- *Corresponding Author:
- M. Khushtar
Department of Pharmacology, Integral University, Lucknow, 226026, India
E-mail: mohdkhushtar@gmail.com
| Date of Received | 10 March 2021 |
| Date of Revision | 07 January 2022 |
| Date of Acceptance | 13 February 2023 |
| Indian J Pharm Sci 2023;85(1):176-188 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Recently, the percentage of Peptic ulcer disease not linked to either Helicobacter pylori or non-steroidal anti-inflammatory drugs has increased and signifies the prominent role of psychophysiological stress in the establishment and advancements of gastric ulcers and other peptic ulcer diseases. The current study was intended not only to develop post-treatment swim stress-induced peptic ulcer disease rat model but also to analyze the curative effects of hydro-alcoholic extract of Triphala in swim stress-induced peptic ulcer disease model. A post-treatment swim stress-induced peptic ulcer disease rat model was developed followed by therapeutic intervention of hydro-alcoholic extract of Triphala. The gross evaluation of gastric tissues showed that swim stress induces significant gastric ulcers in rats that could clearly be observed after 21 d of self-healing. Further, the findings of our interventional investigations revealed that hydro-alcoholic extract of Triphala exerts significant gastro-protective activity in swim stress-induced peptic ulcer disease via decreasing the ulcer index and increasing the protective gastric mucus content, whereas, the level/activities of catalase, superoxide dismutase, glutathione and malondialdehyde were also ameliorated after the administration of Triphala extract in experimental peptic ulcer disease model. In addition, the findings from our biochemical investigations are also well corroborated by histopathological observations. In conclusion, the current study demonstrates that swim-stress results in the development of gastric ulcers and damages the gastric mucosa along with the altered redox homeostasis in rats and Triphala extract exerts significant curative effects in posttreatment swim-stress-induced peptic ulcer disease rat model and may later be investigated and promoted for human clinical applications.
Keywords
Triphala extract, swim stress-induced gastric ulcer, ulcer index, mucus content, malondialdehyde, catalase, superoxide dismutase, oxidative stress.
Peptic Ulcer Disease (PUD) is a long-lasting medical condition affecting a significant proportion of the world’s population[1,2]. The development and the pathogenesis of PUD are influenced by the gastric juice pH and the compromised mucosal-defense[1,3]. Among others, consumption of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and encounter with Helicobacter pylori (H. pylori) are the most widely accepted and studied risk factors for PUDs[4]. Although Warren and Marshall won Noble prize in 2005 for proving the role of H. pylori in PUD[5], the percentage of ulcers independent to either H. pylori or NSAIDs has augmented and this affects the selection as well as efficacy of classical therapeutic regimens used for PUD treatment. Studies indicated that many people developed PUD without having H. pylori infection and NSAIDs history[2,6]. Such ulcers are idiopathic and a lot unmanageable to therapy[4]. Development of PUD without H. pylori is also emphasized by the fact that many H. pylori positive people never developed PUDs[4,7,8]. Conversely, H. pylori infection dropped in response to better control measures but the incidences of PUD are still rising, thus making the impact of H. Pylori in the pathogenesis of PUD less prominent[5,9]. There are clear evidences that psychophysiological stress contributes in etiology of these types of ulcers and association of PUD with stress is strong enough not to be ignored. A study indicated that stress plays a role in 30 %-65 % of PUD cases[6,10]. Distinct stress strategies have been implied to induce PUD in rodents and other animal models that are identical to the pathogenesis of human PUDs[11]. However, studies in both humans and animal models confirmed that oxidative stress potentiates the pathogenesis of PUDs[12,13]. Among distinct strategies i.e., blockers of H2-receptor and proton pumps and anticholinergics, ranitidine has been widely accepted in targeting PUD but it elicits various adverse effects and drug interactions to different cellular components[13-16]. Therefore, various herbal formulations have been assessed for their pharmacological effects, particularly, gastrointestinal ailments[13,17-19].
In contrast, Triphala standing amongst the most common remedies in the Ayurveda, is formulated by mixing of Terminalia chebula Retz (T. chebula), Emblica officinalis Gaertn (E. officinalis) and Terminalia bellirica Roxb (T. bellirica) fruits[12,20]. Triphala is prescribed to treat different gastrointestinal features[21-25] and for its laxative properties of smoothening the stool or fastening the movement of undigested food, diminishing the acidity and regulating the appetite[26]. Triphala is rich in the phytoconstituents i.e., ascorbic acid, gallic acid, chebulinic acid, tannic acid, syringic acid, bellericanin, epicatechin, ellagic acid, and chebulic acid[25,27,28]. Triphala powder has been found effective against cold[29] and noise stress[30] and these effects were attributed to antioxidant potential of Triphala. Further, the generation of free radicals has been strongly associated with stress-induced peptic ulcers[31]. As oxidative imbalance triggers the NSAID- induced injury to gastric mucosa and Triphala is rich in antioxidants[32], here an attempt has been made to develop a post-treatment swim stress-induced PUD rat model followed by the investigation of anti-ulcer/ gastro-protective effects of Triphala extract.
Materials and Methods
Chemicals or reagents:
5,5′-Dithiobis (2-nitrobenzoic acid) (DTNB) and Carboxy Methyl Cellulose (CMC) were purchased from Fisher Scientific, Navi Mumbai, India and Sigma-Aldrich, USA, respectively. Alcian blue and Trichloro Acetic Acid (TCA) were obtained from SD Fine Chem. Ltd., India, whereas, H2O2 was supplied by Loba Chemie Pvt. Ltd, India. The ranitidine was acquired from Cipla Pvt. Ltd, India. Pyrogallol and rest of the analytical grade chemicals were supplied by Himedia, India.
Experimentations:
Preparation of Triphala extract: The dried fruits of three Triphala constituent plants i.e., E. officinalis, T. bellirica and T. chebula were obtained from Lucknow, U.P. (India) and identified by the Faculty of Pharmacy at our University vide accession numbers IU/PHAR/ HRB/14/03, IU/PHAR/HRB/14/04, IU/PHAR/ HRB/14/05, respectively. These fruits were separately grounded and Triphala formulation was obtained by mixing equal proportions of the powdered fruits from each of the above mentioned plants as described in recent report[12]. Triphala extract was prepared by extraction of this Triphala formulation (approx. 25-30 g) through Soxhlet apparatus in 70 % ethanol (Ethanol/ dH2O:70/30) and the extraction was repeated until a colorless condensate appeared in the apparatus[33,34]. Thus, obtained extract was filtered, evaporated and stored at 4° for future use.
Criteria for Triphala extract dose selection: The dose selection for Triphala extract in the current study was based on already published reports demonstrating that the Triphala extract up to 2000 mg/Kg.B.Wt. is safe in rodents[12,22,27]. These higher doses of Triphala showed potent pharmacological activities against distinct pathologies and none of these studies reported any adverse events. Even such higher doses protected the histoarchitecture in sciatic nerve, intestinal as well as hepatic tissues. Moreover, all the constituents of Triphala stand amongst the most commonly used dietary components in Asian counterparts.
Preparation of ranitidine suspension: The reference standard drug ranitidine was dispersed in 1 % CMC suspension and 50 mg/Kg. B.Wt./d was orally administered to rats in respective group[12,35].
In vivo studies:
Animals: The Wistar rats (male 0.2-0.25 Kg) were habituated to the animal house settings for 7 d with unrestricted food and water intake prior to the experimental study. The rats were housed in standard cages (12 h light/dark cycle; 22±2°; humidity 50±15 %). The current in vivo study was dully approved by IAEC of Integral University (Approval no. IU/ Pharm/Ph.D/CPCSEA/12/02).
