- *Corresponding Author:
- N. M. Hoskatte
Department of Botany, Karnatak University, Dharwad, Karnataka 580003, India
E-mail: hnmurthy60@gmail.com
| Date of Received | 11 January 2021 |
| Date of Revision | 04 August 2022 |
| Date of Acceptance | 13 February 2023 |
| Indian J Pharm Sci 2023;85(1):261-266 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The major objective of this investigation was to evaluate the cytotoxic activity of Andrographis lineata Wall. ex Nees var. lawii C.B. Clarke leaf methanol extract on cancer cell lines. In vitro cytotoxicity of Andrographis lineata leaf extract was evaluated against A375 (melanoma), A549 (lung), HCT116 (colon), HeLa (cervix), HepG2 (hepatoma), Jurkat cells (T-cell acute lymphoblastic leukemia), MCF7 (breast cancer), PA1 (ovary), PC3 (prostate) and U87MG (Glioblastoma) cancer cell lines. Andrographis lineata leaf extract showed cytotoxicity activity against all cell lines, however, optimum cytotoxicity was observed against the ovarian cancer cell line (IC50=39.7 μg/ml) cell line. Cell cycle and terminal deoxynucleotidyl transferase dUTP end labeling assays confirmed the cytotoxic activity of Andrographis lineata leaf extract. Reverse phasehigh performance liquid chromatography analysis was performed to recognize the active ingredient in the Andrographis lineata leaf extract and the results reveal that andrographolide was the major component. This investigation is the first report of the anticancer activity of Andrographis lineata leaf extract.
Keywords
Andrographis lineata var. lawii, andrographolide, anti-cancer, reverse phase high performance liquid chromatography
Cancer is a major public health problem in the world and is responsible for the majority of deaths. Various cancer treatments are available including surgery, chemotherapy, radiation therapy, immunotherapy, targeted therapy and hormonal therapy and kind of treatment depends on the type of cancer and how it has advanced in the individuals. Recent research efforts are in search of natural phytochemicals in the prevention and treatment of cancer since natural compounds have advantages like lower side effects and general availability[1]. Andrographis (Acanthaceae) is an ethnobotanical important genus and many species of Andrographis have been utilized in different medicinal systems such as Unani, Siddha, Ayurveda and Traditional Chinese Medicine to cure a broad spectrum of health issues. In the last decade, many scientific proofs were available, that antiinflammatory, anti-microbial, antioxidant, immune stimulant and cytotoxic properties have been attributed to Andrographis species[2]. Andrographolides isolated from Andrographis paniculata (Acanthaceae) are extremely bitter diterpenoid compounds, which are recognized as potential chemotherapeutic agents for the treatment of various kinds of cancers[3]. Several diterpenoid compounds such as 14-deoxy- 11,12-didehydroandrographolide and andrograpanin exhibited significant inhibition of biofilm production by Pseudomonas aeruginosa[4,5]. Andrographis lineata Wall. ex Nees var. lawii C. B. Clarke is a potent species of the genus Andrographis with various medicinal properties. Various extracts (acetone, ethanol, methanol, petroleum ether and chloroform) were found to be showing several biological activities such as antipyretic, anti-inflammatory, antivenom, antidiabetic, antimicrobial, hepatoprotective activity[6]. However, a literature survey revealed the exploration of this plant for its cytotoxicity. Therefore, the current study was undertaken to evaluate the in vitro cytotoxic activity of Andrographis lineata Wall. ex Nees var. lawii C.B. Clarke on selected cancer cell lines and further flow cytometric studies on apoptosis against human ovarian teratocarcinoma cell line expecting new potent anti-cancerous species of genus Andrographis.
Andrographis lineata Wall. ex Nees var. lawii C.B. Clarke plants were collected from Jogimatti, Chitradurga district, Karnataka (14°09'31.9"N 76o23'43.8"E), India. Plants were identified by using Flora of British India and a voucher specimen (DSD- 06A) was deposited at Herbarium, Shivaji University, Kolhapur, India. Healthy leaves of Andrographis lineata Wall. ex Nees var. lawii C.B. Clarke was collected, dried to constant weight under shade and ground into powder form using a blender. The ground plant material (30 g) was extracted with methanol (Analytical grade, HiMedia, Mumbai, India) using soxhlet apparatus at 70° for 8 h.
