- *Corresponding Author:
- D. Selvaraj
Department of Pharmacology, JSS College of Pharmacy, JSS Academy of Higher Education and Research, Ooty, Tamil Nadu 643001, India
E-mail: sayanibh@gmail.com
| Date of Received | 25 June 2021 |
| Date of Revision | 22 July 2023 |
| Date of Acceptance | 24 October 2023 |
| Indian J Pharm Sci 2023;85(5):1224-1233 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Uterine fibroids are highly prevalent understudied myometrial non-cancerous tumors of the female reproductive tract. Fibroids cause increased morbidity and a significant economic burden in women. Despite its high prevalence, the only gold standard treatment for fibroid is hysterectomy. The tumor masses of fibroids are predominantly composed of excessive extracellular matrix when compared to the aberrant tumour cell population. The apoptotic resistance of matrix secreting cells during chronic inflammatory state such as fibroids, leads to deposition of enhanced rigid matrix molecules that are both responsible for tumour cell proliferation via the mechano-transduction process and the manifestation of bulk symptoms such as pain and bleeding. Despite its established fibrotic background and resemblances to pathological fibrosis most of the drugs are introduced as anti-proliferative and not as anti-fibrotic. This review aims in understanding the fibrotic mechanisms associated with the disease and emphasizes on drugs decreasing matrix production and increasing matrix dissolution. Such a modulation could bypass adverse effects associated with current pharmacological interventions that exert its therapeutic effect by modulation of hormones.
Keywords
Uterine fibroids, fibrosis, fibroblast activation protein, uterine leiomyoma, anti-fibrotic, anti-proliferative
Uterine fibroids are the most common non-cancerous reproductive tract tumor prevalent in women between menarche and menopause[1]. It was first discovered in the year 1793 by Matthew Baillie of St. Johns hospital, London[2]. Uterine fibroids are defined as steroid hormone-responsive monoclonal tumours arising from the myometrium of the uterus characterized by increased deposition of disorganized Extracellular Matrix (ECM) and abnormal proliferation of disordered smooth muscle cells[3]. Fibroids are found with a high prevalence rate of 77 %. Though benign, the symptoms of uterine fibroids such as menorrhagia, abnormal menstrual bleeding, dysmenorrhoea, pelvic pressure, infertility, pelvic pain, constipation, urinary incontinence and pregnancy problems pose a considerable impact on quality of life. About 20-50 % of the tumours become clinically apparent only after the appearance of clinical manifestations[4]. Additionally, these tumours also cause a significant economic burden.
The myometrial layer of the uterus is composed of smooth muscle cells and extracellular matrix. These components are responsible for the normal contractility of the uterus. Studies suggest that fibroids are developed from a fibrotic background and contain abundant rigid extracellular matrix. These matrix components are rigid and disorganized due to the deposition of huge quantities of glycosaminoglycan, highly cross-linked interstitial collagens, fibronectin and laminin. This increased stiffness of the ECM can cause abnormal bleeding and pelvic pain. Also, excessive ECM stiffness can trigger mechanotransduction process wherein a cell senses the physical forces and converts them into certain biological and biochemical signals. These signals can change the phenotype of the smooth muscle cell thereby inducing tumorigenesis[5]. Tumors are called monoclonal when all the cells of the tumor are said to be clonally derived from a single progenitor cell whereas a polyclonal tumor is said to develop from multiple progenitor cells[6]. Fibroids are said to be of monoclonal origin as they are developed due to the proliferation of a single clone of smooth muscle cell[7].
Further the ECM also acts as reservoir for profibrotic growth factors[8]. Thus, this review focuses on fibrosis and anti-fibrotic approaches to treat uterine fibroids. Studies suggest that adoption of such approaches can bypass the adverse effects caused by existing hormone modulating pharmacological interventions.