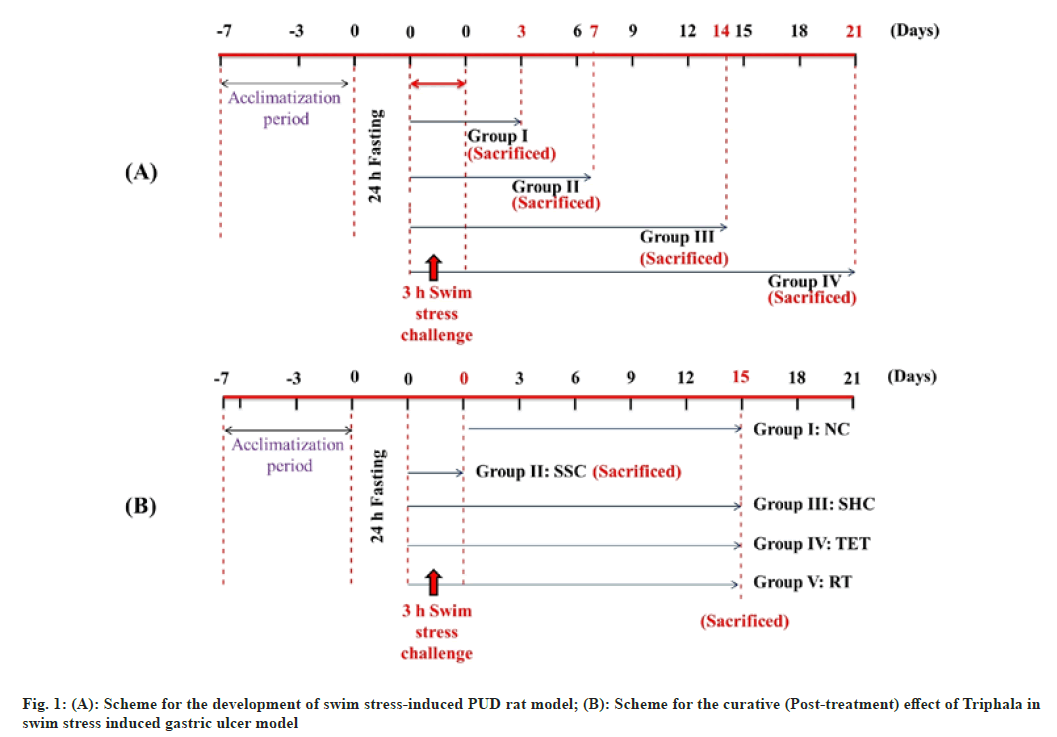
Development of swim stress-induced PUD model: Before inducing the disease, animals were fasted for 24 h with unrestricted drinking water intake in wire mesh cages during fasting to avoid coprophagy. These fasted rats were then forced to swim for 3 h inside the vertical cylinders (height 30 cm; diameter 15 cm; containing water up to 15 cm height; maintained at 23±2°)[36,37] (fig. 1A). The water level was reduced accordingly in order to avoid the drowning of rats. Following the induction procedure, the animals from Group I, II, III and IV were sacrificed by cervical dislocation on 3rd, 7th, 14th and 21st d respectively. Following the sacrifice, the excised stomach from each group was opened through an incision made along the greater curvature and inspected for formation of swim-stress-induced lesions/ ulcer. After confirming the induction of gastric ulcers and self-healing in Group I, II, III and IV, we selected Group III (sacrificed on 14th d) for further therapeutic interventional studies as we observed significant index of gastric ulcers in this group. In contrast, only minute ulcers were seen in Group IV (sacrificed on 21st d) that could have been completely disappeared on administration of standard drug or Triphala extract; making their comparison difficult without visible ulcers.
Scheme for the curative (post-treatment) effect of Triphala in swim stress-induced PUD model: Following the development of gastric ulcer model, the swim stress challenge was repeated for the analysis of the curative effect of Triphala extract along with ranitidine. In this order, twenty-five rats were divided into 5 groups (n=5). 24 h fasted rats were subjected to swim stress exposure as mentioned in previous section except in control group. The rats were removed from the cylinders and feeding and dosing was started. Group I (Normal Control (NC)): Rats received 1 ml/Kg.B.Wt./d oral dose of 1 % CMC; Group II (Swim Stress Control (SSC)): Rats were sacrificed by cervical dislocation immediately after stress procedure on 1st d; Group III (Self-Healing Control (SHC)): Stress challenged rats left untreated till the end of the study; Group IV (Triphala Extract Treated (TET)): Rats received 1000 mg/Kg.B.Wt./d of Triphala extract through gastric intubation and Group V (Ranitidine treated (RT)): Rats received 50 mg/Kg.B.Wt./d of Ranitidine. Following the therapeutic intervention for 15 d, the rats of each group (except SSC; already sacrificed) were fasted for 24 h and sacrificed by cervical dislocation (fig. 1B).
Collection and processing of gastric tissue:
After the prescribed interventional period, the stomach from the sacrificed animals of each group were promptly excised, weighed and washed with ice cold saline (0.9 % NaCl) and stored at 20° till further use. A portion of the gastric tissue was stored in neutral formalin (10 %) for the block preparation.
Homogenization and subcellular fractionation to form Post Mitochondrial Supernatant (PMS):
Portions of gastric tissues were homogenized either in chilled PBS or 0.02 M Ethylenediamine Tetraacetic Acid (EDTA) for distinct biochemical assays using Remi homogenizer (RQ-127-A). Thus, obtained tissue homogenates were filtered with a muslin cloth and centrifuged at 3000 rpm for 10 min to precipitate the nuclear debris. The resulting aliquots were again centrifuged at 10 500 xg for 30 min to get PMS that was required for the estimation of activities of different enzymatic antioxidants.
Calculations for ulcer index:
Excised stomachs from each group were inspected for gastric lesions and indexed as described elsewhere[11,35]. The opened stomachs were placed onto a dissecting tray in such a way so that the greater curvature was exposed clearly and mucosa was checked by a 10x magnifying glass. The lesions appeared mostly in the antrum region of stomach. The ulcers were totaled and their scoring was performed accordingly. Usual coloration was assigned zero, redness was assigned 0.5, individual spot was assigned 1, streak-like hemorrhage was assigned 1.5, deep ulcers were assigned 2 and perforation was assigned 3. The formula used for the calculations of the ulcer index is appended below:
[Ulcer index=UN+US+ UP×10-1]
Where, UN stands for the average no. of ulcers found; US stands for average no. of severity score; and UP denotes the percentage animals having ulcers.
Estimation of gastric mucus:
A section of stomach rich in mucosal glands was stored into deep freezer at -20° following the washing with the chilled physiological saline for the determination of gastric mucus. These tissues from each group were immersed instantly into the 0.1 % solution of alcian blue that was prepared by using 160 mM sucrose in 50 mM CH3COONa and the pH was adjusted to 5.8. 2 h later, the samples were washed with 250 mM sucrose solution in order to remove the unbound dye. After ensuring the removal of unbound dye, the dye-gastric wall mucus complex was dissolved for 2 h in 500 mM magnesium chloride with gentle shaking at regular intervals. Thus, obtained blue solution was shaken dynamically with (C2H5)2O (v/v) and optical density of aqueous layer was taken following the centrifugation at 3000 rpm as described previously. The content of mucus was determined via plotting a calibration curve of varying concentrations of alcian blue[38].
Estimation of lipid peroxide:
The content of Malondialdehyde (MDA) was determined by TBA method as described recently[39,40]. Briefly, 1 ml of the tissue homogenate (prepared in 0.05 M PBS) was mixed with 500 µl of TCA and later on 500 µl of 0.8 % TBA reagent was also added and incubated at 80° for 30 min in dark. After that samples were placed in chilled water followed by centrifugation at 845.32 xg for 15 min. The optical density of thus obtained supernatant was read at 540 nm on Eppendorf Ultraviolet-Visible (UV-VIS) spectroscope. The amount of MDA was determined using the formula:
MDA (nmol)=(OD×Volume of test solution)/0.156
Estimation of Glutathione (GSH) in gastric tissue:
The level of GSH, a non-enzymatic antioxidant, was estimated using previously described standard method[41]. The gastric tissue was homogenized in 20 mM EDTA solution followed by mixing of 50 % TCA and further homogenization, thorough vortexing and then centrifugation at 3381.3 xg. The supernatant was mixed with 400 mM Tris buffer and 10 mM DTNB. The OD of the resulting mixture was taken at 415 nm in order to calculate the GSH content and represented as μg/mg protein.
