Jessy Shaji* and M. Shaikh
Department of Pharmaceutics, Prin. K. M. Kundnani College of Pharmacy, Cuffe Parade, Mumbai-400 005, India.
- *Corresponding Author:
- Jessy Shaji
Department of Pharmaceutics
Prin. K. M. Kundnani College of Pharmacy
Cuffe Parade, Mumbai-400 005, India
E-mail: jessy.shaji@gmail.com
Received Date: 16 Sep 2015; Revised Date: 21 May 2016; Accepted Date: 26 May 2016
Abstract
The pulmonary drug delivery system offers several merits over other drug delivery systems and therefore, this delivery route has been in prime focus for various applications like local and systemic therapeutics delivery. The overall development of drug delivery system depends on its efficacy, quality and safety and to achieve such attributes there is a need of reliable evaluation methods to test them. This review provides an in-depth analysis of the development in the evaluation of pulmonary drug delivery systems. In vitro methods of testing pulmonary products such as particle morphological studies, powder flow characteristics, moisture content test, aerosol turboelectric characterization, particles interparticulate forces measurement and solid state characterizations were discussed. Particle size and zeta potential measurement and evaluation of aerosol performance such as dose uniformity and aerodynamic particle size distribution were reviewed in detail. The development of dissolution methods for pulmonary products is also elaborated. Various cell culture methods for testing pulmonary products were overviewed. The in vivo testing methods including drug administration systems, drug deposition studies and pharmacokinetic studies and ex vivo testing models were also highlighted. Together an overview of current advancement in evaluation and characterization of pulmonary drug delivery system can be analyzed and studied through this review.The pulmonary drug delivery system offers several merits over other drug delivery systems and therefore, this delivery route has been in prime focus for various applications like local and systemic therapeutics delivery. The overall development of drug delivery system depends on its efficacy, quality and safety and to achieve such attributes there is a need of reliable evaluation methods to test them. This review provides an in-depth analysis of the development in the evaluation of pulmonary drug delivery systems. In vitro methods of testing pulmonary products such as particle morphological studies, powder flow characteristics, moisture content test, aerosol turboelectric characterization, particles interparticulate forces measurement and solid state characterizations were discussed. Particle size and zeta potential measurement and evaluation of aerosol performance such as dose uniformity and aerodynamic particle size distribution were reviewed in detail. The development of dissolution methods for pulmonary products is also elaborated. Various cell culture methods for testing pulmonary products were overviewed. The in vivo testing methods including drug administration systems, drug deposition studies and pharmacokinetic studies and ex vivo testing models were also highlighted. Together an overview of current advancement in evaluation and characterization of pulmonary drug delivery system can be analyzed and studied through this review.
Keywords
Pulmonary drug delivery, aerosol, aerodynamic particle size, dissolution studies, cell culture, drug deposition, pharmacokinetics
Pulmonary drug delivery system has been used for the treatment of local and systemic diseases. The local respiratory diseases like chronic obstructive pulmonary disease (COPD) and asthma were the prime focus in the early studies, however the research has been advanced in the treatment of respiratory infectious diseases and also in the transport of therapeutic molecules to the systemic circulation[1]. The merits of pulmonary route such as huge surface area of the lungs, thin barrier of alveolocapillary membrane and high permeability of the lungs paved the way for more research in the treatment of various local and systemic diseases[2-4]. The conventional inhalation products have high dosing frequency and large dose often leads to local and systemic toxicity. Therefore, novel controlled release carrier systems may provide a possible solution to these problems by reducing dosing frequency and increasing drug bioavailability[5]. The decrease in dosing frequency and side effects can lead to improved patient compliance and therefore, it can resolve the problem of nonadherence to prescribed therapy which acts as one of the major obstacle in the control of various respiratory and non-respiratory diseases[6].
These novel carrier systems for pulmonary administration have to be evaluated for the in vitro and in vivo characteristics. The effective pulmonary administration of a drug depends on its deposition in the deep part of the lungs. The deposition of the carrier system is followed by drug release from the system and thereby transportation from the site of deposition to the site of action through the alveolocapillary membrane barrier[7]. The local effects depend on the duration of the drugs in the lungs while the systemic effects depend on the transportation of the drugs to the systemic circulation. The deposition of the drug in the airways depend on the various factors like particle morphology, size, size distribution, moisture content, aerosol turboelectric charge and interparticulate forces[8]. The evaluation of aerosol characteristics such as aerodynamic particle size distribution and dose uniformity gives an idea of drug deposition in the lungs. The particle dissolution studies provide in vitro data of the drug release from the system in the medium mimicking in vivo condition. After the release of drug from the system, it has to be transported into the site of action. The transportation of drug includes the passage through the alveolocapillary membrane. The cell culture study gives an understanding of this transportation of drugs in the presence of the various mammalian alveolar epithelium cells. Various in vivo tests are developed to study drug administration system, drug deposition mechanism and pharmacokinetic action. Some ex vivo testing models has also been developed to study the mechanism of drug transport across lung tissues. Thus, a systemic approach in the evaluation of pulmonary drug delivery system can lead to the successful drug delivery system.
Development in the Evaluation of Pulmonary Drug Delivery System
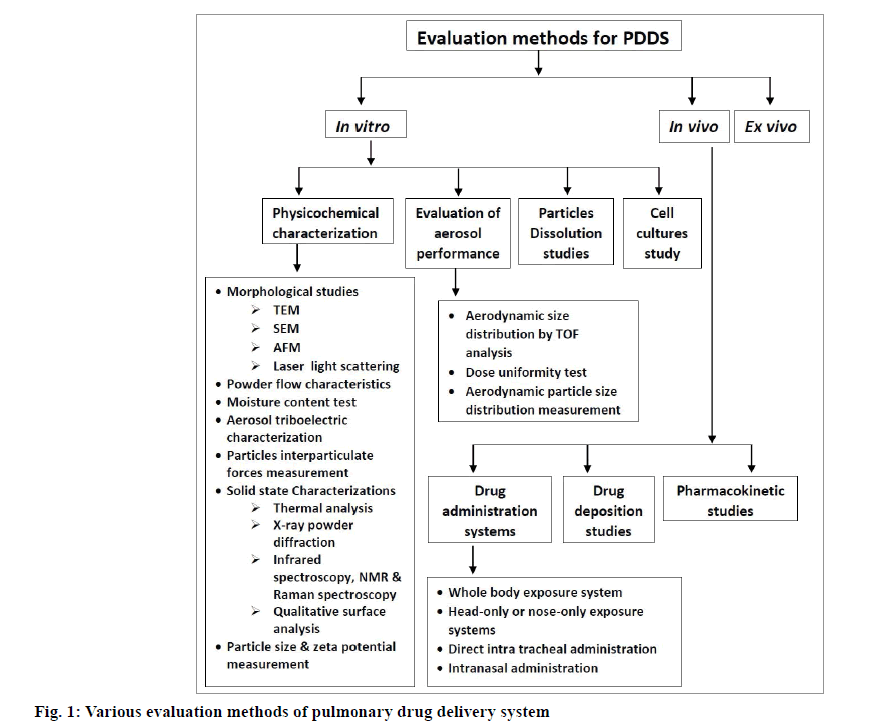
In recent years, there has been a considerable advancement in the field of evaluation and characterization of pulmonary drug delivery system. These methods can be classified as in vitro, in vivo and ex vivo methods. Various in vitro methods like physicochemical characterization of particles, evaluation of aerosol performance, particle dissolution studies and cell cultures study have been evolved. Drug administration system, drug deposition and pharmacokinetic studies are the in vivo methods which are currently in research studies. Ex vivo methods are also developed to check the efficacy and safety parameters of drugs and their delivery mechanisms. Various evaluation methods for pulmonary drug delivery systems are given in fig. 1.