Overview on Uterine Fibroids
Risk factors:
Uterine fibroids are caused due to multiple factors. Menarche at an early age leads to a higher number of cell divisions in the muscle layer of the uterus throughout the fertile years of women leading to increased risk of uterine fibroids[9]. Uterine fibroids are three times highly prevalent in women of African American descent and two times more common in Hispanic women[10]. Such racial differences are risk factors for fibroid probably due to earlier age of finding, severity in sign, and variable intervention responses[10]. Soy is an exogenous phytoestrogen that exerts estrogenic effect[11]. The estrogenic effect caused by soy is higher when compared to the normal endogenous hormone levels. Endocrine-Disrupting Chemicals (EEDC) are estrogens available in the environment that influences the hormonal system by binding steroid receptors, or by influencing its synthesis and metabolism. EEDCs, such as dichlorodiphenyltrichloroethane, bisphenol-A, diethylstilbestrol, genistein, dioxin, soy and polychlorinated biphenyls cause uterine fibroid by developmental reprogramming[12].
Rats exposed to genistein obtained from soy products before puberty showed certain genetic changes that could lead the uterus towards fibroid development[12]. Bisphenol-A, a synthetic oestrogen used in the synthesis of food packing materials, displayed increased fibroid cell proliferation and increased expression of genes involved in fibroid development in vitro. In summary, prepubertal exposure to environmental estrogenic compounds increase risk of uterine leiomyoma and result in reprogramming of reproductive organ[13]. Several studies highlight the link between obesity and fibroid risk. This could be due to the influence of obesity on hormones. Increased adipose tissue associated with obesity produces excessive oestrone by aromatization[14]. Obesity leads to increased unbound active oestrogen due to decreased production of hormone-binding globulin[15]. Age is also found to modulate uterine fibroids. Increased diagnosis at 40 y of age might be possibly due to factors such as additive culmination of hormonal influence from menarche, enhanced symptom establishment by a pre-existing tumour, increased readiness to undertake surgery at a later stage, or due to premenopausal modulators[16]. Chronic inflammation due to an infection, can lead to fibroids by causing excessive proliferation and decreased apoptosis of tumour cells. Further it also leads to abnormal deposition of extracellular matrix leading to tumour bulk[16]. Coffee intake and alcohol intake are directly associated with fibroid risk. Black Women’s Health Study established that an intake of ≥3 cups of coffee/d and ≥500 mg of caffeine/d was associated with increased fibroid risk. The same study also displayed a 60 % increased risk in women who drank more than seven drinks a week when compared to non-alcoholic women. This could be probably due to the elevated oestrogen level associated with such an intake[17]. Parity, cigarette smoking and the use of contraceptives are found to decrease the risk of uterine fibroids[18]. Genetic factors such as translocation between chromosomes 12 and 14, translocations between 6 and 10, translocations in High Mobility Group AT-Hook2 (HMGA2), Mediator of Ribonucleic Acid (RNA) polymerase II transcription subunit 12 (MED 12), and aberrations in the Fumarate Hydratase (FH) gene also leads to fibroids[19,20].
Classification:
The types of fibroids are defined by the position relative to the uterine wall. Subserosal fibroids are located on the myometrium of the uterus and grow towards the serosal layer. They can also become pedunculated, parasitic and intraligamentary fibroid. Intramural fibroids grow within the uterine wall myometrium and distort the uterine wall. Submucosal fibroids begin in the myometrium develop near the endometrium and tend to grow toward the uterine cavity. Pedunculated fibroids grow on a stalk; can either be further classified as subserosal or submucosal depending on their location. Parasitic fibroids tend to obtain blood from omental vessels outside the uterus. Studies report that about 10.6 % of fibroids are subserous, 89.4 % are sub mucous and 74.5 % are intramural fibroids[21].