Estimation of catalase activity:
The activity of Catalase (CAT), an enzymatic antioxidant was measured in gastric tissue PMS via following previously described protocol[33]. Briefly, 50 µl PMS was placed into the quartz cuvette that was pre-filled with 2.95 ml of 20 mM H2O2. The activity of CAT, characterized by conversion of H2O2 into H2O and O2, was monitored through Eppendorf UV-VIS spectroscopy at 240 nm. The CAT activity in gastric PMS was determined using the following formula:
Catalase activity=(Decline in OD per min×Reaction volume)/(0.081×homogenate volume×protein (mg))
Estimation of Superoxide Dismutase (SOD) activity:
Apart from the CAT, the SOD activity was assessed by using the standard protocol[42]. Briefly, 0.1 ml of PMS was mixed with 2.9 ml of Tris HCl and subsequently 25 µl of pyrogallol was added and the variation in OD was monitored at 420 nm. The formula used for the calculation of SOD activity is
SOD (units/ml sample)=[(A–B)×100]/(A×50)
(Where, A=∆OD in control per min; B=∆OD in each test sample per min)
Histopathological evaluation:
At the end of the interventional protocol, a piece of excised stomach was conserved in 10 % neutral buffered formalin solution. The same were embedded in paraffin and the blocks were sectioned followed by double staining with hematoxylin and eosin and microscopic inspection at the magnifications of 100x and 400x[33,34].
Statistical analysis:
Triplicate samples were used for all the experiments and data was represented as mean±SEM. The level of significance was assessed through ANOVA and later on by Post-Hoc Tukey-Kramer test. GraphPad Prism V 4.02 was used for this analysis[43-45].
Results and Discussion
The management of PUD through validation of various therapeutic agents requires the development of suitable animal models but the availability of very few of such ulcer models has delayed the establishment of targeted therapy of gastric ulcers. The most commonly used ulcer models are developed either by psychophysiological, pharmacological (by ethanol, acetic acid, histamine, reserpine and NSAIDs i.e. indomethacin, aspirin and ibuprofen or surgical methods[11]. In the same vein, we for the first time developed a post-treatment swim stress- induced rat PUD model by forcing them to swim for 3 h inside the vertical cylinders. At the end of the induction and self-healing period the stomach from each sacrificed rat was examined for the development of swim stress- induced lesions/ulcer. Gross microscopic examination confirmed that the rats from all four groups developed significant gastric ulcers (data not shown). After self- healing analysis, we selected group III (sacrificed on 14th d) for determination of curative effect of Triphala extract as lesions were easily visible in this group even after 14 d of self-healing.
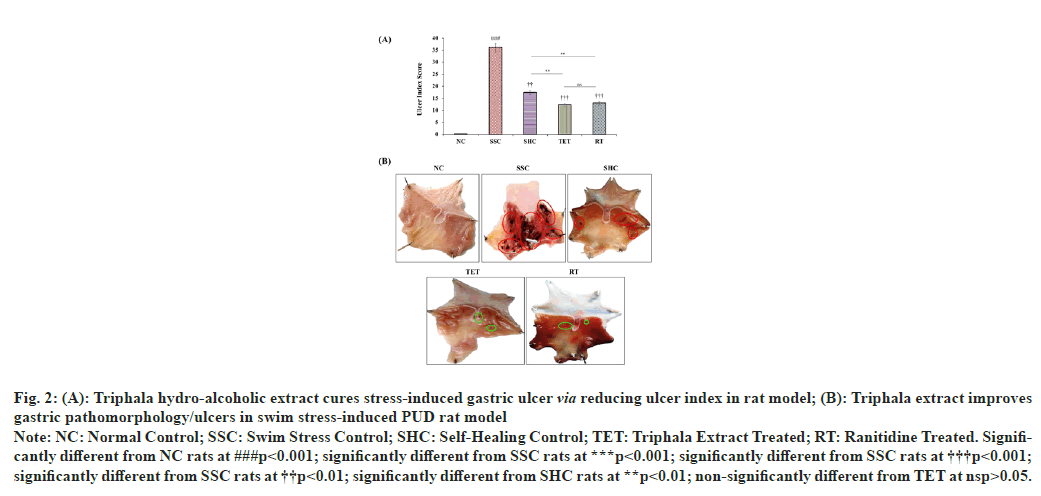
The gastric ulcer index was significantly (p<0.001) higher (from 0.3±0.02 to 36.13±0.25) in SSC group after 3 h swimming stress, when compared to NC rats. The observations from SHC group revealed that the ulcer index was reduced to 17.4±0.85 at the end of the study, when left untreated after swim stress challenge. However, the administration of hydro-alcoholic extract of Triphala for 15 d significantly (p<0.001) diminished the ulcer index by 65.98 % and 29.37 % in TET group, when compared to corresponding SSC and SHC group respectively. These observations reveal that treatment with Triphala extract facilitates the speedy recovery in swim stress challenged SHC rats. The treatment with reference standard drug (RT) also significantly (p<0.001) ameliorated the gastric ulcer index by 63.88 % and 25 %, when compared to ulcer index in SSC and SHC groups, respectively (fig. 2).
Fig. 2 :(A): Triphala hydro-alcoholic extract cures stress-induced gastric ulcer via reducing ulcer index in rat model; (B): Triphala extract improves
gastric pathomorphology/ulcers in swim stress-induced PUD rat model
Note: NC: Normal Control; SSC: Swim Stress Control; SHC: Self-Healing Control; TET: Triphala Extract Treated; RT: Ranitidine Treated. Significantly
different from NC rats at ###p<0.001; significantly different from SSC rats at ***p<0.001; significantly different from SSC rats at †††p<0.001;
significantly different from SSC rats at ††p<0.01; significantly different from SHC rats at **p<0.01; non-significantly different from TET at nsp>0.05.
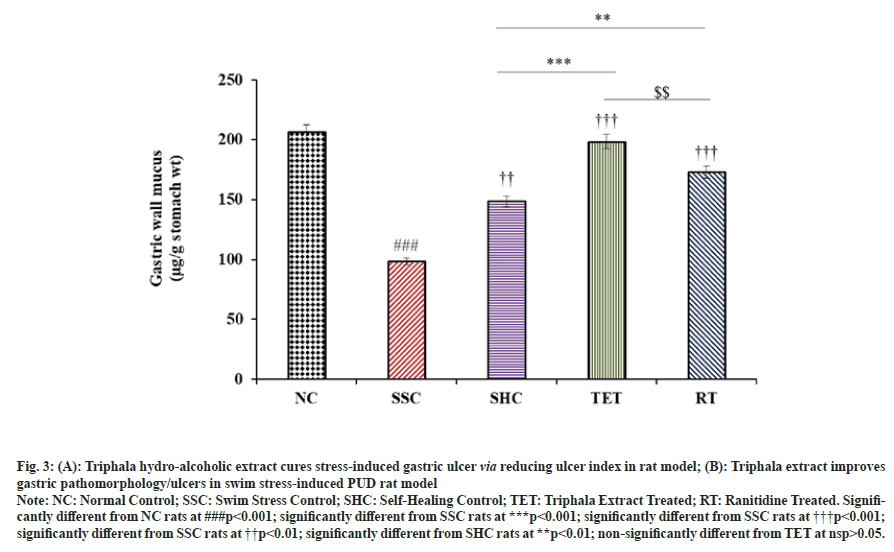
Our results showed that the mucus content was significantly (p<0.001) decreased from 206.35±3.34 to 98.38±2.31 µg/g stomach wt. (-52.32 %) in SSC group, when compared to corresponding NC rats. We observed a slight improvement in gastric wall mucus content in untreated SHC rats (148.61±2.26). In contrast, the treatment with Triphala extract significantly (p<0.001) elevated the gastric wall mucus by 50 % and 33.57 % in TET group, when compared to corresponding SSC and SHC rats respectively. In contrast, the mucus content in TET group was almost equal to that of NC group (nsp>0.05), which signifies the curative effect of TET against swim stress-induced peptic ulcers. In addition, administration of standard drug also showed a marked increase in the mean gastric wall mucus in RT group, when compared with SSC and SHC group (fig. 3).
