over-course of treatment and contraindications.
To standardize the use of drugs with respect to severe irrational and large amounts of use, such as proton pump inhibitors and human albumin, we formulated the hospital-use specification according to the literature and embedded a clinical pathway to assist doctors in drug selection decisions.
Efficacy evaluation and pharmacoeconomic analysis of adjuvant drugs of the same type is described below. We finally included three guides[8-10], two meta-reviews[11,12], two expert consensus reports[13,14] and three other highquality reports[15-17]. Furthermore, the cost and medical insurance status of the two drugs were compared. The comprehensive evaluation results of edaravone injection and GM1 are presented in Table 4.
| Evaluation items | Conclusion |
|---|---|
| Efficacy | Edaravone is preferred in the treatment of brain injury and stroke; GM1 is preferred in the treatment of central nervous system tumor like demyelinating lesions for nerve repair and cervical spondylosis. |
| Safety | Edaravone: The incidence of adverse reactions was 4.57 %. The main manifestations are abnormal liver function, increased liver enzyme (incidence>5 %), rash, red tide, red blood cell reduction, leukocytosis, leucopenia, thrombocytopenia, proteinuria, hematuria, etc. Japan’s Ministry of health, labour and welfare reported that the incidence of edaravone aggravating acute renal insufficiency or renal failure was about 0.02 %. Can cause liver and kidney function damage aggravation, liver and kidney function damage patients use with caution. Heart disease, elderly patients (over 80 y old) should be used with caution. Pregnant and lactating women are prohibited. |
| GM1: The common adverse reactions were shivering, fever, rash, dyspnea, palpitation, vomiting, etc. It is found that acute inflammatory demyelinating polyneuropathy (also known as Guillain Barre syndrome) may be caused by post marketing monitoring of drugs at home and abroad. The patients with hereditary glycolipid metabolism abnormality and Guillain Barre syndrome are forbidden. | |
| Economy | Both of them are not national essential drugs, edaravone belongs to class B of the national medical insurance catalogue and GM1 does not belong to the national medical insurance catalogue. Edaravone is the first choice for the patients with medical insurance; Edaravone (20 ml/30 mg) has lower cost per day and per course of treatment than GM1 (20 mg/2 ml). Edaravone is preferred under the same conditions. |
Table 4: Comprehensive Evaluation of Edaravone Injection and GM1
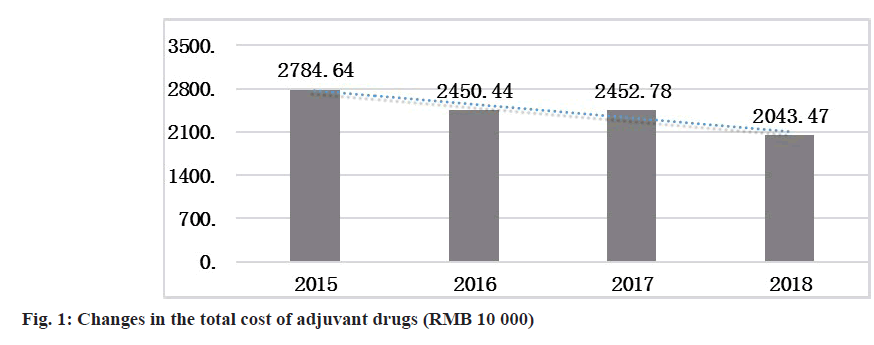
To evaluate the overall effect, the consumption of adjuvant drugs was assessed from multiple perspectives, including the total amount used, the proportion of income of adjuvant drugs and the cost of adjuvant drugs per capita. In 2018, the total cost of the use of adjuvant drugs decreased by 7.4117 million Renminbi (RMB) compared with that in 2015 (fig. 1).