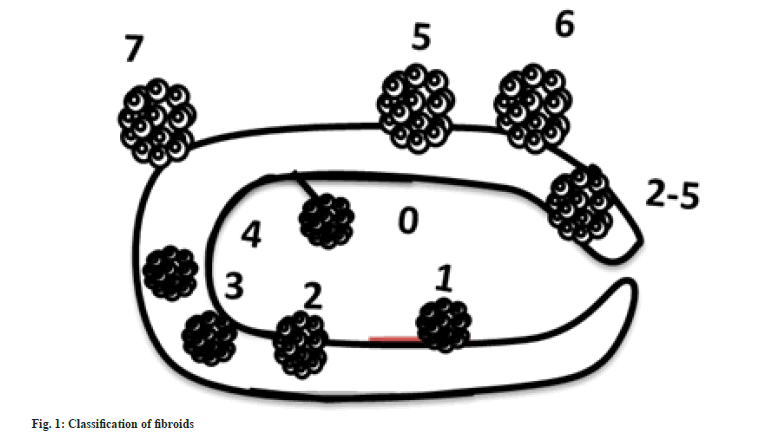
European Society for Gynaecological Endoscopy (ESGE) has classified uterine fibroids into three classes, G0-pedunculated intrauterine myoma, G1- >50 % in the uterine cavity, and G2 - >50 % in the myometrium[22]. International Federation of Gynaecology and Obstetrics (FIGO) has classified uterine fibroids into 8 types along with a hybrid type in PALM-COEN that contains conditions such as polyp, adenomyosis, leiomyoma, malignancy, hyperplasia, coagulopathy, ovulatory dysfunction, endometrial iatrogenic and not classified[23] (fig. 1 and Table 1).
| Types | Subtypes | Description |
|---|---|---|
| Submucosal: Immediate proximity to the endometrium. Distorts uterine cavity | Type 0: Pedunculated fibroid | Attached to the myometrium by a pedicle |
| Type 1: Sessile with intramural extension =50 % | Less than 50 % of the mass in the myometrium, or more than 50 % in the uterine cavity | |
| Type 2: Sessile with intramural extension =50 % | More than 50 % of the mass within the myometrium | |
| Other | 100 % intramural | Located within the myometrium |
| Does not distort the uterine cavity | ||
| Less than 50 % protrudes into the serosa | ||
| Intramural | Located within the myometrium | |
| Does not distort the uterine cavity | ||
| Less than 50 % protrudes into the serosa | ||
| Subserosal =50 % | Beneath peritoneum of the uterine corpus | |
| Subserosal ?50 % | Beneath peritoneum of the uterine corpus | |
| Subserosal pedunculated | Beneath peritoneum of the uterine corpus | |
| Other (parasitic) | Extend into the peritoneal cavity | |
| Outgrows blood supply |
Table 1: Types of Uterine Fibroids–Figo classification.
Symptoms associated with uterine fibroids:
Uterine fibroids cause significant quality of life impairment and morbidity owing to the symptoms associated with disease[24]. It includes menstrual cycle abnormalities such as heavy menstrual bleeding, menstrual cramping and metrorrhagia. Excessive bleeding also leads to iron deficiency anaemia in most women. Heavy menstrual bleeding can occur with submucous fibroids due to an expansion of the uterine cavity area, increased vascularity of the uterus, pressure on veins of the myometrium, uterine contraction disturbances and ulcers in an endometrial layer of the uterus. Though not a characteristic symptom, inter-menstrual bleeding occurs when the fibroid undergoes necrosis, ulceration in the endometrial layer, or pressure exertion in the veins of the myometrium[25].
Pain and pressure symptoms depend on the anatomical position of the fibroids. Fibroids in the anterior position cause discomfort leading to a constant need to urinate, posterior fibroids cause lower backache and fibroids that occupy the entire pelvic region causes pressure symptoms like urine and faeces leakage. Acute pain may also be caused due to torsion in the pedunculated fibroid, distension of cervix owing to the extension of lower uterus, red degeneration, ischemia, and necrosis. Women often complain of voiding difficulties with difficulty emptying the bladder. A large posterior myoma can cause constipation and altered bowel behaviour[26]. Blockage of uterine tubes, damage of uterine cavity, ischemia, atrophy and ulceration in the cavity due to fibroids can cause fertility and implantation issues. Certain reports suggest that miscarriages during the first and second trimester are common in women with fibroids. Recurrent pregnancy loss is also associated with uterine fibroids[27]. It also causes sudden miscarriages, premature delivery, premature membrane breakage, placental abruption, foetal growth restriction, post-delivery bleeding, and can be a cause for Caesarean section. The location of fibroid near the placenta also leads to postpartum haemorrhage and premature delivery[26-28] (Table 2).