Fig. 3: (A): Triphala hydro-alcoholic extract cures stress-induced gastric ulcer via reducing ulcer index in rat model; (B): Triphala extract improves
gastric pathomorphology/ulcers in swim stress-induced PUD rat model
Note: NC: Normal Control; SSC: Swim Stress Control; SHC: Self-Healing Control; TET: Triphala Extract Treated; RT: Ranitidine Treated. Significantly
different from NC rats at ###p<0.001; significantly different from SSC rats at ***p<0.001; significantly different from SSC rats at †††p<0.001;
significantly different from SSC rats at ††p<0.01; significantly different from SHC rats at **p<0.01; non-significantly different from TET at nsp>0.05.
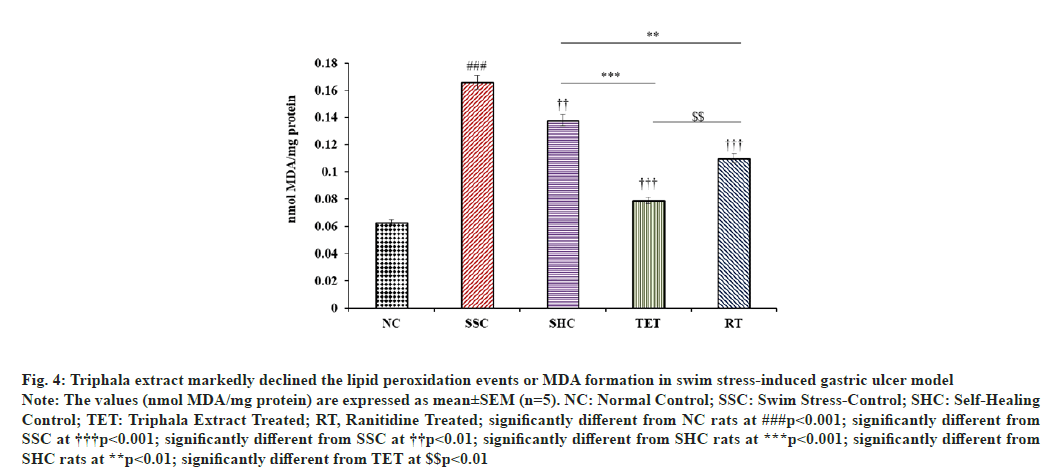
The levels of MDA in gastric mucosa was significantly (p<0.001) increased from 0.063±0.011 to 0.166±0.02 nmol MDA/mg protein in SSC group, when compared to MDA content in NC group. However, MDA content in self-healing rats was reduced only by 16.86 % at the end of the interventional study, when compared to the MDA level in SSC rats. In contrast, subsequent administration of Triphala extract lead to a marked decline in MDA content in gastric mucosa (-52.41 %), when compared to SSC group. Most strikingly, the treatment of Triphala extract speeded up the pace of amelioration of MDA content in PUD rat model (16.86 % to 42.75 %), when compared to untreated SHC rats. Likewise, the treatment with reference drug significantly diminished the gastric mucosal MDA content in RT group by 33.73 % and 20.29 %, when compared with SSC and SHC groups, respectively. In contrast, the curative impact of Triphala on MDA content in swim-stress-induced PUD model was greater than that of the standard drug, when compared to both SSC and SHC group (fig. 4).
Fig. 4: Triphala extract markedly declined the lipid peroxidation events or MDA formation in swim stress-induced gastric ulcer model
Note: The values (nmol MDA/mg protein) are expressed as mean±SEM (n=5). NC: Normal Control; SSC: Swim Stress-Control; SHC: Self-Healing
Control; TET: Triphala Extract Treated; RT, Ranitidine Treated; significantly different from NC rats at ###p<0.001; significantly different from
SSC at †††p<0.001; significantly different from SSC at ††p<0.01; significantly different from SHC rats at ***p<0.001; significantly different from
SHC rats at **p<0.01; significantly different from TET at $$p<0.01
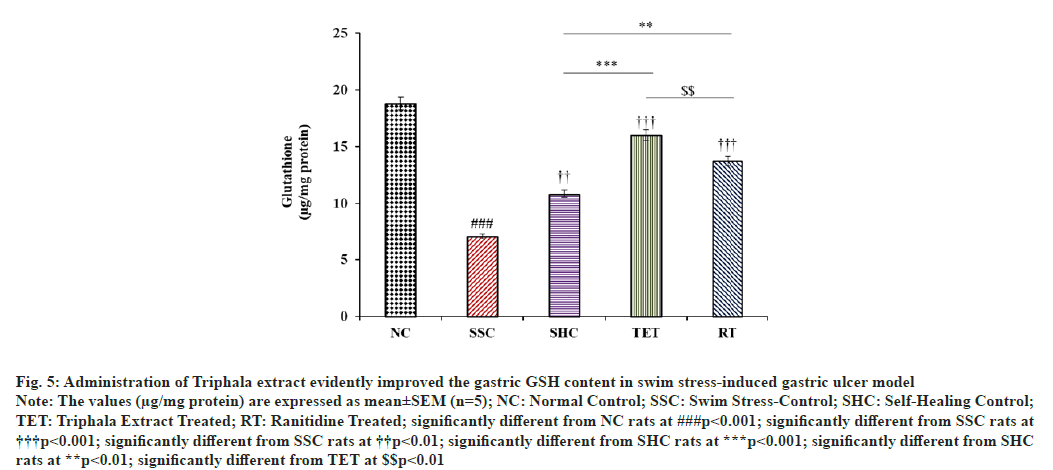
In the current study, we observed that the level of non- enzymatic antioxidant i.e., GSH in gastric tissue was significantly diminished by 62.41 % in SSC rats after 3 h swim stress challenge, when compared to NC rats. Self- healing of PUD model for designated duration resulted in comparatively lesser improvement in GSH content (52.75 %), when compared to SSC rats. However, treatment with Triphala extract significantly potentiated the alleviation in the gastric GSH content by +126.59 %, when compared to SSC rats and this improvement in GSH level in TET group was 1.48 folds higher than that of reported in untreated SHC group. Further, treatment with RT also elevated the gastric glutathione content by +94.48 % and + 27.07 %, when compared to SSC and SHC rats. In contrast, the effect of TET on gastric GSH content in swim stress-induced gastric ulcer model was better than that observed in RT treated rats (fig. 5).