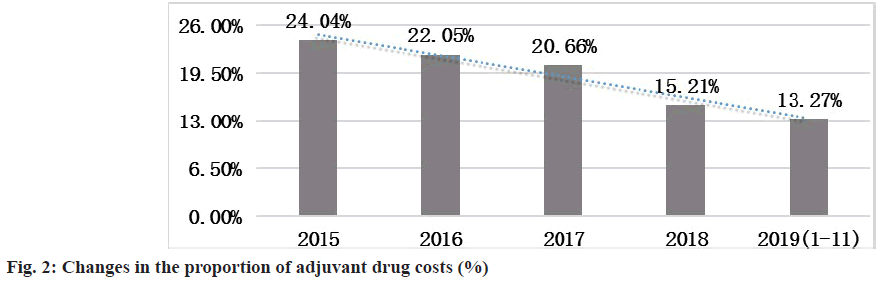
In the first half of 2019, compared with the same period in 2015, the proportion of revenue from adjuvant drugs (revenue from adjuvant drugs/total drug use) decreased by 10.77 % (fig. 2).
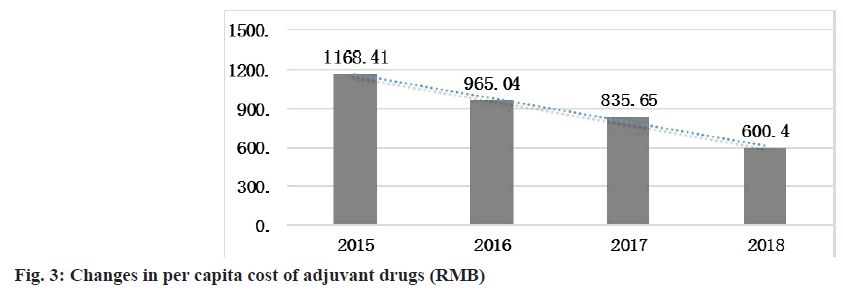
From 2015 to 2018, the per capita drug cost of adjuvant drugs gradually decreased and in 2018, this decrease resulted in savings of 568.41 RMB compared with that in 2015 (fig. 3).
SPSS 21.0 software was used for one-way ANOVA to assess the per capita adjuvant drug cost of inpatients. The data include the per capita adjuvant drug cost of inpatients in 4 y (2015-2018) and 48 mo, which is expressed in the form of mean±standard deviation (x±s). From the results in fig. 3, the per capita cost of adjuvant drugs for inpatients exhibited a downward trend in 2015-2018. Based on the results of one-way ANOVA, the p-value is less than the significance level of 0.01, which is statistically significant, indicating that the per capita cost of adjuvant drugs for inpatients decreased significantly in these 4 y (Table 5).
| Item | Sample | Inpatients (RMB) | Outpatients (RMB) | Minimum value (RMB) | Maximum value (RMB) |
|---|---|---|---|---|---|
| 2015 | 12 | 1169.1114 | 136.7361 | 794.19 | 1316.60 |
| 2016 | 12 | 965.0380 | 103.8738 | 820.03 | 1100.97 |
| 2017 | 12 | 835.6525 | 84.9700 | 701.28 | 998.66 |
| 2018 | 12 | 600.4793 | 64.2809 | 421.09 | 670.70 |
| Total | 48 | 892.5703 | 230.2923 | 421.09 | 1316.60 |
| F value | 66.715 | ||||
| p value | 0.000 |
Table 5: Single Factor Anova Results of Per Capita Adjuvant Drugs Cost of Inpatients
Evidence-based medicine should be used to scientifically manage all the drugs that satisfy the definition of adjuvant drugs to improve the safety of drug use and end the use of contraindications and reduce unreasonable drug use, including over-indications. The exploration and formation of an adjuvant drug information management platform to improve the work efficiency and coverage, and the formation of a new management mode have been realised. We compared the pharmacotherapeutics and pharmacoeconomics of the same adjuvant drugs with large dosage to provide scientific references for clinical drug selection.