| Anatomical position | Symptoms |
|---|---|
| Intramural and submucosal (Impacting uterine cavity) |
Menstrual disorders - Menorrhagia, Metrorrhagia, Abnormal uterine bleeding, Fertility issues, Iron deficiency anemia |
| Adverse pregnancy outcomes - Repeated abortions, Placenta previa, Pregnancy malpresentation, Premature labor, C section, and blood loss after vaginal delivery | |
| Intramural and subserosal (That do not impact uterine cavity) |
Bulk symptoms - Pelvic pressure, Pelvic ache, Painful sexual intercourse, prolonged constipation, and urine leakage |
Table 2: Symptoms as per Anatomical position.
Current pharmacological interventions:
Ever since the adaptation of hysterectomy in medical science, it is the only definitive treatment for uterine fibroids as it instantly cures the symptoms and prevents recurrence in women who do not wish to preserve future fertility[29,30]. Despite the procedure involves decreased loss of blood when compared to myomectomy it is not suitable for women desiring further pregnancies. Removal of the uterus can also cause damages to nearby organs causing complications such as vaginal vault prolapse, bowel, bladder, and ureteric damage[30]. Myomectomy preserves the fertility potential of women but it requires extra surgical intervention within a term of 5 y due to its recurrence potential[31]. Despite it being associated with lower blood loss the surface should be sealed securely as secondary haemorrhage is a potential complication[32].
Endometrial ablation is the destruction of the endometrium by using heat, laser and radiofrequency. Though it is performed to reduce heavy bleeding its actions are considered inferior and hence are associated with diminished patient satisfaction. Further, this technique is also associated with ectopic pregnancies, abortions, and placental disorders. Myolysis a method of destruction of the uterus or its blood supply preserves the uterus but not fertility. But such a method might compromise the integrity of the uterine wall leading to uterine rupture[33]. Uterine artery embolization is a relatively less invasive approach that involves the introduction of thrombi into uterine arteries thereby cutting off the blood supply to the tumour vessels. But yet it leads to pregnancy complications like abortions, placenta previa, premature delivery, adhesions, and post-delivery bleeding[34]. Oral contraceptives are used for decreasing the excessive menstrual bleeding associated with fibroids but fails to influence the tumour as such. It decreases the size of endometrial cells thereby reducing bleeding. Levonorgestrel releasing Intrauterine Device (IUD) decreases menorrhagia by decreasing the proliferation of endometrial cells. But increased chances of expulsion limit its efficacy[35].
Gonadotropin Releasing Hormone (GnRH) agonists are used as a short-term treatment in decreasing tumour volume either to improve surgical outcome or in perimenopausal women. Administration of agonists causes a temporary increase in gonadotropins leading to increased Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH). But continuous administration inhibits the pituitary gonad axis leading to decreased oestrogen associated reduction in tumour volume. Despite shrinkage in tumour volume use of such drugs creates menopause-like symptoms, bone demineralization, and an increase in tumour size after cessation of therapy[36]. Selective oestrogen receptor modulators bind to oestrogen and display stimulation and inhibition effects based on the tissue. Certain report suggests increase in fibroid growth and endometrial hyperplasia following treatment with tamoxifen and no effect in fibroid volume after treatment with raloxifene[37]. GnRH antagonists like cetrorelix bind to the GnRH receptors and block the binding of endogenous naturally occurring exogenous ligands and agonists thereby creating a hypo-estrogenic state. But the short half-life of the drug and lack of depots demands for frequent administration[37].