Fig. 5: Administration of Triphala extract evidently improved the gastric GSH content in swim stress-induced gastric ulcer model
Note: The values (μg/mg protein) are expressed as mean±SEM (n=5); NC: Normal Control; SSC: Swim Stress-Control; SHC: Self-Healing Control;
TET: Triphala Extract Treated; RT: Ranitidine Treated; significantly different from NC rats at ###p<0.001; significantly different from SSC rats at †††p<0.001; significantly different from SSC rats at ††p<0.01; significantly different from SHC rats at ***p<0.001; significantly different from SHC
rats at **p<0.01; significantly different from TET at $$p<0.01
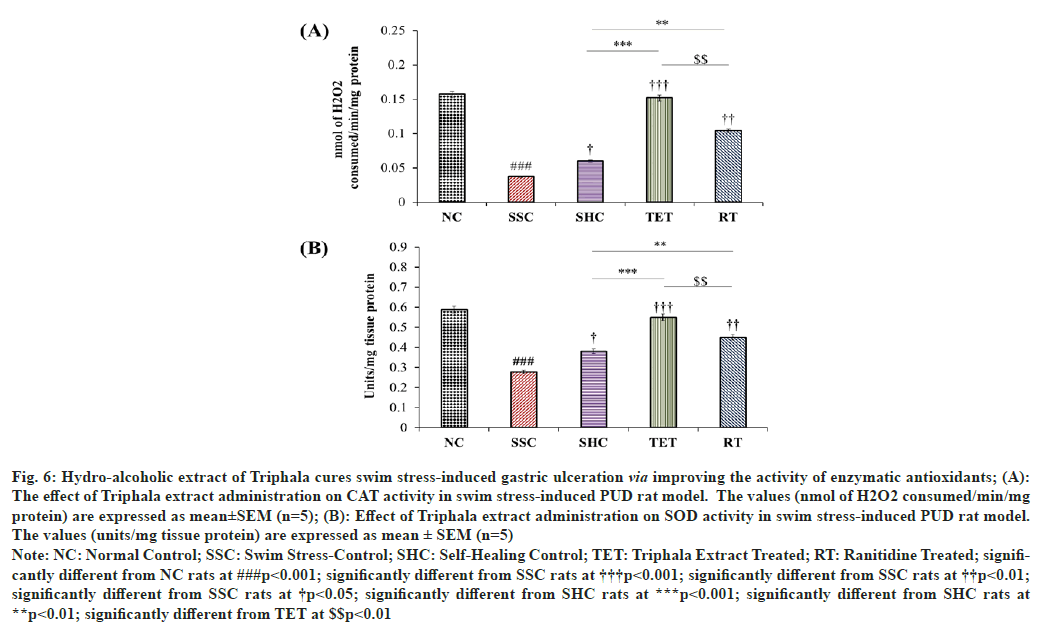
In addition to the non-enzymatic antioxidant (GSH) level, the activity of enzymatic antioxidant components was also estimated in the current interventional study. As a result, we observed that the activity of CAT in gastric tissue was significantly declined by 76.43 %, when compared to SSC group. The effect of self-healing of PUD rat model till the end of the interventional study showed a slight elevation in the gastric CAT activity (1.62 folds), when compared to the CAT activity in corresponding SSC group. However, supplementation of Triphala extract significantly potentiated the gastric CAT activity with an increase of 310.81 %, when compared to SSC group, as well as the effect of TET on CAT activity was also found to be 2.53 folds higher than that of reported in SHC group. Most fascinatingly observation to be noted in this case is the effect of Triphala extract brought the CAT activity up to the level that found in NC rats (nsp>0.05). The treatment with RT also alleviated the gastric CAT activity with an increase of 181.08 % and 73.33 %, when compared to SSC and SHC rats respectively, but these effects were not as significant as reported in TET group (fig. 6A).
Fig. 6: Hydro-alcoholic extract of Triphala cures swim stress-induced gastric ulceration via improving the activity of enzymatic antioxidants; (A):
The effect of Triphala extract administration on CAT activity in swim stress-induced PUD rat model. The values (nmol of H2O2 consumed/min/mg
protein) are expressed as mean±SEM (n=5); (B): Effect of Triphala extract administration on SOD activity in swim stress-induced PUD rat model.
The values (units/mg tissue protein) are expressed as mean ± SEM (n=5)
Note: NC: Normal Control; SSC: Swim Stress-Control; SHC: Self-Healing Control; TET: Triphala Extract Treated; RT: Ranitidine Treated; significantly
different from NC rats at ###p<0.001; significantly different from SSC rats at †††p<0.001; significantly different from SSC rats at ††p<0.01;
significantly different from SSC rats at †p<0.05; significantly different from SHC rats at ***p<0.001; significantly different from SHC rats at
**p<0.01; significantly different from TET at $$p<0.01
Similarly, 3 h swim stress challenge also led to a marked decline in gastric SOD activity by 52.72 %, when matched to corresponding NC rats. Although, the rats left for SHC showed a mild elevation in the gastric SOD activity by 36.69 % in PUD model, when compared to SSC rats, but the amelioration in SOD activity in SHC group was nowhere comparable to the SOD activity reported in NC rats. In contrast, the treatment with Triphala extract markedly increased the activity of gastric SOD by 97.54 %, when compared to SSC group. Statistical analysis showed that TET also potentiated the self-healing process as SOD activity was raised by 1.45 folds, when compared to the SOD activity in SHC rats. However, no significant difference was reported in the SOD activity among the rats of NC and TET group, which further signifies the curative effects of this extract in targeting stress-induced PUD. Further, the treatment with RT also improved the SOD activity in gastric tissue but the effect was not comparable to that observed in TET group (fig. 6B).
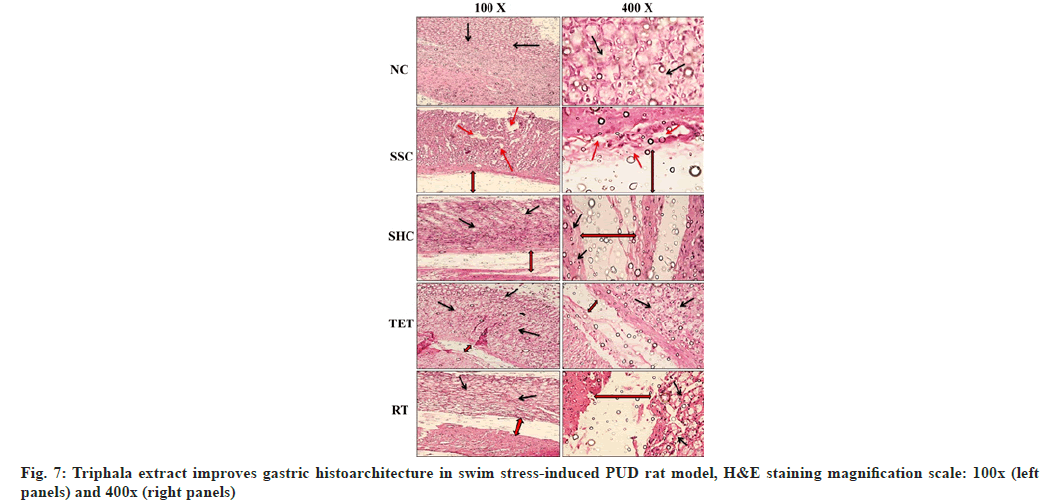
Apart from the biochemical parameters, our H&E staining studies revealed that the gastric histoarchitecture was well maintained in NC rats at the end of the study. In contrast, the histopathological observations in SSC group showed significant destruction to gastric mucosa, the surface epithelium and oedema of the sub-mucosal layer. The micrographs from the untreated rats left for self-healing also exhibited a markedly disrupted gastric mucosa, surface epithelium and oedematous gaps of the sub-mucosal layer. However, the treatment with Triphala extract cured the swim stress-induced PUD- associated abnormal histoarchitecture in TET group by amelioration of damaged gastric mucosa, sub-mucosal oedema and epithelial cell losses, when compared to micrograph from both SSC and SHC group. Conversely, the cure provided in TET group was also better than that observed in SHC group, which signifies the curative effects of Triphala extract in combating swim stress-induced gastric ulcers. The histopathological section from the standard drug treated group (RT) also showed normalization of above mentioned gastric histoarchitecture but the curative effects of RT were not comparable to the effects reported in TET group (fig. 7).
The clear role of H. pylori in PUDs may not be disregarded as H. pylori exists in majority of the cases diagnosed with intestinal/gastric ulcers but relatively a small proportion (10 %-20 %) of individuals worldwide infected with H. pylori apparently develop PUDs[2,6,8]. Development of PUD without H. pylori is also emphasized by fact that 16 %-30 % people developed PUD without having H. pylori infection and NSAIDs history[2,4,6,8]. The current argument is further strongly supported by the fact that psychophysiological stress contributes to the etiology of these types of ulcers and association of PUD with stress is strong enough not to be ignored. Till date, few animal models have been developed using different stresses in order to unravel the contribution of psychophysiological stress on the development of PUD and subsequent therapeutic modulation of PUD pathologies by distinct strategies[11,12]. Therefore, the present study was premeditated not only to develop a post-treatment swim-stress-induced PUD model, first of its kind, but also to uncover the curative effect of Triphala extract on various parameters related to PUD pathologies in swim-stress-induced PUD model.