This study focused on evaluating the drug use scientifically and reasonably in the management of adjuvant drugs. The use of evidence-based medicine to evaluate the rationality of drug use can provide cuttingedge safe and rational drug use services for clinical use and resolve the issues facing the multiple drug delivery schemes at present, which cannot be evaluated in terms of efficacy and economics. This result can strengthen the medical practice regarding the use of adjuvant drugs and improve the comprehensive level and affect their management[18,19]. Next, we used information technology to adapt to the actual workings of the existing design to expand the coverage of drug evaluation for saving time and effort. Advocating the use of information technology to manage rational drug use is also one of the highlights of drug use management in our hospital. Finally, through the collection of drug efficacy cases for the purposes of economic comparison, if a scientific and orderly evaluation standard for the same types of drugs is established, the selection of clinical drugs can provide suitable suggestions for clinical needs and gradually achieve the ultimate goal of individualised drug use and refined drug treatment.
In April 2019, China’s Health Commission issued a notice on drug use monitoring and clinical comprehensive evaluation, which requires detailed evaluation of drug use and applies the evaluation results to the improvement of local medical support system, clinical diagnosis and treatment service quality. We have used a decision-tree, namely Markov model, to simulate and analyse the drug use. Considering the long-term cost-effectiveness during the realworld study observation period, the cost-effectiveness analysis based on the prospective or retrospective cohort studies, the establishment of intervention groups and control groups, and collection of cost and clinically effective data for analysis, studies have focused on improving the scientific comprehensive evaluation of drugs according to the general principles of PICOS at home and abroad.
Adjuvant drugs are the agents that aid or increase the action of the principal drug (drug synergism) or drugs that affect the absorption, mechanism of action, metabolism or excretion of the primary drug (pharmacokinetics) to enhance its effects. It is commonly used in the prevention or treatment of cancer, liver, cardiovascular and cerebrovascular diseases. Appropriate use of adjuvant drugs is beneficial for the recovery of patients from the disease and it can not only shorten the time of hospitalisation but also reduce the cost of hospitalisation so that the country’s medical resources can be more effectively allocated. Conversely, it can increase the risk of adverse drug reactions because of the increased use of combination of drugs or unnecessary use of drugs. It can increase the risk of new adverse effects, prevent the rehabilitation of the original diseases, prolong the length of stay and increase the economic burden on patients and the healthcare system.
Strengthening the management of rational drug use and reducing the economic burden of patients has become a public concern. The management of adjuvant drugs has become a critical part of the management of rational drug use. The current excessive use of adjuvant drugs can not only easily lead to an increased incidence of adverse drug reactions and bodily damage but also increase the economic burden on patients, resulting in wastage of medical resources. In 2017, the office of the Chinese state council issued a number of opinions on reforming and improving the policy on drug production and circulation. The document requires monitoring the use of antibiotics, adjuvant drugs and nutritional drugs, publicising the limits on irrational prescriptions, and establishing an interview system.
The clinical use of adjuvant drugs has been standardized by the scientific management system of adjuvant drugs based on the prescription review, administrative intervention, information management and pharmacoeconomic evaluation. The use of adjuvant drugs in our hospital has become increasingly standardized, the level of drug treatment has improved, the proportion of adjuvant drugs has decreased and the unnecessary drug expenses for patients have been saved. This study has practical and effective reference value for the scientific management of adjuvant drugs in other hospitals.
Author’s contributions:
Lei Zheng and Zhihao Yang contributed equally to this work.
Funding:
This study was supported by the Key projects of China Pharmaceutical Association for promoting the dissemination of precision medicine science and technology (Grant No. CMEI2019KPYJ(JZYY)00204); the special fund project for clinical research for therapeutic drug monitoring of the Shandong Medical Association (YXH2020ZX047), Project of Shandong ADR monitoring center (2021SDADRKY03), the special fund project for clinical pharmacy scientific research of the Shandong Medical Association (YXH2019ZX016) and Shandong traditional Chinese medicine science and technology project (2019-0329, 2021-M199).
Acknowledgements:
We acknowledge the editors and the anonymous reviewers for insightful suggestions on this work. This study was funded and supported by the Chinese Pharmaceutical Association. We would also like to acknowledge our outstanding colleagues working on the front lines of our hospital.
Conflict of interests:
The authors declared no conflict of interest.
References
- Han S. The application status of adjuvant drugs in our country and the management countermeasures study. Chin Pharm J 2016:678-82.