Anti-progesterone, like mifepristone blocks progesterone receptors and control fibroid-related bleeding without influencing the tumour volume. But its influence over endometrium makes its use controversial. Selective progesterone receptor modulators like asoprisnil and ulipristal possess both stimulatory and inhibitory effects in a tissue-specific manner. Despite proven tumour reduction in short management to improve surgical outcome, its long-term endometrial safety is yet to be established[38]. Non-hormonal therapies, like Non-Steroidal Anti-Inflammtory Drugs (NSAIDS) and tranexamic acid decrease prostaglandin production and fibrin degradation respectively leading to decreased blood loss. But it fails to influence tumor volume[38]. This shows that the available treatments are only for symptom management, short-term treatment to improve surgical outcome, and are strategies with un-established safety efficacy reports.
Pathogenesis of Uterine Fibroids
The exact initiator of uterine fibroid is not known. There are many theories that support the initiation of tumorigenesis [39]. Some of the theories are described below.
Hormones and growth factors:
One theory suggests that the increased levels of estrogen and progesterone increases the mitotic rate of fibroid cells by enhancing somatic maturations. Estrogen upregulates both estrogen receptors and progesterone receptors during the follicular phase, followed by progesterone mediated mitogenesis during the luteal phase. The deficiency of the estrogen-metabolizing enzyme 17β-hydroxysteroid dehydrogenase in fibroids causes the accumulation of estradiol in these tumors thereby leading to growth promoting effects while the overexpression of estradiol 4-hydroxylase results in a metabolite that possesses long-acting estrogenic activity. The growth-promoting effects of these hormones are also mediated through the mitogenic effects of growth factors secreted by smooth muscle cells and fibroblasts. Growth factors are polypeptides or proteins that are produced by various cell types and, have different biologic effects. They function in an autocrine or paracrine manner and control the multiplication of cells. Overexpression of either the growth factor or its receptor may contribute to tumorigenesis.
The Transforming Growth Factor-β (TGF-β) and TGF-β receptors classified as I-III has been detected in human myometrial tissue. These factors can lead to the upregulation cell proliferation and synthesis of matrix components. Although some reports suggest TGF-β3 to be an important factor in uterine fibroid growth by stimulating cellular proliferation and the production of extracellular matrix. The effects of TGF-β may be controversial, depending upon the target, the amount of TGF-β and the presence of other regulatory factors. Basic Fibroblast Growth Factor (bFGF) causes the proliferation of smooth muscle cells and fibroid cells and promotes angiogenesis. Increased expression of bFGF messenger RNA (mRNA) was found in the leiomyomas when compared with the myometrium.
Epidermal Growth Factor (EGF) causes the proliferation of smooth muscle cells and fibroid cells. The EGF is elevated in leiomyomas during the luteal phase. Platelet-Derived Growth Factor (PDGF) has the capacity to bind to heparin, hence they may become sequestered in the extracellular matrix and serve as a reservoir for these growth factors. More PDGF receptors are expressed in leiomyomas than in the myometrium.
Almost all the Vascular Endothelial Growth Factor (VEGF), contrarian-binding regions and hence can mediate binding to the extracellular matrix and serve as a reservoir. VEGF stimulates angiogenesis and increases capillary permeability, which could enhance the growth of fibroids by increasing their nutrient supply. VEGF also indirectly induces the proliferation of endothelial cells, which further produces a number of growth factors. In addition, VEGF acts synergistically with Fibroblast Growth Factor (FGF) and release bFGF from its storage on heparan sulfates of the extracellular matrix. The resulting combinations have a combined effect on angiogenesis while bFGF also exerts proliferative effects on smooth muscle cells.
Insulin-like Growth Factor (IGF) is structurally related to proinsulin and promote cellular proliferation, differentiation, and cell survival. Some studies indicate mRNAs for IGF-I and IGF-II and their receptors to be expressed in myometrium and fibroid tumors.