In the same vein, we for the first time developed a post-treatment swim stress-induced rat PUD model by forcing them to swim for 3 h inside the vertical cylinders and different intervals of self-healing. We selected group III (sacrificed on 14th d) as a model for the determination of curative effect of Triphala extract as lesions were clearly visible in this group after 14 d of self-healing. At the end of the therapeutic intervention, we reported that the gastric ulcer index was significantly increased in SSC rats after 3 h swim- stress challenge, when compared to NC rats, which may be attributed to the enhanced acid secretion under the influence of swim-stress as stress is a well- recognized factor for the enhanced acid production and development of PUDs[11,46,47]. Stress also increases the level of neurotransmitter Acetylcholine (ACh) which stimulates the phospholipase pathway leading to the activation of phosphokinase-C (PKC), which ultimately activates hydrogen/potassium ATPase and secretes acid from parietal cells[46,47]. The group under self-healing observation exhibited a decreased ulcer index, when compared to SSC group. However, treatment with Triphala extract significantly ameliorated swim stress- induced gastric ulcers, when compared to both SSC and SHC groups. This curative effect of Triphala extract on ulcer index could be attributed to its antioxidant as well as anti-inflammatory potential[12,21,48]. Similarly, the effects of ranitidine on ulcer index were also well justified by the previously published reports[12,49].
On the other hand, mucus produced by mucus neck cells, functions as the first-line of defense against distinct ulcerogens/pathogens and shields the whole gastrointestinal or epithelial mucosa thus preventing physical/chemical scratches and ulcer production as well as other vascular diseases[12,50,51]. Owing to the composition and thickness of glycocalyx (a thick and viscous matrix layer of glycoproteins, proteoglycans and hyaluronan that covers the endothelium of most of the organs)[51], it defends against the injury caused by endogenous releases (acids, pepsin, oxidized lipids and bile juice) and by exogenous irritants such as alcohol, aspirin and other NSAIDs[50]. The results from the current study demonstrated that the level of gastric wall mucus was markedly decreased in SSC rats, when compared to gastric wall mucus content from NC rats. Our results are well justified by recent reports which also demonstrated the diminished gastric wall mucus in distinct gastric ulcer models, particularly, indomethacin- induced PUD models[12,13]. However, the altered level of gastric wall mucus was slightly improved in SHC but the administration of Triphala extract speeded up the recovery process as evident by significantly increased gastric mucus in TET group, when compared to both SSC and SHC groups. The beneficial effects of Triphala extract against post-treatment swim stress- induced PUD model may be attributed to its potent antioxidant secondary metabolites i.e., ascorbic acid, gallic acid, tannic acid, syringic acid, epicatechin, and ellagic acid and these beneficial effects are verified by earlier reports[12,13,48]. On the other hand, RT also ameliorated the gastric wall mucus content in current PUD model and these effects are in agreement with earlier reports[12,13].
The oxidative modification of lipids involves the generation of conjugated dienes and lipid hydroperoxides and their degradation results in the formation of MDA, a highly reactive and toxic carbonyl compound[39,40]. The carbonyl species are well reckoned to induce toxic response/stress in both in vitro and in vivo systems via a non-enzymatic glycation reaction with cellular macromolecules (i.e., proteins, lipids and DNA) to produce modified protein adducts i.e., advanced glycation end products[52–55]. The level of gastric mucosal MDA has been widely used to assess the oxidative damage to membranes in human subjects as well as PUD animal models[12,31]. In the same vein, we also measured the levels of MDA in gastric tissue and reported that the MDA content was significantly elevated in SSC group which signifies the role of physiological stress in Reactive Oxygen Species (ROS) production and subsequent lipoxidation and membrane damage. The SHC group showed an improvement in MDA to some extent; however, the aforementioned aberrations were significantly cured by the administration of Triphala extract that may be attributed to its strong antioxidant potential as evident by earlier reports[12,48].
The role of ROS can never be denied as it plays foremost part in the establishment of various diseases but not limited to cardiovascular diseases (atherosclerosis) [40,44], diabetes[34,54], neurological disorders[56], renal pathologies[57], PUD and gastritis[12,31] and cancer[48]. ROS are normally produced in response to distinct stimuli and physiological necessities and counterbalanced by endogenous antioxidant system, both enzymatic (CAT and SOD) and non- enzymatic (GSH)[58]. However, an imbalance amongst the oxidants and antioxidants (increased oxidants and decreased antioxidant) leads to lipoxidation, cellular and histological destruction and subsequent augmentation of gastric ulcers[12,47]. Similarly, we also reported diminution of non-enzymatic antioxidant i.e., GSH in gastric tissue of SSC group, which clearly shows that the rats challenged to swim-stress were not able to tackle the increased free radical generation and subsequent pathologies due to declined level of the endogenous GSH. Even, self-healing strategy was also not quite enough to overcome the damage caused by swim-stress-induced oxidative stress in this study. Additionally, various plant based remedies have been implicated in the treatment of oxidative stress-mediated PUD in recent decade to get rid of undesired effects of synthetic drugs[12,13,59]. Similarly, we also reported that administration of Triphala extract significantly elevated the level of gastric GSH level at the end of the interventional study (better than that reported in SHC group). Furthermore, ranitidine supplementation also ameliorated the level of GSH in gastric tissue of swim stress-induced PUD rat model and our outcomes are well supported by previous reports[12,13].
A part from status of the non-enzymatic antioxidant (GSH), the activity of enzymatic antioxidants like CAT and SOD also affects the redox homeostasis as these enzymes neutralize the highly reactive and toxic H2O2 and superoxide anion radicals, respectively[33,34,60]. Therefore, the activities of CAT and SOD were investigated in gastric tissue of interventional groups. In this attempt, we reported that the activities of CAT and SOD were significantly diminished in SSC group, which may be attributed to swim stress-triggered enhanced ROS generation and inflammatory cascades. The rats under self-healing showed some improvement in the activity of gastric CAT and SOD but these effects were not significant, when compared to corresponding SSC rats, thus signified the need of surplus therapy. In contrast, the treatment with Triphala extract markedly increased the activities of both CAT and SOD in gastric tissue of TET rats, when compared to untreated SSC and SHC groups. This amelioration in CAT and SOD activities in swim stress-challenged PUD rat model was possibly achieved via potent antioxidant metabolites of Triphala extract, which reduced the oxidative stress and subsequently protected against free radical-mediated damage to the cellular membranes. The outcomes from the current study are well supported by the previously published reports demonstrating the enhanced activities of CAT and SOD in different PUD models by the administration of different plant extracts[12-14].
Apart from the biochemical parameters, physiological/ oxidative stress is well reckoned to induce histoarchitectural abnormalities in gastric as well as other tissues[12,33,34]. Similarly, the histopathological investigations of gastric tissue from SSC group showed extensively damaged gastric mucosa, surface epithelium and oedema of the sub-mucosal layer. These abnormalities were possibly mediated by swim stress-induced acid secretion and oxidative stress (characterized by altered level of GSH and MDA; diminished CAT and SOD activities) which ultimately led to the destruction of the tissue. Furthermore, decreased gastric mucus also made it more prone to be attacked by increased acid secretions and free radicals leading to the irregularities and destructions in gastric histoarchitecture. Though, self-healing was not enough to mend these pathologies completely, however, adjunct therapy with Triphala extract cured the above- mentioned irregularities by reducing the sub-mucosal oedema and epithelial cell losses. Conversely, the cure provided by Triphala extract (TET) was also better than that observed in SHC group, which signifies the curative effects of Triphala extract in combating swim stress-induced gastric ulcers. However, further clinical studies are required in order to validate the findings from our current in vivo study and to promote this formulation for the management of PUDs.