Morphological studies:
The morphological studies of nanoparticles can be done with transmission electron microscopy (TEM), which uses different techniques for analysis of particles like cryogenic transmission methods, negative staining method and freeze-fracture method. The particles shape, structure and arrangement can be identified by interpretation of the morphological data of TEM analysis. Similarly, particle size can also be estimated[9]. Scanning electron microscopy (SEM) is used to evaluate the particle size and surface morphology of the particles. It uses high magnification for visualizing the surface morphology of the particles. The distinctive surface characteristic can be studied using the varied resolutions[10]. Measurement of interparticulate forces and surface energy and imaging surface nanotopography of particles can be done using atomic force microscopy (AFM)[11]. The surface roughness of the particles is evaluated by this method and the scanned image is quantified by image analysis software. The degree of surface corrugation can also be expressed by surface fractal dimension determined by a light scattering method[12-14]. A real-time aerodynamic measurement of particles ranging from 0.5 to 20 microns with high resolution was done using the Aerodynamic Particle Sizer® 3321. The light-scattering intensity in the equivalent optical size range of 0.37 to 20 microns can be measured using these particle sizers. The paired data for each particle have been provided by these particle sizers and it helps the researcher in studying the aerosol makeup.
Powder flow characteristics:
Powder flow characteristics of inhalable powders are done to evaluate the flow characteristics and its relationship with the aerodynamic properties. The angle of repose, bulk density, tapped density, apparent density and porosity are some of the powder flow characteristics need to be measured for quality control. The angle that the side of the conical heap of powders makes with the horizontal plane is termed as angle of repose[15]. Apparent density and porosity values of inhalable powders were determined by mercury porosimeter (Micromeritics AutoPore IV 9500; Micromeritics Instrument Corporation, Norcross, USA) [16]. A tap density tester (Stampfvolumeter, STAV 2003) are used to measure tap density. Carr’s compressibility index and Hausner ratio were determined by the values of bulk and tapped density[17].
The powders are tested for their moisture content by using a dynamic vapour sorption instrument (Surface Management Systems, UK)[18].
Aerosol turboelectric characterization:
It has been seen that electrostatic charge develops during the handling of powders and aerosolization tends to control the deposition of drug particles in the respiratory tract thereby affecting the aerosolization characteristics of inhaled drug products. Static charge in aerosol has shown an improved delivery into the deep part of the lungs and can be used to target particles in the required area in the respiratory system. Direct measurement and indirect measurement are the two methods used to measure aerosol electrostatic properties. Devices like Electrical Low Pressure Impactor (ELPI™)[19,20], electrical Next Generation Impactor (eNGI)[21,22] and Twin-Stage Impinger (TSI) uses the direct measurement while E-SPART uses the indirect measurement method. Out of which E-SPART uses laser Doppler velocimeter theory to measure pressurized metered dose inhaler (pMDI) and Dry Powder Inhaler (DPI), while ELPI and eNGI uses Faraday cage theory to determine the static charge of aerosol in pMDI and DPI, respectively. ELPI device has some limitations with DPI particles measurement due to the absence of pre-separator and possesses low flow rate of 30 l/min. This limitation has been overcome by using Andersen Cascade Impactor (ACI) with preseparator attachment with modified TSI. Modified TSI can be used to measure static charge on particles in DPI[19,23].
Particles interparticulate forces measurement:
In DPI powder formulation, the adhesive forces between the carrier and drug particles are crucial as the dose deposition in the lungs depends on the separation of the particles from the carrier. Fewer studies have been carried out in the field of interparticulate interactions with that of aerozolisation behavior of the particles. Inverse gas chromatography, centrifugation and particle detachment rate measurement are some of the methods to determine adhesive forces in the system. These methods have been estimating in indirect way and are limited to the determination of a bulk characteristic of powders. However, a direct measurement of these adhesive forces and balance between adhesive and cohesive forces between particles and that of a substrate can be measured with the AFM colloid probe technique[24]. The cohesive/adhesive balance (CAB) measurement is a technique which uses the AFM colloid probe in studying uniformity of the blend, drug particle segregation, and characteristics of powder dispersion in DPI systems.
Solid state characterizations:
Differential scanning calorimetry (DSC) is a routine thermal analytical method used for the analysis of polymorphic changes in a drug-carrier matrix. Drug encapsulation within the matrix can be evaluated with this technique. Stability of the formulation can be determined by structural changes in the drug-carrier matrix. Parameters such as melting and recrystallization can be used to determine polymorphic changes in the matrix. A comprehensive thermal analytical study on one of the matrix system have been described by Bunjes et al[25-27]. Thermogravimetric analysis (TGA) was also performed using a Q50 TGA from TA Instruments. Data analysis was completed using Universal Analysis 2000 (Version 4. 3A) software that was provided by TA instruments[15]. X-ray powder diffraction (XRPD) is a widely used tool for evaluation of the crystalline state of the drug and excipients in the formulation. This method can ensure the drug encapsulation in the carrier system[28]. Fourier Transformed infrared spectroscopy, Nuclear magnetic resonance and Raman spectroscopy are the other advanced technique for the physicochemical characterization of nano and microparticulate system[28]. Qualitative surface analysis is done by Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS). It uses image spectral data (surface chemical mapping based on mass fragment analysis) of the sample powder to provide Rayleigh- Gans-Debye (RGD) scattering theory that explains the structure of each single particle in an aggregate[14].
Particle size and zeta potential measurement:
Particle size and zeta potential are crucial factors which decide the efficacy and stability of the delivery system as the particle size tends to control the drug deposition in the lung, thereby penetration into the systemic delivery whereas zeta potential helps in the stability management of the system. Particles of size <5 μm have the greatest possibility of deposition in the lung[29] and particle with size <2 μm can reach up to the alveoli[30,31]. Deposition of large particles with an aerodynamic diameter of >5 μm is held in upper airways by inertial impaction. Gravitational settling of particles with aerodynamic diameter 1 to 5 μm takes place in the central and distal tract. Particles with <1 μm are largely exhaled out as they remain suspended in the air during respiration. Ultrafine particles of <100 nm size can largely deposit in the respiratory tract by random Brownian motion and these particles can reach the alveolar region while particles <10 nm get deposited in the tracheo-bronchial region due to their high diffusion coefficients[32]. One micrometer and 10 μm diameter particles can be taken up by macrophages present in the respiratory system[33,34]. Particles having size between 3 μm and 6 μm are optimum to be taken up by the alveolar macrophages[35] while particles with 0.3 μm to 1.1 μm diameter are taken up by peritoneal macrophages and peripheral blood mononuclear cells[36-38]. Large porous particles of 10-20 μm diameter avoids the macrophages engulfment due to their large size and gets deposited into the deep regions of the lungs[39-40]. However, there have been reports of macrophages surrounding the large inhaled particles thereby showing attachment and surface digestion of these particles[39]. Particles in nanometer range can also be taken up by the macrophages[41]. The uptake of these nanoparticles mainly takes place by pinocytosis and it is dependent on the concentration of particle in extracellular region and period of contact between macrophages and the particles. Therefore, estimation of particle size can give an idea of the drug deposition and penetration within the body. Besides particle size, a polydispersibility index is significant in determining the uniformity of the particles in the formulation. The value higher than 0.2 shows the multiple sizes of particles in the formulation while smaller polydispersibility index value shows more uniform size particles in the given formulation system. Zeta potential measurements are useful for estimating the particles stability in the formulation. The higher value dictates better stability due to repulsion between particles and less aggregation helping in a uniform distribution of particles in the system thereby establishing better shelf life of the formulation.