Genetic and/or epigenetic changes:
Genetic and/or epigenetic changes can also lead to fibroids. Karyotypic abnormalities have been identified in surgically removed uterine leiomyomas. The most common of these are the translocation t(12;14) and the deletionn of 7q. There may be more than one genetic pathway to the formation of fibroids.
Fibrosis and fibroblasts in uterine fibroids:
The fibrosis theory is one interesting theory wherein a fibroid is said to develop as a response to injury. This occurs when the myometrial cells undergo a change from a contractile phenotype to a proliferative phenotype as response to an injury[39].
Fibrosis is defined as enhanced deposition of the extracellular matrix due to an imbalance in the wound healing process during chronic inflammatory state[40]. Myofibroblasts are matrix secreting cells that are responsible for the contractility of scar tissue. It plays a key role in both wound healing and fibrotic processes[41]. During an injury fibroblasts present in the connective tissue of the organ transform into myofibroblasts. The myofibroblasts produce extracellular matrix that leads to wound closure. The restoration of the integrity of the organ after wound closure is followed by apoptosis of myofibroblasts[42]. But in chronic inflammation, the myofibroblast fails to undergo apoptosis and continues to persist leading to fibrosis similar to hypertrophic scarring. A similar state was reported in fibroids. Certain studies suggest that tissue macrophages were abundant in leiomyoma tissue when compared to normal, myometrium. As tissue macrophages are also known to be involved with fibrosis and fibroblastic transformation, it was concluded that chronic inflammatory state in the uterus activates the fibrotic response and production of excessive extracellular matrix by activation of myofibroblasts[43].
A microarray study found an association between abnormal wound healing disorder keloid and uterine fibroid as both the diseases involved similarities in myofibroblast apoptotic resistance, matrix accumulation, deregulated extracellular matrix genes, ultrastructure and ethnic prevalence. Though steroid-responsive hormone tumour genes involving in hormone action were not differentially expressed, it was the extracellular matrix genes that were differentially expressed suggesting the involvement of a myofibroblast phenotype. The study highlighted that about 30 % of under-expressed genes and 20 % of over expressed genes encoded extracellular proteins or were closely involved in the synthesis or secretion of matrix. This finding was further confirmed by reverse transcription polymerase chain reaction[44]. The first link between leiomyoma and keloids was established by comparing the similarities in decreased dermapontin expression in both the diseases. Both the diseases showed similar disoriented collagen fibrils[45]. Uterine fibroids and keloid scars resemble each other as both conditions are non-cancerous fibrous overgrowths characterized by increased deposition of disorganized extracellular matrix and not increased proliferation of cells. Both the diseases can be inherited and have ethnic similarities of prevalence. Molecular similarities like enhanced uterine TGF-β levels, increased glycosaminoglycans and decreased dermatopontin levels are observed with both the diseases. These resemblances confirm the fibrotic background of uterine fibroids[46]. Uterine leiomyoma cells when co-cultured with leiomyoma-derived fibroblasts led to the proliferation of leiomyoma cells and extracellular matrix due to increased downstream signalling pathways and growth factors production in co-culture. This effect was not seen with uterine smooth muscle co culture or leiomyoma culture alone[47]. This confirms the involvement of activated fibroblasts in tumour development.
Fibroblast Activation Protein (FAP):
FAP is a fibroblast activation marker, especially for cancer-associated fibroblasts. It has pro-tumourogenic activity by its enzymatic and non-enzymatic activities[48]. FAP mRNA expressions were higher in uterine fibroids when compared to normal myometrium. The over expressed FAP was also associated with increased expression of proliferation and invasion genes such as survivin, livin, B-cell lymphoma 2, Snail, N-cadherin, and Matrix Metalloproteinase (MMP2) leading to proliferation and invasion of uterine fibroids[49]. As a steroid hormone-responsive tumour, Oestrogen plays a crucial role in fibroblast proliferation, cell proliferation signalling pathways by phosphorylating Mitogen activated protein Kinase (MEK), Extracellular Regulated Kinase (ERK1/2), and AKT, and extracellular matrix production. Silencing of FAP by small interfering RNA attenuated proliferation of fibroblasts, decreased phosphorylation of cell proliferation pathways and decreased extracellular matrix components. This displayed that estrogens exerts its activities partially via FAP[50].