Ten different cancer cell lines were considered for the current study viz. melanoma cancer line (A375, ATCC #CRL-1619), lung cancer cell line (A549, ATCC #CCL-185), colon cancer cell line (HCT116, ATCC #CCL-247), cervix cancer cell line (HeLa, ATCC #CCL-2), hepatoma cell line (HepG2, ATCC #HB-8065), T cell acute lymphoblastic leukemia cell line (Jurkat cells, ATCC #TIB-152), breast cancer cell line (MCF7, ATCC #HTB-22), ovary cancer cell line (PA1, ATCC #CRL-1572), prostate cancer cell line (PC3, ATCC #CRL-1435) and Glioblastoma (U87MG, ATCC #HTB-14) were obtained from National Centre for Cell Science, Pune, India. A375, A549, HCT116, HeLa, HepG2, PA1, PC3 and U87MG lines were maintained in Dulbecco’s Modified Eagles Medium (DMEM, HiMedia, Mumbai, India) supplemented with 10 % Fetal Bovine Serum (FBS). Jurkat cell lines were maintained in Roswell Park Memorial Institute medium (RPMI, HiMedia, Mumbai, India) with 10 % FBS, whereas, MCF7 cell lines were maintained in Minimum Essential Medium (MEM, HiMedia, Mumbai, India) containing 10 % FBS. The cell lines were maintained at 37° in a humidified incubator (95 % air and 5 % CO2).
The plant extract was tested for its in vitro cytotoxicity activity using the 3-(4,5-dimethylthiaxo- 2yl) 2,5-diphenyl tetrazolium bromide (MTT) assay. The cell lines were maintained at 37° in a humidified incubator (95 % air and 5 % CO2) and plated into 96- well microplates (10 000 cells/200 μl). The extract was dissolved in Dimethyl Sulphoxide (DMSO) and further diluted with the respective medium (DMEM/ RPMI/MEM medium) to obtain a stock solution that gave a DMSO final concentration of 0.5 %. Concentrations of plant extract ranging from 100- 500 μg/ml were prepared by serial dilutions with the medium. After 24 h of incubation, test samples were added to the wells containing the cells and incubated for 24 h. 200 μl of MTT solution (500 μg/ ml) was added to each well and incubation was continued for another 3 h. DMSO solution was used as a positive control during the assay. After the incubation formazan crystals formed were dissolved in 100 μl DMSO. The Optical Density (OD) was measured with a microplate reader (Bio-Rad, model 3550, USA) at 570 nm (OD570-620). The percentage of net growth of the cells was calculated using the formula (T-T0/T0)×100 where T is the OD 570 nm of the cells at the end of the experiment and T0 is the OD 570 nm of the cell at the time of loading cells. IC50 was generated from the dose-response curve for each cell line. For A375 and PA1 cancer cells, the IC50 ranged below the experimental scale of concentrations. Thus, the whole experiment was repeated for the lower concentration of extract ranging from 10-160 μg/ ml.
Annexin V apoptosis assay was conducted by flow cytometry[7]. PA1 cells were cultured in a 6-well plate at a density of 3×105 cells/2 ml and incubated in a CO2 incubator at 37°. After incubation of 24 h, the spent medium was removed and cells were washed with 1X Phosphate Buffered Saline (PBS). Cells were treated with the required concentration of experimental test compound (IC50=39.7 μg/ml) which was prepared in 2 ml of culture medium, well with the only medium considered as negative control and incubated for 24 h. The medium was removed from all the wells and washed with 500 μl PBS. After removing the PBS 200 μl of the trypsin-EDTA solution was added and incubated at 37° for 3-4 min. Cells were harvested along with the medium and centrifuged at 300 xg at 25°. The supernatant was discarded and the pellet of cells was washed with PBS twice. 1X binding buffer was prepared from the stock 10X buffer using distilled water. 100 μl of that solution was taken and stained by 5 μl of Fluorescein Isothiocyanate (FITC) Annexin V, mixed gently and incubated at room temperature for 15 min in dark. Secondary staining was done with 5 μl of Propidium Iodide (PI) and analyzed by flow cytometer (Cytomics FC 500, Beckman Coulter, USA) immediately. FITC-Annexin V fluorescence was collected in the FL1 detector using a 525 nm bandpass filter while PI fluorescence was collected in FL3 using a 620 nm band pass filter.