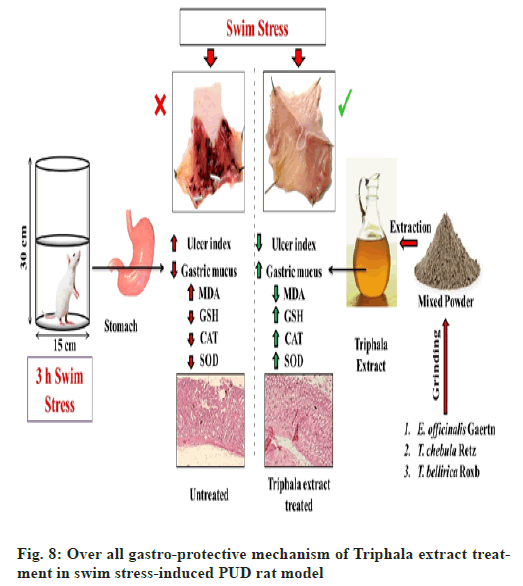
Considering the usefulness and demand of animal models in treatment and management of PUD, we developed a post-treatment swim stress-induced PUD rat model. Furthermore, the results from current therapeutic interventional study demonstrated that hydro-alcoholic extract of Triphala exerts significant gastro-protective activity in swim stress-induced PUD rat model via decreasing the ulcer index and increasing the protective gastric mucus content, whereas, the level/activities of enzymatic (CAT and SOD) and non- enzymatic (GSH) antioxidants were also ameliorated after the administration of Triphala extract in experimental PUD model. In addition, the findings from our biochemical investigations are also well validated by our histopathological observations. In contrast, the curative effects of Triphala extract were superior to the effects caused by RT (fig. 8). To sum up the whole, the current study demonstrates potent curative effects of Triphala extract in targeting swim-stress-induced PUD rat model and may later be investigated and promoted for human clinical applications.
Acknowledgements:
We would like to appreciatively acknowledge the efforts of Prof. S.W. Akhtar, Honorable Chancellor, Integral University, for providing the state-of the-art research laboratory and Animal House Facilities at Department of Pharmacy.
Conflicts of interests:
Authors declare that they have no conflict of interest.
References
- Kuna L, Jakab J, Smolic R, Raguz-Lucic N, Vcev A, Smolic M. Peptic ulcer disease: A brief review of conventional therapy and herbal treatment options. J Clin Med 2019;8(2):179.
[Crossref] [Google Scholar] [PubMed]
- Lanas A, Chan FK. Peptic ulcer disease. Lancet 2017;390(10094):613-24.
- Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med 2019;132(4):447-56.
[Crossref] [Google Scholar] [PubMed]
- Chung CS, Chiang TH, Lee YC. A systematic approach for the diagnosis and treatment of idiopathic peptic ulcers. Korean J Int Med 2015;30(5):559-70.
[Crossref] [Google Scholar] [PubMed]
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374(9699):1449-61.
[Crossref] [Google Scholar] [PubMed]
- Levenstein S, Rosenstock S, Jacobsen RK, Jorgensen T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2015;13(3):498-506.
[Crossref] [Google Scholar] [PubMed]
- Schmitt A, Schmitt J, Münch G, Gasic-Milencovic J. Characterization of advanced glycation end products for biochemical studies: Side chain modifications and fluorescence characteristics. Anal Biochem 2005;338(2):201-15.
[Crossref] [Google Scholar] [PubMed]
- Charpignon C, Lesgourgues B, Pariente A, Nahon S, Pelaquier A, Gatineau‐Sailliant G, et al. Peptic ulcer disease: One in five is related to neither Helicobacter pylori nor aspirin/NSAID intake. Aliment Pharmacol Ther 2013;38(8):946-54.
[Crossref] [Google Scholar] [PubMed]
- Iijima K, Kanno T, Koike T, Shimosegawa T. Helicobacter pylori-negative, non-steroidal anti-inflammatory drug: negative idiopathic ulcers in Asia. World J Gastroenterol 2014;20(3):706.
[Crossref] [Google Scholar] [PubMed]
- Deding U, Ejlskov L, Grabas MP, Nielsen BJ, Torp-Pedersen C, Bøggild H. Perceived stress as a risk factor for peptic ulcers: a register-based cohort study. BMC Gastroenterol 2016;16:1-2.
- Adinortey MB, Ansah C, Galyuon I, Nyarko A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers 2013;2013.
- Khushtar M, Siddiqui HH, Dixit RK, Khan MS, Iqbal D, Rahman MA. Amelioration of gastric ulcers using a hydro-alcoholic extract of Triphala in indomethacin-induced Wistar rats. Eur J Integrat Med 2016;8(4):546-51.
- Tahir M, Rahman MA, Khushtar M. Gastroprotective effect of Hyssopus officinalis L. leaves via reduction of oxidative stress in indomethacin-induced gastric ulcer in experimental rats. Drug Chem Toxicol 2022;45(1):291-300.
[Crossref] [Google Scholar] [PubMed]
- Khushtar M, Ahmad A, Rahman MA. Gastroprotective effect of hydro-alcoholic extract of Polygonum bistorta Lin root in indomethacin-induced gastric ulcers in sprague dawley rats. Indian J Pharm Educ Res 2018;52:618-25.
- Weissman S, Siu M, Ferm S, Hassan A. Common antacid medication–ranitidine causing a rare serious adverse effect. Cureus 2018;10(11).
[Crossref] [Google Scholar] [PubMed]
- Werbel T, Cohen PR. Ranitidine-associated sleep disturbance: Case report and review of H2 antihistamine-related central nervous system adverse effects. Cureus 2018;10(4):e2414.
[Crossref] [Google Scholar] [PubMed]
- Fahmy NM, Al-Sayed E, Michel HE, El-Shazly M, Singab AN. Gastroprotective effects of Erythrina speciosa (Fabaceae) leaves cultivated in Egypt against ethanol-induced gastric ulcer in rats. J Ethnopharmacol 2020;248:112297.
[Crossref] [Google Scholar] [PubMed]
- Taha MS, El-Sherbiny EM, Osman HF. Anti-ulcerogenic activity of Gum Arabic in gastric mucosal injury induced by ethanol in male albino rats. Appl Physiol Nutr Metab 2020;45(7):731-6.
[Crossref] [Google Scholar] [PubMed]
- Ahmad P, Alvi SS, Khan MS. Functioning of organosulfur compounds from garlic (allium sativum linn) in targeting risk factor-mediated atherosclerosis: A cross talk between alternative and modern medicine. Natural Bio-active Compounds 2019:561-85.
- Chouhan B, Kumawat RC, Kotecha M, Ramamurthy A, Nathani S. Triphala: A comprehensive ayurvedic review. Int J Res Ayurveda Pharm 2013;4(4):612-7.
- Peterson CT, Denniston K, Chopra D. Therapeutic uses of triphala in ayurvedic medicine. J Altern Complement Med 2017;23(8):607-14.
[Crossref] [Google Scholar] [PubMed]
- Nariya MB, Shukla VJ, Ravishankar B, Jain SM. Comparison of gastroprotective effects of triphala formulations on stress-induced ulcer in rats. Indian J Pharm Sci 2011;73(6):682-7.
[Crossref] [Google Scholar] [PubMed]
- Baliga MS, Meera S, Mathai B, Rai MP, Pawar V, Palatty PL. Scientific validation of the ethnomedicinal properties of the Ayurvedic drug Triphala: A review. Chin J Integr Med 2012;18(12):946-54.
[Crossref] [Google Scholar] [PubMed]
- Khlaj A, Hamid KS, Reza KA, Ranjbar SH, Esfehani MM, Mohammad K, et al. A systematic review of the antioxidant, anti-diabeticand anti-obesity effects and safety of triphala herbalformulation. J Med Plants Res 2013;7(14):831-44.
- Tarasiuk A, Mosińska P, Fichna J. Triphala: Current applications and new perspectives on the treatment of functional gastrointestinal disorders. Chin Med 2018;13:39.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Rai S, Bhattachar S, Kumar DP, Biswas TK, Jana U, et al. Clinical study of ‘TRIPHALA’–A well known phytomedicine from India. Iran J Pharmacol Ther 2006;5(1):51-4.
- Sabina EP, Rasool M. An in vivo and in vitro potential of Indian ayurvedic herbal formulation Triphala on experimental gouty arthritis in mice. Vascul Pharmacol 2008;48(1):14-20.
[Crossref] [Google Scholar] [PubMed]
- Singh DP, Govindarajan R, Rawat AK. High‐performance liquid chromatography as a tool for the chemical standardisation of Triphala: An Ayurvedic formulation. Phytochem Anal 2008;19(2):164-8.