Photon correlation spectroscopy (PCS) and laser diffraction (LD) are the most common principles used for measuring particle size and polydispersibility index. PCS can measure particles from a few nanometers to 3 μm while LD measures particles from the nanometer to a few millimeter size range. A nano tracking analysis (NTA) is a novel method for the measurement of size and zeta potential of nanoparticles for inhaled drug delivery[42]. NTA method consists of a direct visualization and analysis of the particle by particle movement in the nanosuspension. Particle in the range of 10-2000 nm can be measured by this technique. Nanoparticles diffuse laser light during the Brownian motions and this phenomenon is tracked by a charge-coupled device (CCD) camera attached to the microscope which operates at 30 frames per second, capturing a video of the moving particles under Brownian motion. The particles are tracked individually and hydrodynamic diameters of the particles have been calculated by the Stokes-Einstein equation using the software[43,44].
Delivered dose uniformity test:
Dosage Unit Sampling Apparatus (DUSA) for desired testing device are used to perform Delivered Dose Uniformity test[45]. “Delivered dose uniformity” and “Delivered dose uniformity over the entire contents” are the content uniformity test specified by the Pharmacopoeias. The total quantity of drug emitted from the device, thereby accessible to the user is termed as the delivered dose. The delivered dose is retained by the filter in the sampling apparatus after the firing of the test device. The active drug from the retained dose is dissolved in a suitable solvent and the sample aliquot is analyzed with the chromatographic analytical method. Simulation of inhalation is brought about by drawing air through the sampling apparatus and air drawing manner depends on the device under test condition. Delivered dose uniformity is a critical quality attribute (CQA) in determining the safety, quality and efficacy of inhaled drug products. DUSA for metered dose inhalers (MDIs) has been designed by Charles Thiel in 3M’s laboratories in Minneapolis, USA specifically for the sampling and testing of MDIs. DUSA for dry powder inhalers (DPIs) is a bigger version of MDIs sampling apparatus, which is available for use with flow rates up to 100 l/min for sampling. DUSA for Nebulizers consists of a filter holder, suitable mouthpiece adapter and a breath simulator to simulate the inhalation pattern during the test.
Aerodynamic particle size distribution measurement:
Beside delivered dose, CQA also acknowledges the aerodynamic particle size distribution (APSD) as an in vitro characterization parameter for inhaled drug products. Both the regulators and Pharmacopoeias consider the cascade impactor as the instrument of choice for measuring the aerodynamic size distribution of inhaled drug products. Cascade impactor defines the aerodynamic characteristics of aerosol particles by separating the dose with respect to its sizes in impactor plates of the equipment. Fine particle fraction and mass median aerodynamic diameter are aerosol parameters determined by Cascade impactors[46]. The analytical data helps in determining the pharmaceutical products quality and it is also used for product development. A result from impactors predicts human lung deposition data as aerosol deposition in the human respiratory tract depend on particle aerodynamic size. However, in cascade impactors dimensions are at room temperature and humidity is low which differs from human airway’s ambient environment. MSP Corporation, USA had developed Nano-MOUDI™ device, a nano/ micro orifice with uniform deposition impactor and some added features over conventional impactors[47]. Aerodynamic size distribution by time-of-flight analysis determines the aerodynamic diameter and distributions of particle size of the nanoparticulate dry powders using an Aerosizer LD[15]. The Aerosizer is a dual purpose, time-of-flight instrument that can rapidly size powders as well as aerosol particles.
Current pharmacopoeial specifications recommend various marketed impactors like next generation impactor (NGI) and ACI which are used globally for the testing of MDIs, DPIs and liquid droplet Inhalers. Various impactors and their pharmacopoeial specifications are given in Table 1. For the testing of nebulizer systems, special modified versions are available as per the Pharmacopoeial requirements. These impactors are usually used with the European Pharmacopoeia (Ph. Eur.)/ United States Pharmacopeia (USP) induction port and a pre-separator. In 2002, EPAG (European Pharmaceutical Aerosol Group) put forth some study on a calibration of the NGI to 15 l/ min for use in the nebulizer’s testing. NGI cooler is believed to solve the problem associated with the heat related droplet size reduction and production of artificially low particle size measurements. In 2004, Food and Drug Administration (FDA) put Guidance on Process Analytical Technology which imparts quality by design (QbD) application to pharmaceutical testing and analysis. This approach put forth the concept of abbreviated impactor measurement (AIM) in APSD determination. Several marketed versions of Abbreviated impactors are available with reduced stage models of the ACI and NGI.
| Impactor | European Pharmacopeia | US Pharmacopeia | Manufacturer |
|---|---|---|---|
| Twin Impinger (Glass) | Apparatus A: pMDI, DPIs, and nebulizers | Not Specified | Westech Scientific Instruments (UK), Erweka® (UK), Copley Scientific Limited (UK) |
| Andersen Cascade Impactor (ACI) | Apparatus D: pMDIs | USP Apparatus 1: MDIs | Westech Scientific Instruments (UK), Erweka® (UK), Copley Scientific Limited (UK) |
| Marple Miller Impactor (MMI) | Not Specified | USP Apparatus 2: DPIs | Copley Scientific Limited (UK) |
| Andersen Cascade Impactor (ACI) + Preseparator | Apparatus D: DPIs | USP Apparatus 3: DPIs | Westech Scientific Instruments (UK), Erweka® (UK), Copley Scientific Limited (UK) |
| Multi- Stage Liquid Impinger (MSLI) | Apparatus C: pMDI and DPIs | USP Apparatus 4: DPIs | Westech Scientific Instruments (UK), Erweka® (UK), Copley Scientific Limited (UK) |
| Next Generation Impactor (NGI) + Preseparator | Apparatus E: DPIs | USP Apparatus 5: DPIs | Westech Scientific Instruments (UK), Copley Scientific Limited (UK) |
| Next Generation Impactor (NGI) | Apparatus E: MDI and nebulizer (as per Chapter 2. 9. 44) | USP Apparatus 6: MDIs and nebulizer (as per Chapter <1601>) | Westech Scientific Instruments (UK), Copley Scientific Limited (UK) |
TABLE 1: CURRENT PHARMACOPOEIAL SPECIFICATIONS FOR CASCADE IMPACTORS
In 2006, a new regulatory guidance on the nebulizers “Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products” was issued by European Medicines Agency (EMA). These guidelines ensure that the nebulizer’s safety and efficacy are not independent but depends on a combination of nebulizer and drug. After EMA guidelines, the European and US pharmacopoeias added a new Chapter (Ph. Eur. 2.9.44 and USP Chapter <1601>) on “Preparations for Nebulisation: Characterisation”. The new Pharmacopoeial chapters became the base for the introduction of the new ISO 27427 requirements for the “safety, performance and testing for general purpose nebulising systems intended for continuous or breath-actuated delivery of liquids in an aerosol form, to humans through the respiratory system” specified in Annex C. Over the last 10-15 years the regulatory framework and pharmacopoeia monographs for nebulizers have changed considerably with the need. Recently updated monographs includes nebulizer and a new draft of USP monographs for the testing of pressurized metered dose inhalers (pMDIs) with spacers and valve holding chambers. These reviews update the capabilities of breathing simulators and their application in a nebulizer, dry powder inhaler and pMDI testing. For speeding the research and development in the pulmonary drug delivery system an AIM is used for fast screening of novel formulations in product development stage. Fast throughputs, ease of handling and easier automation are the main merits of the abbreviated impactors. These impactors give detailed in vitro and in vivo performance and help in reduced dependence on expensive and lengthy clinical trials. Alberta Idealised Throat (AIT) shows realistic human-like design being replaced with the existing Ph. Eur./USP Induction Port for improved in nitro in vivo correlation. The use of Breath simulators in place of conditions like constant flow rate in testing can simulate more in vivo condition. The Mixing Inlet also helps mimic in vivo testing condition. Fast screening Andersen (FSA) is an AIM model of the standard ACI modified for quality control (FSA-QC) or product development (FSA-HRT). The reduced next generation impactor (rNGI) is an abbreviated method for utilizing the NGI for both AIM-QC and AIM-HRT applications. Fast screening impactor (FSI) is a purpose made approach to AIM that help in separation of the dose into fine particle mass and coarse particle mass i.e. NGI Preseparator technology, making it suitable for AIM-HRT application (i.e. FSI-HRT) for MDIs, DPIs and nasal sprays. Accessories required along with the DUSA and cascade impactor for measuring the delivered dose uniformity and aerodynamic particle size distribution of inhaled drug products are shown in Table 2.