The activity of caspase 3 was determined by the flow cytometric method as explained by Dai et al.[8]. PA1 cells were cultured, treated with an appropriate concentration of test samples and then harvested after the incubation as explained previously. Fixation of the cells before the immunofluorescent staining was done by adding 0.5 ml 2 % Paraformaldehyde (PFA) solution followed by 20 min of incubation. After washing with 0.5 % Bovine Serum Albumin (BSA) in 1X PBS, 0.1 % of Triton-X 100 (prepared in 0.5 % BSA) was added and incubated for 10 min to remove the traces of Triton-X 100 cells are washed with 0.5 % BSA in 1X PBS 2 times. Along with 0.5 % BSA, 20 μl FITC labeled Rabbit Anti- Active Caspase-3 IgG antibodies were added, mixed well and incubated for 30 min in dark at room temperature. Then those are washed with 1X PBS to remove unbound antibodies, flowcytometric analysis was done after adding and mixing with 0.5 ml of PBS. Active Caspase-3 expression was detected by measuring the cells positive for green fluorescence in the FL1 detector using a 525 nm band pass filter .
Determination of Bcl-2 activity was carried out by flow cytometry[8]. PA1 cells were cultured, treated with an appropriate concentration of test samples and then harvested after the incubation as explained previously. Fixation of the cells before the immunofluorescent staining was done by adding 0.5 ml 2 % Paraformaldehyde (PFA) solution followed by 20 min of incubation. After washing with 0.5 % BSA in 1X Phosphate Buffered Saline (PBS), 0.1 % of Triton-X 100 (prepared in 0.5 % BSA) was added and incubated for 10 min to remove the traces of Triton-X 100 cells are washed with 0.5 % BSA in 1X PBS 2 times. Along with 0.5 % BSA, 20 μl PE Mouse Anti-Human Bcl-2 antibodies were added, mixed well and incubated for 30 min in dark at room temperature. Then those are washed with 1X PBS to remove unbound antibodies, Flowcytometric analysis was done after adding and mixing with 0.5 ml of PBS.
Flow cytometric analysis was done to study the effect of ALE on the arrest of the cell cycle as explained by Pozarowski et al.[9] . PA-1 cells were seeded into 6-well plates and incubated at 37° for 24 h. Cells were treated with the IC50 concentration of the test compounds for 16 h, trypsinized and taken into 15 ml tubes. Cells were washed with 1X DPBS, fixed in chilled 70 % ethanol (-20°), washed with 1X DPBS twice, resuspended in 400 μl PI-RNase solution per million cells, and taken into 1.5 ml tubes. Samples were mixed well and analyzed by Cytomics FC500 Flow cytometer. Along with 0.5 ml of PBS, the cells were subjected to detect Bcl-2 expression by measuring orange (PE) fluorescence in the FL2 detector using a 575 nm band pass filter.
Detection of DNA damage was assessed by Terminal deoxynucleotidyl transferase dUTP End Labeling (TUNEL) assay[10]. The treated cells were resuspended and were washed with 1 ml of wash Buffer, centrifuged at 300 xg and removed the supernatant. Cell pellets were then suspended in 50 μl of the DNA labeling solution and incubated for 60 min at 37°. At the end of the incubation, 1 ml of rinse buffer was added and centrifuged at 300 xg for 5 min; repeat the same one more time, and the supernatant was removed. 0.5 ml of PI/RNase was added to stain the labeled ends, incubated in dark for 30 min at room temperature and then analyzed.
To verify the active ingredient in ALE, andrographolide content was estimated in the methanol extract of ALE by Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) as per the protocol of Dalawai et al.[11].