[Crossref] [Google Scholar] [PubMed]
- Dhanalakshmi S, Devi RS, Srikumar R, Manikandan S, Thangaraj R. Protective effect of Triphala on cold stress-induced behavioral and biochemical abnormalities in rats. Yakugaku Zasshi 2007;127(11):1863-7.
[Crossref] [Google Scholar] [PubMed]
- Srikumar R, Parthasarathy NJ, Manikandan S, Narayanan GS, Sheeladevi R. Effect of Triphala on oxidative stress and on cell-mediated immune response against noise stress in rats. Mol Cell Biochem 2006;283:67-74.
[Crossref] [Google Scholar] [PubMed]
- Demir S, Yılmaz M, Akalin N, Aslan D, Aydin A. Role of free radicals in peptic ulcer and gastritis. Turk J Gastroenterol 2003;14(1):39-43.
[Google Scholar] [PubMed]
- Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mishra KP, Banavalikar MM, et al. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res 2005;19(7):582-6.
[Crossref] [Google Scholar] [PubMed]
- Hashim A, Alvi SS, Ansari IA, Khan MS. Phyllanthus virgatus forst extract and it’s partially purified fraction ameliorates oxidative stress and retino-nephropathic architecture in streptozotocin-induced diabetic rats. Pak J Pharm Sci 2019;32(6):2697-708.
[Google Scholar] [PubMed]
- Akhter F, Alvi SS, Ahmad P, Iqbal D, Alshehri BM, Khan MS. Therapeutic efficacy of Boerhaavia diffusa (Linn.) root methanolic extract in attenuating streptozotocin-induced diabetes, diabetes-linked hyperlipidemia and oxidative-stress in rats. Biomed Res Ther 2019;6(7):3293-306.
- Abebaw M, Mishra B, Gelayee DA. Evaluation of anti-ulcer activity of the leaf extract of Osyris quadripartita Decne.(Santalaceae) in rats. J Exp Pharmacol 2017:1-11.
[Crossref] [Google Scholar] [PubMed]
- Hatware KV, Sharma S, Patil K, Shete M, Karri S, Gupta G. Evidence for gastroprotective, anti-inflammatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in Wistar rats. Biomed Pharmacother 2018;103:317-25.
[Crossref] [Google Scholar] [PubMed]
- Zhang S, Xu Z, Gao Y, Wu Y, Li Z, Liu H, et al. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PLoS One 2012;7(12):e51148.
[Crossref] [Google Scholar] [PubMed]
- Come SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol 1974;242(2):116P-7P.
[Google Scholar] [PubMed]
- Alvi SS, Ansari IA, Khan I, Iqbal J, Khan MS. Potential role of lycopene in targeting proprotein convertase subtilisin/kexin type-9 to combat hypercholesterolemia. Free Radic Biol Med 2017;108:394-403.
[Crossref] [Google Scholar] [PubMed]
- Alvi SS, Ansari IA, Ahmad MK, Iqbal J, Khan MS. Lycopene amends LPS induced oxidative stress and hypertriglyceridemia via modulating PCSK-9 expression and Apo-CIII mediated lipoprotein lipase activity. Biomed Pharmacother 2017;96:1082-93.
[Crossref] [Google Scholar] [PubMed]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25:192-205.
[Crossref] [Google Scholar] [PubMed]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47(3):469-74.
[Crossref] [Google Scholar] [PubMed]
- Nabi R, Alvi SS, Shah A, Chaturvedi CP, Iqbal D, Ahmad S, et al. Modulatory role of HMG-CoA reductase inhibitors and ezetimibe on LDL-AGEs-induced ROS generation and RAGE-associated signalling in HEK-293 Cells. Life Sci 2019;235:116823.
[Crossref] [Google Scholar] [PubMed]
- Wang G, Liu Z, Li M, Li Y, Alvi SS, Ansari IA, et al. Ginkgolide B mediated alleviation of inflammatory cascades and altered lipid metabolism in HUVECs via targeting PCSK-9 expression and functionality. BioMed Res Int 2019;2019:7284767.
[Crossref] [Google Scholar] [PubMed]
- Ahmad P, Alvi SS, Iqbal J, Khan MS. Identification and evaluation of natural organosulfur compounds as potential dual inhibitors of α-amylase and α-glucosidase activity: An in silico and in vitro approach. Med Chem Res 2021;30:2184-202.
- Bourgonje AR, Feelisch M, Faber KN, Pasch A, Dijkstra G, van Goor H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med 2020;26(11):1034-46.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94(2):329-54.
[Crossref] [Google Scholar] [PubMed]
- Prasad S, Srivastava SK. Oxidative stress and cancer: chemopreventive and therapeutic role of triphala. Antioxidants 2020;9(1):72.
[Crossref] [Google Scholar] [PubMed]
- Moharram FA, Al-Gendy AA, El-Shenawy SM, Ibrahim BM, Zarka MA. Phenolic profile, anti-inflammatory, antinociceptive, anti-ulcerogenic and hepatoprotective activities of Pimenta racemosa leaves. BMC Complemen Alternat Med 2018;18:1-5.
[Crossref] [Google Scholar] [PubMed]
- Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 2013;10(6):352-61.
[Crossref] [Google Scholar] [PubMed]
- Ahmad P, Alvi SS, Iqbal D, Khan MS. Insights into pharmacological mechanisms of polydatin in targeting risk factors-mediated atherosclerosis. Life Sci 2020;254:117756.
[Crossref] [Google Scholar] [PubMed]
- Nabi R, Alvi SS, Shah MS, Ahmad S, Faisal M, Alatar AA, et al. A biochemical & biophysical study on in-vitro anti-glycating potential of iridin against D-Ribose modified BSA. Arch Biochem Biophys 2020;686:108373.
[Crossref] [Google Scholar] [PubMed]
- Nabi R, Alvi SS, Khan RH, Ahmad S, Ahmad S, Khan MS. Antiglycation study of HMG-R inhibitors and tocotrienol against glycated BSA and LDL: A comparative study. Int J Biol Macromol 2018;116:983-92.
[Crossref] [Google Scholar] [PubMed]
- Nabi R, Alvi SS, Saeed M, Ahmad S, Khan MS. Glycation and HMG-CoA reductase inhibitors: Implication in diabetes and associated complications. Curr Diabetes Rev 2019;15(3):213-23.
[Crossref] [Google Scholar] [PubMed]
- Alvi SS, Nabi R, Khan M, Akhter F, Ahmad S, Khan MS. Glycyrrhizic acid scavenges reactive carbonyl species and attenuates glycation-induced multiple protein modification: An in vitro and in silico study. Oxid Med Cell Longev 2021;2021.
[Crossref] [Google Scholar] [PubMed]
- Alvi SS, Ahmad P, Ishrat M, Iqbal D, Khan MS. Secondary metabolites from rosemary (Rosmarinus officinalis L.): Structure, biochemistry and therapeutic implications against neurodegenerative diseases. Natural Bio-active Compounds 2019:1-24.
- Nabi R, Alvi SS, Alouffi S, Khan S, Ahmad A, Khan M, et al. Amelioration of neuropilin-1 and RAGE/matrix metalloproteinase-2 pathway-induced renal injury in diabetic rats by rosuvastatin. Arch Biol Sci 2021;73(2):265-78.
- Nabi R, Alvi SS, Shah A, Chaturvedi CP, Faisal M, Alatar AA, et al. Ezetimibe attenuates experimental diabetes and renal pathologies via targeting the advanced glycation, oxidative stress and AGE-RAGE signalling in rats. Arch Physiol Biochem 2021:1-6.
[Crossref] [Google Scholar] [PubMed]
- Sidahmed HM, Hashim NM, Abdulla MA, Ali HM, Mohan S, Abdelwahab SI, et al. Antisecretory, gastroprotective, antioxidant and anti-Helicobcter pylori activity of zerumbone from Zingiber zerumbet (L.) Smith. PloS one 2015;10(3):e0121060.
[Crossref] [Google Scholar] [PubMed]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 2018;54(4):287-93.