| Accessories/Marketed Brand | Functions | Regulatory and Pharmacopoeial specifications |
|---|---|---|
| Breath simulators: Model BRS 1000-500ml version (Copley Scientific) |
Produces the adult breathing pattern needed for the dose uniformity testing of nebulizers. | ISO 27427:2010, Ph. Eur. Chapter 2. 9. 44. and USP <1601> |
| Model BRS 2000 - 1l version (Copley Scientific) | Produces the neonate, infant, child, adult 1 and adult 2 breathe pattern. | Ph. Eur. 2. 9. 44 and USP <1601> |
| Model BRS 3000 - 5l version (Copley Scientific) | Testing of MDIs and DPIs as well as the nebulizers, spacers and holding chambers. | |
| Critical Flow Controller: MODEL TPK and MODEL TPK 2000 (Copley Scientific) |
Record and control the critical parameters related with the delivered dose uniformity testing and APSD measurement of DPIs. | Ph. Eur. in Chapters 0671 and 2. 9. 18, USP Chapter 601. |
| TP-DG-DPI 1000 (ERWEKA®) | Used to adjust flow rate and flow duration. | Ph. Eur. and USP |
| Critical Flow Controller (Westech Scientific Instruments) | Establishing the required pressure drop, flow rate. | EP Apparatus B |
| GCE-3 (Westech Scientific Instruments) | Revolutionize the testing of DPIs capability to perform 4kPa tests from a programmed menu. | NA |
| GCE-4 MDI Controller (Westech Scientific Instruments) | Uses an air driven vacuum system to provide and control the flow for testing of MDIs. | NA |
| Data Analysis function: Copley Inhaler Testing Data Analysis Software (CITDAS) Version 3. 10 |
Easy and fast processing of data generated from impactor drug deposition, inaccordance with Pharmacopoeia requirements and compliance with 21 CFR 11. | USP Chapter <601> and Ph. Eur. Chapter 2. 9. 18 |
| Flow Meters: DFM3 and DFM 2000 (Copley Scientific) | Provide range and accuracy for flow. | USP 33 and Ph. Eur. 6. 0 |
| Flowmeters (Westech Scientific Instruments) | Digital flowmeters with adaptors. | Adapts to USP Induction port |
| DFM Stand Alone Unit (ERWEKA®) | Used with the instrumentation employed in testing pharmaceutical inhalers. | NA |
| Mouthpiece Adapters, Tubing and Quick Release Connectors | Help in connecting the several components of the system. | NA |
| Pump: LCP5, HCP5 and SCP5 (Copley Scientific) | Suitable for MDIs, Nasal Sprays and Nebulizers. | Ph. Eur. and USP |
| VP 1000 (ERWEKA®) | Suitable for testing MDIs and DPIs. | NA |
| HPV (ERWEKA®) | Achieves sonic flow. | NA |
TABLE 2: ACCESSORIES REQUIRED ALONG WITH THE DOSAGE UNIT SAMPLING APPARATUS AND CASCADE IMPACTOR
Particle In Vitro Dissolution Studies
Dissolution testing of the inhaled drug products varies from the other drug delivery systems as it depends on the emitted dose and the deposition in the respiratory tract as oppose to the drug release or dissolution of the whole system like solid dosage forms. Till now the inhaled drug product lacks any prescribed and established dissolution testing method because of their different nature of drug administration and deposition to the system. However many studies using conventional dissolution method have been used in dissolution testing of inhaled dosage forms, but these studies are crucial to existing approved products. Using such system for testing novel controlled release system required more emphasis[48]. Simulating the in vivo condition of lungs has been difficult as due to low fluid content and also the presence of biological surfactants. Other difficulty arising in the dissolution testing of inhaled drug products is the quantification of the deposited drug from the site of the impactors and impingers. Thus for overcoming these difficulties in dissolution testing using conventional dissolution apparatus[49] various testing methods have been employed such as a horizontal diffusion cell[50], a dissolution cell[51,52], and a TSI[53]. However, no single in vitro test system has yet emerged as the ideal choice for performing dissolution measurements for inhalation formulations. Some novel methods have been developed using USP flow-through cell system for determining dissolution behavior of inhaled drug products[54]. Further Davis et al. had tested the deposited aerosol particles on a filter membrane in an abbreviated Andersen cascade impactor. These membranes were placed in a two different dissolution systems like a Franz diffusion cell and a flow through cell apparatus[55]. The whole emitted dose have been taken into the study and not just those fine fraction of particles, also the dissolution medium lacks lung surfactant which can stimulate in vivo condition. To overcome these issues, Son and McConville at the University of Texas have developed a testing method with the use of NGI collection cups, which captures the fine fractions of emitted dose following aerodynamic separation, the separated fractions on their respective membranes were covered with another presoaked membrane. A sealed disc is formed by clamping these two membranes with membrane holder and can be placed in a vessel of a conventional dissolution apparatus like paddle over disc. The dissolution testing results of hydrocortisone from these membrane holder showing major deviation among the aerodynamically separated dose and that of bulk formulation[56]. Son et al.[57] customized a modified version of prototype membrane holder especially for the NGI which showed better dose collection.