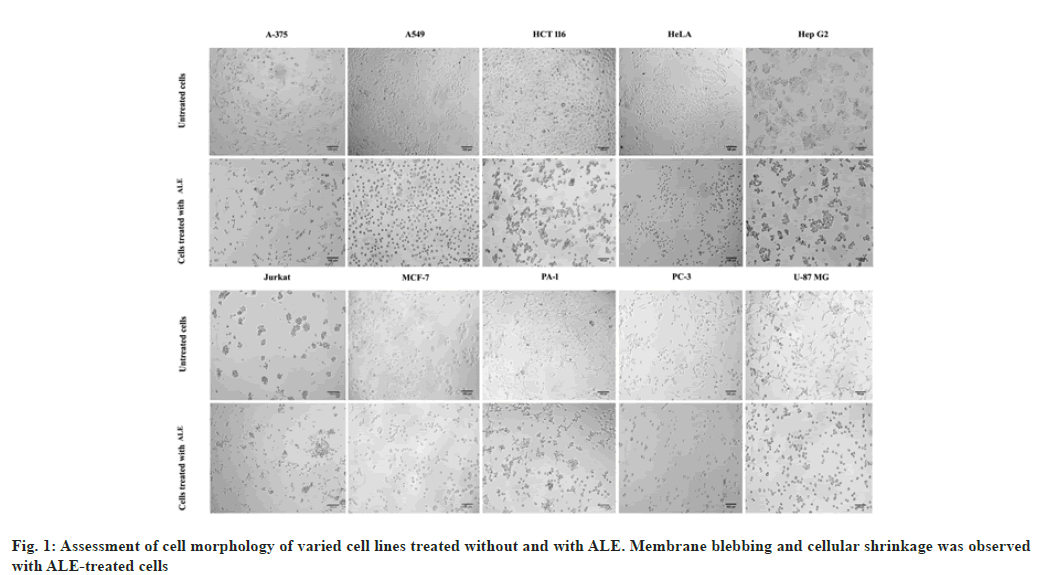
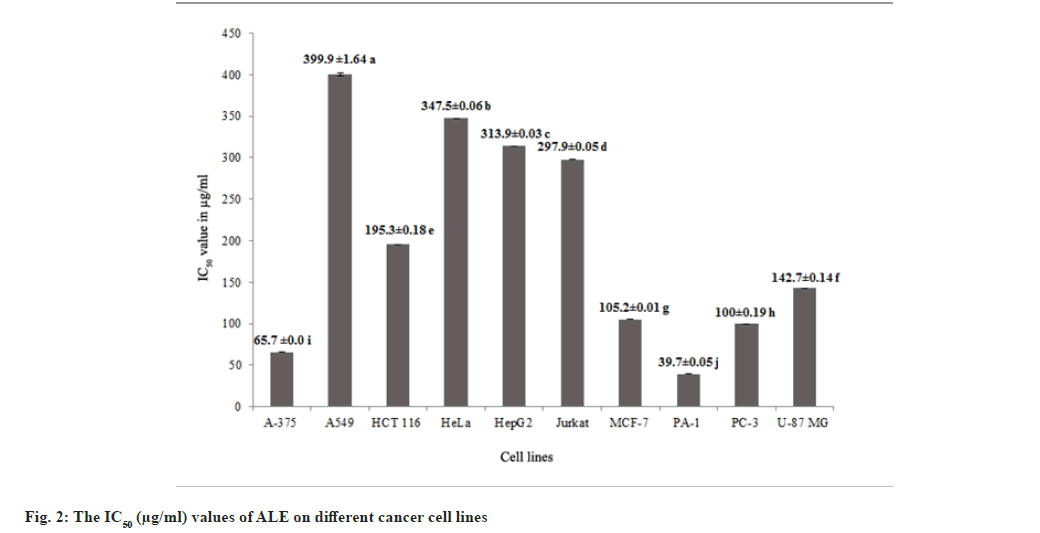
Methanol leaf extracts of Andrographis lineata Wall. ex Nees var. lawii C.B. Clarke (ALE) was evaluated for its in vitro inhibitory activities against ten human cancer cell lines A375 (melanoma), A549 (lung cancer), HCT116 (colon cancer), HeLa (cervix cancer), HepG2 (hepatoma), Jurkat cells (T cell acute lymphoblastic leukemia), MCF7 (breast cancer), PA1 (ovary cancer), PC3 (prostate cancer) and U87MG (Glioblastoma) and cytotoxicity was observed with all the cell lines. The results revealed morphological changes in different cancer cell lines such as membrane blebbing and shrinkage of cells with the ALE treatment leading to cell death (fig. 1). ALE potentially interrupts the cell proliferation among different cancer cell lines ranging from IC50 values of 39.7 μg/ml to 399.9 μg/ml (fig. 2). The results affirm that ALE had a strong cytotoxicity against PA1 (IC50=39.7 μg/ml), A375 (IC50=65.7 μg/ ml) and PC3 (IC50=100 μg/ml) cell lines.