- Herxheimer A. Educating doctors to use drugs well. Br J Clin Pharmacol 1976;3:111-2.
[Crossref] [Google Scholar] [PubMed]
- U.S. National Library of Medicine. Adjuvants, Pharmaceutic. NLM drug information portal, MeSH Database; 2016.
- Steurer J, Roner S, Gnannt R, Hodler J. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: A systematic literature review. BMC Musculoskelet Disord 2011;12(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Zhào H, Liu Y, Zeng J, Li D, Huang Y. Troxerutin cerebroprotein hydrolysate injection ameliorates neurovascular injury induced by traumatic brain injury via endothelial nitric oxide synthase pathway regulation. Int J Neurosci 2018;128(12):1118-27.
[Crossref] [Google Scholar] [PubMed]
- Yu-Xing YI, Zhang P, Wang R. Observation on the effect of vinpocetine injection in treatment of the elder cerebral infarction. Chin J Mod Drug Appl 2013;83:327-31.
- Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, et al. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med 2009;103(3):434-41.
[Crossref] [Google Scholar] [PubMed]
- Neuroimmunology group of neuroimmunology branch of Chinese society of immunology, neuroimmunology group of neurology branch of Chinese medical association, neuroimmunology group of neurology professional committee of science and technology committee of Chinese people's liberation army. Diagnosis and treatment guide of central nervous system tumor like demyelinating disease. Chin J Neuroimmunol Neurol 2017;5:23-90.
- Jin H, Wang S, Hou L, Pan C, Li B, Wang H, et al. Clinical treatment of traumatic brain injury complicated by cranial nerve injury. Injury 2010;41(9):918-23.
[Crossref] [Google Scholar] [PubMed]
- Wu S, Sena E, Egan K, Macleod M, Mead G. Edaravone improves functional and structural outcomes in animal models of focal cerebral ischemia: A systematic review. Int J Stroke 2014;9(1):101-6.
[Crossref] [Google Scholar] [PubMed]
- Xiaochen W, Liqin Z, Chunge W, Qi D, Xin L. Systematic review of the efficacy and safety of gangliosides in preventing chemotherapy-induced peripheral neurotoxicity. Chin J Clin Pharmacol 2015;24:2462-4.
- Min L, Zhen-yu T, Xiao-ping S. Efficacy and safety of low molecular weight heparin combined with edaravone in the treatment of progressive cerebral infarction: A systematic review. J Evid Based Med 2013;13(4):218-24.
- Ruxiang X. Expert consensus on neurological damage and repair of brain injury. Chin J Neurotrauma Surg 2016;2;100-4.
- Department of neurological injury, Traumatology branch of Chinese medical association. monosialic acid tetrahexose ganglioside sodium salt injection: Expert consensus on treating patients with brain and spinal cord injury. Chin J Traumatol 2010;1:6-8.
- Shu-cheng G, Zhan-you X. Edaravone: Good outcome as supplementary treatment for patients presenting with crescendo transient ischemic attack. J Chin Clin Med 2009;4(8):444-7.
- Jin W, Lisheng Z, Li Y, Chen Jun, Shengfeng W, Jian Z, et al. Therapeutic effect of monosialic acid tetrahexose ganglioside sodium combined with edaravone on acute cerebral infarction. Evaluation and analysis of drug-use in hospitals of China 2018;169:61-2.
- Liao X, Zhang Y, Xie YM, Liu Y, Yi DH, Zhang YL, et al. Analysis of Diemailing Kudiezi injection use in real world in 7189 patients with cerebral infarction. China J Chin Mater Med 2016; 41(23):4442-50.
[Google Scholar] [PubMed]
- Yang J Zheng L Chen L, Song C. Establishment of clinical use management mode of adjuvant therapy drugs in our hospital. China Pharm 2017;28:3545-8.
- Yang J, Zheng L, Guan Y, Song C. Analysis of the impact of antimicrobial management and rational use of antibiotics. Eur J Hosp Pharm 2020;27(5):286-91.