Designing a standardized dissolution method applicable to the lung is not an easy task, because the lung has unique features that are difficult to replicate in vitro, such as the extremely small amount of aqueous fluid and lung surfactant. Marques et al.discussed various simulated lung fluids used in dissolution testing of inhaled particles. Simulation of lungs interstitial environments has been achieved by lysosomal fluid (ALF) and Gamble’s solution together termed as SLF1. ALF mimics the condition inside of alveolar and interstitial macrophages while, Gamble’s solution simulate the interstitial fluid condition deep inside the lungs[58]. SLF2 is a modified version of Gamble’s solution and simulate extracellular lung fluids condition[59]. SLF3 a simulating the interstitial fluid[60]was used to determine insulin’s in vitro release from the porous particles intended for lung delivery[61].Son and McConville[56]used a SLF3 and SLF4 in standard test method to determine dissolution characteristics of various formulations intended for inhalation. SLF4 is a modified type of SLF3 with the addition of 0.02% [w/v] dipalmitoylphosphatidyl choline (DPPC). Itraconazole in vitro release from inhaled nanoparticles is evaluated with the use of SLF4[62]. SLF5 is used by Cheng et al. to evaluate the dissolution of titanium tritide particles used as components of neutron generators[63]. DPPC were found to form large size liposomes and constrict the diffusion of SLF4 through the membrane thereby preventing it to reach the drug particles[56]. Therefore biological buffers like phosphate buffer and phosphate buffer saline were used to determine the novel controlled release products[50,56,64-66]. The standardized dissolution media has not been developed yet because the media selection for lung dissolution is quite dependent on the test apparatus, the chemical/physical properties of active pharmaceutical ingredients (APIs) as described above. Thus, careful and thorough examinations and validation in the selection of media must be made, especially if one seeks to establish valid in vitro-in vivo correlations.
Cell Culture Study
The formulations must have to be surpassed through the cell culture testing methods before the ex vivo and in vivo testing. The last two decades have seen a numerous cell models obtained from human and murine tissues of pulmonary epithelium[67,68]. Continuous cell cultures provide ease in usage in contrast to primary cell cultures with a lack of distinguished morphology and characteristics of biochemicals of the original tissue. It arises from alveolar epithelial cells. Human lung adenocarcinoma originates a type II alveolar epithelial cell line A549. It is less interesting as a drug delivery model because A549 cells do not form stretched monolayers, but very helpful in metabolic and toxicological studies[69]. Primary cell cultures like alveolar epithelial cells are used for cell culture studies. Type II pneumocytes for primary culture can be obtained from the different lungs species. Cells from human are less available than cells from other mammals and are representatives of the clinical circumstances. Airinterface cultures aerosol particles are placed straight onto the semi-dry apical cell surface. The deposition of drug and its dissolution held in a small volume of cell lining fluid, mimics the in vivo condition. The culture show more resemblance to airways epithelial morphology[70]. Recently, Stephanie et al. developed a pharmaceutical aerosol deposition device on cell cultures known as PADDOCC to simulate in vivo conditions in which aerosol dose emitted on monolayers of epithelial cell are exposed to an air-liquid interface. PADDOCC minimizes testing of animals and helps in the establishment of early clinical trials[71].
Drug Administration System
In whole-body exposure system a sealed plastic chamber is attached to a dry powder aerosol generator or a nebulizer and the animals are placed inside the chamber[72,73]. Pulmonary administration is less stressful as compared to other exposure system[74]; but some absorption of drug through the skin, from the nasal route and from the gastrointestinal tract of the animals. In head-only or nose-only exposure systems system the animal is restrained in the exposure chamber and the aerosol is in contact with only the head or nose of the animal. One or more animals can be administered through specific design of this system. It offered several merits over other exposure system like exposure of drug to the skin and its uptake through it can be avoided. The amount of drug needed to generate the aerosol is less due to low volume of the exposure chamber. Drug reactivity with excreta is also prevented[75]. There are commercially available exposure systems in the market, however some homemade models of head-only or nose-only aerosol exposure systems are made and evaluated[76-82]. Intratracheal liquid instillation are achieved by oral gavage needles and the MicroSprayer®[83-85]. The oral gavage needle delivers dose in the form of a liquid bolus while the MicroSprayer® gives spray instillation. Dry powders can be delivered intratracheally by using a powder-insufflator or by the generation of powder aerosol. It is simple method of pulmonary drug delivery and significant changes in site of drug deposition within the lung can be achieved by small modification in the method and thereby affecting systemic absorption of drug. Control of delivery of drug dose, the absence of drug loss in the instrument, the avoidance of nasal passages and the possible targeting of different regions within the respiratory organ are some of the merits of this exposure system. The powder insufflators are used to deliver various drugs to the lungs in different respiratory and non-respiratory diseases like COPD[86], asthma[50], pulmonary embolism[87], lung cancer[88-90], tuberculosis[72,91-98], diabetes[99-101], osteoporosis[102] and cancer[103,104]. The delivery of some immunosuppressant[105] and immunostimulant agents[106] were also reported. Intranasal administration is used for local nasal drug delivery but it can also be used for intrapulmonary drug administration in animals[107,108].
Drug Deposition Studies
The amount of drug deposited in the lung and its distribution are key parameters in the evaluation of the formulation and performance of devices for pharmaceutical aerosols. Deposition of the drugs can be measured by invasive and noninvasive methods in experimental animals. Noninvasive technique provides merit as it gives in vivo pharmacokinetic parameters with respect to the time course of drug administration. Dolovich[109] studied the use and principles of singlephoton emission computed tomography (SPECT), gamma scintigraphy and positron emission tomography (PET) as imaging methods in animal studies. These imaging methods are successfully employed in pulmonary drug delivery and inhalation toxicology. Invasive techniques such as broncho alveolar lavage (BAL) method can be employed for insoluble, inert particles when the non-invasive methods are inadequate to measure the drug deposition.
Pharmacokinetic Studies
Compartmental or non-compartmental methods are used to calculate pharmacokinetic parameters after inhalation of the drug. A mathematical model describing the disposition of a given drug can be obtained by incorporating the physiologically based pharmacokinetics (PBPK)[110]. PBPK consists of different compartments with each representing a particular organ. Byron[111] and Gonda[112] developed a mathematical and compartmental model to address lung residence time of soluble aerosols.
Ex Vivo Tests
The mechanisms of drug transport across lung tissue can be studied by ex vivo lung model. These models can also help in vitro-in vivo correlation. Isolated perfused lung (IPL) and precision cut lung slices (PCLS) are different ex vivo models. Sakagami[113] and Beck-Broichsitter et al. described IPL ex vivo model showing uptake of particles. PCLS are another ex vivo model used conveniently. Some Toxicity testing are performed by PCLS model[114-117].
The development of novel carrier systems for the pulmonary drug delivery gives rise to the need of advancement in the field of characterization technology for evaluation of these inhalation products. The efficacy, safety and quality of the drug and drug products can be estimated and established by the current compendial and standardized testing methods, which are the need evolving with time. The better understanding of the product testing and characterization methods can lead to the successful development of pulmonary drug delivery system.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov 2007;6:67-74.
- Salama R, Traini D, Chan H, Young P. Preparation and characterisation of controlled release co-spray dried drug-polymer microparticles for inhalation 2: Evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur J Pharm Biopharm 2008;70:145-52.
- Ugwoke, Michael IV, Ingrid JL, Henrik. New Trends and Opportunities Microparticles and Liposomes as Pulmonary Drug Delivery Systems: What Are the Recent Trends?. In: Karolinc B-P, Hcnrik L, editors. Pulmonary Drug Delivery Basics, Applications and Opportunities for Small Molecules and Biopharmaceutics. 1st ed. Aulendorf Germany: Editio Cantor Verlag; 2007. p. 308-31.