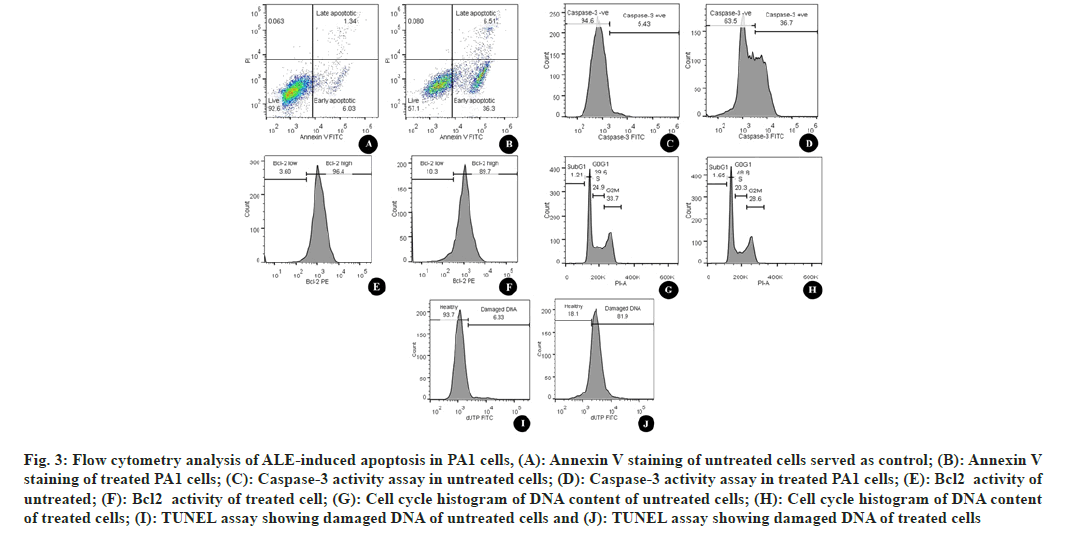
To confirm the apoptotic destruction of cells Annexin V-FITC/PI double staining was performed. Fig. 3 represents the cells treated with ALE in comparison with DMSO-treated cells (fig. 3A). The number of high FITC and FITC+PI was spotted to be increased after the treatment (fig. 3B). This indicated that the cells that lead to apoptosis are due to the interference of phytoconstituents present in the extract.
Cysteinyl Aspartate Proteinases (Caspases) are the enzymes that are responsible to cleave specific intracellular targets that result in the downfall of cell division. Thus, the up-regulation of caspases is the indication of apoptotic cell death[12]. Flow cytometric estimation of caspase 3 showed an increased number of caspase-3 indicating the increased amount of executioner caspases (fig. 3C and fig. 3D), hence an increased level of apoptosis.
Fig. 3: Flow cytometry analysis of ALE-induced apoptosis in PA1 cells, (A): Annexin V staining of untreated cells served as control; (B): Annexin V staining of treated PA1 cells; (C): Caspase-3 activity assay in untreated cells; (D): Caspase-3 activity assay in treated PA1 cells; (E): Bcl2 activity of untreated; (F): Bcl2 activity of treated cell; (G): Cell cycle histogram of DNA content of untreated cells; (H): Cell cycle histogram of DNA content of treated cells; (I): TUNEL assay showing damaged DNA of untreated cells and (J): TUNEL assay showing damaged DNA of treated cells
Bcl- 2 is the antiapoptotic constituent of the Bcl- 2 protein family. It protects the cell by apoptosis through mitochondrial localization. Unlike normal cells; cancer cells are characterized by continuous cell division. Thus, increased activity of Bcl-2 is the indication of blocked apoptotic cell death in the case of cancer cells[13]. In the current study, the Bcl-2 was quantified using PE Mouse Anti-Human Bcl-2 and it was found that there was a considerable increase in the number of cells with a low level of Bcl-2 after the treatment with ALE. ALE was found to be responsible for shifting the percentage of Bcl-2 low cells from 3.6 (fig. 3E) to 10.3 (fig. 3F) indicating that ALE was promoting apoptosis by increasing the level of antiapoptotic protein.