- Liang Z, Ni R, Zhou J, Mao S. Recent advances in controlled pulmonary drug delivery. Drug Discov Today 2015;20:380-9.
- Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev 2015;85:83-99.
- Kaur IP, Singh H. Nanostructured drug delivery for better management of tuberculosis. J Control Release 2014;184:36-50.
- Todoroff J, Vanbever R. Fate of nanomedicines in the lungs. Curr Opin Colloid Interface Sci 2011;16:246-54.
- Yang W, Peters JI, Williams RO. Inhaled nanoparticles - a current review. Int J Pharm 2008;356(1-2):239-47.
- Mühlfeld C, Rothen-Rutishauser B, Vanhecke D, Blank F, Gehr P, Ochs M. Visualization and quantitative analysis of nanoparticles in the respiratory tract by transmission electron microscopy. Part Fibre Toxicol 2007;4:11.
- Chen AZ, Yang YM, Wang SB, Wang GY, Liu YG, Sun QQ. Preparation of methotrexate-loaded, large, highly-porous PLLA microspheres by a high-voltage electrostatic antisolvent process. J Mater Sci Mater Med 2013;24:1917-25.
- Bunker M, Davies M, Roberts C. Towards screening of inhalation formulations: measuring interactions with atomic force microscopy. Expert Opin Drug Deliv 2005;2:613-24.
- Chew NY, Tang P, Chan HK, Raper JA. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res 2005;22:148-52.
- Tang P, Chan HK, Raper JA. Validation of computation method to predict aerodynamic diameter of particles with rough surface. Powder Technol 2009;192:74-84.
- Tang P, Chew NYK, Chan HK, Raper JA. Limitation of determination of surface fractal dimension using N-2 adsorption isotherms and modified Frenkel-Halsey-Hill theory. Langmuir 2003;19:2632-8.
- El-Gendy N, Pornputtapitak W, Berkland C. Nanoparticle agglomerates of fluticasone propionate in combination with albuterol sulfate as dry powder aerosols. Eur J Pharm Sci 2011;44:522-33.
- Maretti E, Rossi T, Bondi M, Croce MA, Hanuskova M, Leo E, et al.Inhaled Solid Lipid Microparticles to target alveolar macrophages for tuberculosis. Int J Pharm 2014;462:74-82.
- Chan HK, Chew NY. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev 2003;55:793-805.
- Chan JG, Chan HK, Prestidge CA, Denman JA, Young PM, Traini D. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur J Pharm Biopharm 2013;83:285-92.
- Telko MJ, Kujanpaa J, Hickey AJ. Investigation of triboelectric charging in dry powder inhalers using electrical low pressure impactor (ELPI™). Int J Pharm 2007;336:352-60.
- Young P, Sung A, Traini D, Kwok P, Chiou H, Chan H. Influence of humidity on the electrostatic charge and aerosol performance of dry powder inhaler carrier based systems. Pharm Res. 2007;24:963-70.
- Hoe S, Traini D, Chan HK, Young PM. Measuring charge and mass distributions in dry powder inhalers using the electrical next generation impactor (eNGI). Eur J Pharm Sci 2009;38:88-94.
- Hoe S, Young PM, Chan HK, Traini D. Introduction of the electrical next generation impactor (eNGI) and investigation of its capabilities for the study of pressurized metered dose inhalers. Pharm Res 2009;26:431-7.
- Zhu KW, Ng WK, Shen SC, Tan RB, Heng PW. Design of a device for simultaneous particle size and electrostatic charge measurement of inhalation drugs. Pharm Res 2008;25:2488-96.
- Begat P, Morton DA, Staniforth JN, Price R. The cohesive-adhesive balances in dry powder inhaler formulations I: Direct quantification by atomic force microscopy. Pharm Res 2004;21:1591-7.
- Bunjes H. Lipid nanoparticles for the delivery of poorly water-soluble drugs. J Pharm Pharmacol 2010;62:1637-45.
- Bunjes, H. Structural properties of solid lipid based colloidal drug delivery systems. Curr Opin Colloid Interface Sci 2011;16:405-11.
- Bunjes H, Unruh T. Characterization of lipid nanoparticles by differential scanning calorimetry, X-ray and neutron scattering. Adv Drug Deliv Rev 2007;59:379-402.
- MuellerR. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur J Pharm Biopharm 2000;50:161-77.
- Rees PJ, Clark TJ, Morén F. The importance of particle size in responses to inhaled bronchodilators. Eur J Respir Dis 1982;63:73-8.
- Zanen P, Go TL, Lammers J. Optimal particle size for beta 2 agonist and anticholinergic aerosols in patients with severe airflow obstruction. Thorax 1996;51:977-80.
- Zanen P, Go TL, Lammers J. The optimal particle size for β-adrenergic aerosols in mild asthmatics. Int J Pharm 1994;107:211-7.
- Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005-15 µm. J Aerosol Sci 1986;17:811-25.
- Makino K, Yamamoto N, Higuchi K, Harada N, Ohshima H, Terada H. Phagocytic uptake of polystyrene microspheres by alveolar macrophages: effects of the size and surface properties of the microspheres. Colloid Surf B: Biointerfaces 2003;27:33-9.
- Hasegawa T, Hirota K, Tomoda K, Ito F, Inagawa H, Kochi C, et al. Phagocytic activity of alveolar macrophages toward polystyrene latex microspheres and PLGA microspheres loaded with anti-tuberculosis agent. Colloid Surf B: Biointerfaces 2007;60:221-8.
- Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, et al. Optimum conditions for efficient phagocytosis of rifampicin loaded PLGA microspheres by alveolar macrophages. J Control Release 2007;119:69-76.
- Pratten MK, Lloyd JB. Pinocytosis and phagocytosis: the effect of size of a particulate substrate on its mode of capture by rat peritoneal macrophages cultured in vitro. Biochim Biophys Acta 1986;881:307-13.
- Seymour L, Schacht E, Duncan R. The effect of size of polystyrene particles on their retention within the rat peritoneal compartment, and on their interaction with rat peritoneal macrophages in vitro. Cell Biol Int Rep 1991;15:277-86.
- Kawaguchi H, Koiwai N, Ohtsuka Y, Miyamoto M, Sasakawa S. Phagocytosis of latex particles by leucocytes. I. Dependence of phagocytosis on the size and surface potential of particles. Biomaterials 1986;7:61-6.
- Edwards DA, Ben-Jebria A, Langer R. Recent advances in pulmonary drug delivery using large, porous inhaled particles. J Appl Physiol 1998;84:379-85.
- Edwards D, Dunbar C. Bioengineering of Therapeutic Aerosols. Annu Rev Biomed Eng 2002;4:93-107.
- He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010;31:3657-66.
- Mann J, McKay P, Arokiasamy S, Patel R, Klein K, Shattock R. Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J Control Release 2013;170:452-9.
- Jab?czy?ska K, Janczewska M, Kulikowska A, Sosnowski T. Preparation and Characterization of Biocompatible Polymer Particles as Potential Nanocarriers for Inhalation Therapy. Int J Polym Sci 2015;2015:1-8.
- Konde D, Sosnowski T. Changes in the activity of the pulmonary surfactant after contact with bentonite nanoclay particles. Chem Eng Trans 2012;26:531-6.
- Mali A, Pawar A, Purohit R. Development of Budesonide Loaded Biopolymer Based Dry Powder Inhaler: Optimization, In vitroDeposition, and Cytotoxicity Study. J Pharm 2014;2014:1-12.
- Newman SP, Chan HK. In vitro/in vivocomparisons in pulmonary drug delivery. J Aerosol Med Pulm Drug Deliv 2008;21:77-84.
- Fujitani Y, Hasegawa S, Fushimi A, Kondo Y, Tanabe K, Kobayashi S, et al. Collection characteristics of low-pressure impactors with various impaction substrate materials. Atmos Environ 2006;40:3221-9.
- Grey VA, Hickey AJ, Balmer P, Davies NM, Dunbar C, Foster TS, et al. The Inhalation Ad Hoc Advisory Panel for the USP performance tests of inhalation dosage forms. Pharmacopeial Forum 2008;34:1068-74.
- Batycky R, Deaver D, Dwivedi S, Hrkach J, Johnston L, Olsen T, et al. The development of large porous particles for inhalation drug delivery. In: Rathbone MJ, Hadgraft J, Roberts MS, editors. Modified-Release Drug Delivery Technology. 1st ed. New York: Marcel Dekker; 2003. p. 891-902.
- Cook RO, Pannu RK, Kellaway IW. Novel sustained release microspheres for pulmonary drug delivery. J Control Release 2005;104:79-90.
- Ansoborlo E, Guilmette RA, Hoover MD, Chazel V, Houpert P, Henge-Napoli MH. Application of in vitrodissolution tests to different uranium compounds and comparison with in vivodata. Radiat Prot Dosim 1998;79:33-7.
- Sdraulig S, Franich R, Tinker RA, Solomon S, O’Brien R, Johnston PN. In vitrodissolution studies of uranium bearing material in simulated lung fluid. J Environ Radioact 2008;99:527-38.
- McConville JT, Patel N, Ditchburn N, Tobyn MJ, Staniforth JN, Woodcock P. Use of a novel modified TSI for the evaluation of controlled-release aerosol formulations. I. Drug Dev Ind Pharm 2000;26:1191-8.
- Taylor MK, Hickey AJ, VanOort M. Manufacture, characterization, and pharmacodynamics evaluation of engineered ipratropium bromide particles. Pharm Dev Technol 2006;11:321-36.
- Davies NM, Feddah MIR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm 2003;255:175-87.
- Son YJ, McConville JT. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int J Pharm 2009;382:15-22.
- Son Y, Horng M, Copley M, McConville J. Optimization of an in vitrodissolution test method for inhalation formulations. Dissolution Technol. 2010;17:6-13.
- Colombo C, Monhemius A, Plant J. Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotox Environ Safe 2008;71:722-30.
- Gray JE, Plumlee GS, Morman SA, Higueras PL, Crock JG, Lowers HA,et al. In vitrostudies evaluating leaching of mercury from mine waste calcine using simulated human body fluids. Environ Sci Technol 2010;44:4782-8.
- Moss OR. Simulants of lung interstitial fluid. Health Phys. 1979;36:447-8.
- Ungaro F, Bianca RE, Giovino C, Miro A, Sorrentino R, Quaglia F, et al. Insulin-loaded PLGA/cyclodextrin large porous particles with improved aerosolization properties: in vivodeposition and hypoglycaemic activity after delivery to rat lungs. J Controlled Release 2009;135:25-34.
- Yang W, Tam J, Miller DA, Zhou J, McConville JT, Johnston KP, et al. High bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizers. Int J Pharm 2008;361:177-88.
- Cheng YS, Dahl AR, Jow HN. Dissolution of Metal Tritides in a Simulated Lung Fluid. Health Phys 1997;73:633-8.
- Learoyd TP, Burrows JL, French E, Seville PC. Chitosan-based spray-dried respirable powders for sustained delivery of terbutaline sulfate. Eur J Pharm Biopharm 2008;68:224-34.
- O’Hara P, Hickey AJ. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharm Res 2000;17:955-61.
- Sung JC, Padilla DJ, Garcia-Contreras L, VerBerkmoes JL, Durbin D, Peloquin CA, et al. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res 2009;26:1847-55.
- Forbes B, Shah A, Martin GP, Lansley AB. The human bronchial epithelial cell line 16HBE14o− as a model system of the airways for studying drug transport. Int J Pharm 2003;257:161-7.
- Sakagami M. In vivo, in vitroand ex vivomodels to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev 2006;58:1030-60.
- Lin H, Li H, Cho HJ, Bian S, Roh HJ, Lee MK, et al. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitromodel for airway drug transport studies. J Pharm Sci 2006;96:341-50.
- Blank F, Rothen-Rutishauser B, Schurch S, Gehr P. An optimized in vitromodel of the respiratory tract wall to study particle cell interactions. J Aerosol Med 2006;19:392-405.
- Hein S, Bur M, Schaefer U, Lehr C. A new Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) to evaluate pulmonary drug absorption for metered dose dry powder formulations. Eur J Pharm Biopharm 2011;77:132-8.
- Garcia-Contreras L, Fiegel J, Telko MJ, Elbert K, Hawi A, Thomas M, et al. Inhaled large porous particles of capreomycin for treatment of tuberculosis in a guinea pig model. Antimicrob Agents Chemother 2007;51:2830-6.
- Garcia-Contreras L, Sung JC, Muttil P, Padilla D, Telko M, Verberkmoes JL, et al. Dry powder PA-824 aerosols for treatment of tuberculosis in guinea pigs. Antimicrob Agents Chemother2010;54:1436-42.
- McConville JT, Williams RO, Carvalho TC, Iberg AN, Johnston KP, Talbert RL, et al. Design and evaluation of a restraint-free small animal inhalation dosing chamber. Drug Dev Ind Pharm 2005;31:35-42.
- Fernandes CA, Vanbever R. Preclinical models for pulmonary drug delivery. Expert Opin Drug Deliv 2009;6:1231-45.
- Denkers ND, Seelig DM, Telling GC, Hoover EA. Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J Gen Virol 2010;91:1651-8.
- Sinha B, Mukherjee B. Development of an inhalation chamber and a dry powder inhaler device for administration of pulmonary medication in animal model. Drug Dev Ind Pharm 2012;38:171-9.
- Yang W, Johnston KP, Williams RO 3rd. Comparison of bioavailability of amorphous versus crystalline itraconazole nanoparticles via pulmonary administration in rats. Eur J Pharm Biopharm 2010;75:33-41.
- Sharma R, Saxena D, Dwivedi AK, Misra A. Inhalable microparticles containing drug combinations to target alveolar macrophages for treatment of pulmonary tuberculosis. Pharm Res 2001;18:1405-10.
- Yuan Y, Li F, Wang YM, Zhang RQ, Wei LP, Wang HC. Respiratory face mask: a novel and cost-effective device for use during the application of myocardial ischemia in rats. J Zhejiang Univ Sci B 2009;10:391-4.
- Tam JM, McConville JT, Williams RO 3rd, Johnston KP. Amorphous cyclosporin nanodispersions for enhanced pulmonary deposition and dissolution. J Pharm Sci2008;97:4915-33.
- Kaur J, Muttil P, Verma RK, Kumar K, Yadav AB, Sharma R, et al. A hand-held apparatus for "nose-only" exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur J Pharm Sci 2008;34:56-65.