To detect the effect of ALE on the arrest of cell proliferation, we studied the progression of the cell cycle. In contrast to the untreated cells (33.7 %) (fig. 3G), there was a considerable hike in the number of cells in the G2/M phase (38.9 %) (fig. 3H). The percentage of cells in the sub-G1 phase increased from 1.21 to 1.65 evidencing the destructed DNA after ALE treatment. There was a rise in the percentage of cells in the G0/G1 phase after ALE treatment from 39.6 % to 48.8 % indicating the cell cycle arrest at this phase.
TUNEL was used to determine the extent of DNA fragmentation. The results showed an elevated level of cells with dUTP FITC indicating the nicks were due to DNA damage after the ALE treatment. The healthy cells were found at 93.7 % (fig. 3I) in the control cells whereas it was decreased to 18.1 % (fig. 3J) after the effect of ALE.
Further, the amount of andrographolide present in ALE was quantified using RP-HPLC, the amount of andrographolide present in ALE was estimated to be 21.24±0.12 mg/g dry weight. Andrographolide thus can be concluded as a major active compound present in the ALE. The present study demonstrated a promising effect of ALE against ovary cancer and melanoma cell lines. Additionally, the study also provides details about the apoptotic pathway proving the destructive effect of the ALE against ovarian cancer.
Acknowledgments:
This work was supported UGC-BSR mid-career award grant [No. F.19-223/2018/ (BSR)]. One of the authors (MB) is thankful to Karnatak University, Dharwad for awarding University Research Studentship.
References
- Pratheeshkumar P, Son YO, Korangath P, Manu KA, Siveen KS. Phytochemicals in cancer prevention and therapy. Biomed Res Int 2015;2015:324021.
[Crossref] [Google Scholar] [PubMed]
- Okhuarobo A, Falodun JE, Erharuyi O, Imieje V, Falodun A, Langer P. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: A review of its phytochemistry and pharmacology. Asian Pac J Trop Dis 2014;4(3):213-22.
[Crossref] [Google Scholar] [PubMed]
- Islam MT, Ali ES, Uddin SJ, Islam MA, Shaw S, Khan IN, et al. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett 2018;420:129-45.
[Crossref] [Google Scholar] [PubMed]
- Majumdar M, Dubey A, Goswami R, Misra TK, Roy DN. In vitro and in silico studies on the structural and biochemical insight of anti-biofilm activity of andrograpanin from Andrographis paniculata against Pseudomonas aeruginosa. World J Microbiol Biotechnol 2020;36:1-8.
[Crossref] [Google Scholar] [PubMed]
- Majumdar M, Misra TK, Roy DN. In vitro anti-biofilm activity of 14-deoxy-11, 12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa. Braz J Microbiol 2020;51:15-27.
[Crossref] [Google Scholar] [PubMed]
- Alagesaboopathi C. Phytochemical analysis and antimicrobial evaluation of Andrographis lineata Nees leaves and stem extracts. Afr J Pharm Pharmacol 2011;5(9):1190-5.
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994;84(5):1415-20.
[Crossref] [Google Scholar] [PubMed]
- Dai C, Krantz SB. Interferon γ induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood 1999;93(10):3309-16.
[Crossref] [Google Scholar] [PubMed]
- Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol 2004;281:301-11.
[Crossref] [Google Scholar] [PubMed]
- Walker PR, Kokileva L, LeBlanc J, Sikorska M. Detection of the initial stages of DNA fragmentation in apoptosis. Biotechniques 1993;15(6):1032-40.
[Google Scholar] [PubMed]
- Dalawai D, Aware C, Jadhav JP, Murthy HN. RP-HPLC analysis of diterpene lactones in leaves and stem of different species of Andrographis. Nat Prod Res 2021;35(13):2239-42.
[Crossref] [Google Scholar] [PubMed]
- Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene 2003;22(53):8543-67.
[Crossref] [Google Scholar] [PubMed]
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990;348:334-6.
[Crossref] [Google Scholar] [PubMed]