- Lee J, Oh YJ, Lee SK, Lee KY. Facile control of porous structures of polymer microspheres using an osmotic agent for pulmonary delivery. J Control Release 2010;146:61-7.
- Varshosaz J, Minaiyan M, Forghanian M. Prolonged hypocalcemic effect by pulmonary delivery of calcitonin loaded poly(methyl vinyl ether maleic acid) bioadhesive nanoparticles. Biomed Res Int 2014;2014:1-13.
- Beck-Broichsitter M, Gauss J, Gessler T, Seeger W, Kissel T, Schmehl T. Pulmonary targeting with biodegradable salbutamol-loaded nanoparticles. J Aerosol Med Pulm Drug Deliv 2010;23:47-57.
- Yoo NY, Youn YS, Oh NM, Oh KT, Lee DK, Cha KH, et al.Antioxidant encapsulated porous poly(lactide-co-glycolide) microparticles for developing long acting inhalation system. Colloids Surf B Biointerfaces2011;88:419-24.
- Rawat A, Majumder QH, Ahsan F. Inhalable large porous microspheres of low molecular weight heparin: in vitro and in vivoevaluation. J Control Release2008;128:224-32.
- Dhanda DS, Tyagi P, Mirvish SS, Kompella UB. Supercritical fluid technology based large porous celecoxib-PLGA microparticles do not induce pulmonary fibrosis and sustain drug delivery and efficacy for several weeks following a single dose. J Control Release 2013;168:239-50.
- Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT, Shin BS, et al. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained- release inhalation system for the treatment of metastatic lung cancer. Biomaterials 2012;33:5574-83.
- Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT, Shin BS, et al. Doxorubicin-loaded porous PLGA microparticles with surface attached TRAIL for the inhalation treatment of metastatic lung cancer. Biomaterials 2013;34:6444-53.
- Lu D, Garcia-Contreras L, Muttil P, Padilla D, Xu D, Liu J, et al. Pulmonary Immunization Using Antigen 85-B Polymeric Microparticles to Boost Tuberculosis Immunity. AAPSJ 2010;12:338-47.
- Lu D, Garcia-Contreras L, Xu D, Kurtz SL, Liu J, Braunstein M, McMurray DN, Hickey AJ. Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitroT cell response to recombinant Mycobacterium tuberculosisantigen 85B. Pharm Res 2007;24:1834-43.
- Suarez S, O'Hara P, Kazantseva M, Newcomer CE, Hopfer R, McMurray DN, et al. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm Res 2001;18:1315-9.
- Doan TV, Olivier JC. Preparation of rifampicin-loaded PLGA microspheres for lung delivery as aerosol by premix membrane homogenization. Int J Pharm2009;382:61-6.
- Suarez S, O'Hara P, Kazantseva M, Newcomer CE, Hopfer R, McMurray DN, et al. Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J Antimicrob Chemother 2001;48:431-4.
- Sung JC, Padilla DJ, Garcia-Contreras L, Verberkmoes JL, Durbin D, Peloquin CA, et al. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res 2009;26:1847-55.
- Ohashi K, Kabasawa T, Ozeki T, Okada H. One-step preparation of rifampicin/poly (lactic-co-glycolic acid) nanoparticle-containing mannitol microspheres using a four-fluid nozzle spray drier for inhalation therapy of tuberculosis. J Control Release 2009;135:19-24.
- Fiegel J, Garcia-Contreras L, Thomas M, VerBerkmoes J, Elbert K, Hickey A, et al. Preparation and in vivoevaluation of a dry powder for inhalation of capreomycin. Pharm Res 2007;25:805-11.
- Ungaro F, De Rosa G, Miro A, Quaglia F, La Rotonda MI. Cyclodextrins in the production of large porous particles: Development of dry powders for the sustained release of insulin to the lungs. Eur J Pharm Sci2006;28:423-32.
- Surendrakumar K, Martyn GP, Hodgers EC, Jansen M, Blair JA. Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J Control Release 2003;91:385-94.
- Dong Z, Hamid KA, Gao Y, Lin Y, Katsumi H, Sakane T, et al. Polyamidoamine dendrimers can improve the pulmonary absorption of insulin and calcitonin in rats. J Pharm Sci 2010;100:1866-78.
- Tewes F, Gobbo OL, Amaro MI, Tajber L, Corrigan OI, Ehrhardt C, et al. Evaluation of HPβCD-PEG microparticles for salmon calcitonin administration via pulmonary delivery. Mol Pharm 2011;8:1887-98.
- Alipour S, Montaseri H, Tafaghodi M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids Surf B Biointerfaces 2010;81:521-9.
- Koushik K, Dhanda DS, Cheruvu NP, Kompella UB. Pulmonary delivery of deslorelin: Large-porous PLGA particles and HPCD complexes. Pharm Res 2004;21(7):1119-26.
- Onoue S, Sato H, Kawabata Y, Mizumoto T, Hashimoto N, Yamada S. In vitroand in vivocharacterization on amorphous solid dispersion of cyclosporine A for inhalation therapy. J Control Release 2009;138:16-23.
- Li YZ, Sun X, Gong T, Liu J, Zuo J, Zhang ZR. Inhalable microparticles as carriers for pulmonary delivery of thymopentin-loaded solid lipid nanoparticles. Pharm Res 2010;27:1977-86.
- Seong JH, Lee KM, Kim ST, Jin SE, Kim CK. Polyethylenimine-based antisense oligodeoxynucleotides of IL-4 suppress the production of IL-4 in a murine model of airway inflammation. J Gene Med2006;8:314-23.
- Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv Drug Deliv Rev 2005;57:411-30.
- Dolovich MB. Measuring Total and Regional Lung Deposition Using Inhaled Radiotracers. J Aerosol Med 2001;14 suppl 1:35-44.
- Chiu W, Barton H, DeWoskin R, Schlosser P, Thompson CM, SonawaneB, et al. Evaluation of physiologically based pharmacokinetic models for use in risk assessment. J Appl Toxicol 2007;27:218-37.
- Byron P. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci 1986;75:433-8.
- Gonda I. Drugs administered directly into the respiratory tract:Modeling of the duration of effective drug levels. J Pharm Sci 1988;77:340-6.
- Deuse T, Blankenberg F, Haddad M, Reichenspurner H, Phillips N, Robbins RC, et al. Mechanisms behind local immunosuppression using inhaled tacrolimus in preclinical models of lung transplantation. Am J Respir Cell Mol Biol 2010;43:403-12.
- Niven RW. Modulated drug therapy with inhalation aerosols. In: Hickey AJ, editor. Pharmaceutical inhalation aerosol technology. New York, NY: Marcel Dekker; 1992. p. 321-359.
- Florea B, Cassara M, Junginger H, Borchard G. Drug transport and metabolism characteristics of the human airway epithelial cell line Calu-3. J Control Release2003;87:131-8.
- Khanna C, Waldrep JC, Anderson PM, Weischelbaum RW, Hasz DE, Katsanis E, et al. Nebulized interleukin 2 liposomes: Aerosol characteristics and biodistribution. J Pharm Pharmacol 1997;49(10):960-71.
- Kreyling WG, Blanchard JD, Godleski JJ, Haeussermann S, Heyder J, Hutzler P, et al. Anatomic localization of 24- and 96-h particle retention in canine airways. J Appl Physiol 1999;87:269-84.