Antifibrotic drugs for the treatment of fibroids:
Pirfenidone is an anti-fibrotic agent studied for its therapeutic benefits in pulmonary fibrosis patients. Oral administration of pirfenidone in clinical trials is led to severe side effects such as vomiting, fever, abnormality of hepatic function, dizziness, facial paralysis, hepatoma and skin photosensitivity. It is also studied for its anti-fibrotic role in uterine fibroids as both pulmonary fibrosis and uterine fibroids involved tissue fibrosis. Pirfenidone blocked the proliferation of both leiomyoma and myometrium cells by down regulating several growth factors. It also led to diminished mRNA levels of collagen 1 and 3[51]. Currently Pirfenidone is in the second phase of the clinical trial for its anti-fibrotic activity against uterine fibroids (Clinical trial.gov number NCT00332033)[52].
Antifibrotic agent halofuginone is an alkaloid predominantly used as a coccidiostat to prevent infection of the gastrointestinal tract. Halofuginone blocks leiomyoma and myometrial smooth muscle cells proliferation via inhibition of DNA synthesis and eventually leading to apoptosis. Halofuginone also significantly down regulates TGFβ 1 mRNA. Oral administration of halofuginone, when taken in solid tumours, led to nausea, vomiting, and fatigue[53,54]. Collagenase C histolyticum breaks the increased collagen present in uterine fibroids leading to diminished matrix stiffness[55]. Decreased stiffness also decreased tumour bulk symptoms such as pain and bleeding due to decreased tumour size associated with changed mechanotransduction. Purified collagenase upon injection reduced tissue stiffness of fibroids in a proof of principle study[56].
Role of extracellular matrix mediated mechanotransduction in uterine fibroids:
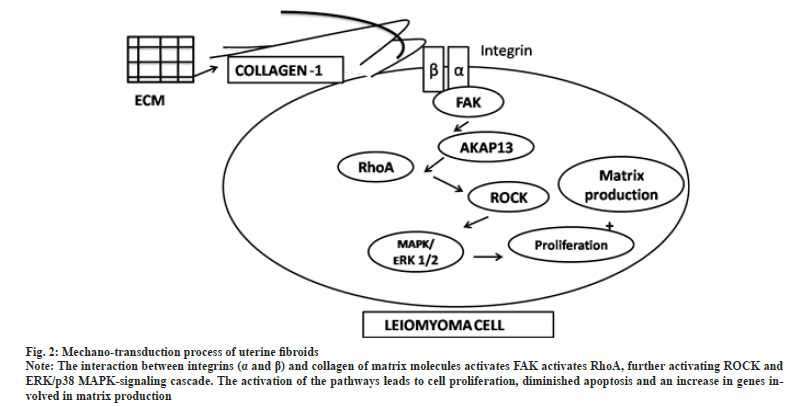
Uterine fibroids are characterized by increased deposition of extracellular matrix leading to matrix rigidity. The stiffness of the extracellular matrix leads to tumourogenesis owing to increased mechanical stress[57]. Mechanotransduction is a process whereby mechanical signals are converted into biochemical signals by extracellular matrix components and transmembrane molecules. In uterine fibroids the mechanical force elicited by the stiff matrix stimulates mechanical signalling pathways. The interaction between integrin (α and β) and matrix molecules activates Focal Adhesion Kinase (FAK) leading to actin polymerization and activation of Ras family member homology A (RhoA), further activating Rho-associated protein Kinase (ROCK) and Extracellular Regulated Kinase/p38 Mitogen Activated Protein Kinase (ERK/p38 MAPK)-signalling cascade. The activation of the pathways leads to cell proliferation, diminished apoptosis, and an increase in genes involved in matrix production[58] (fig. 2). As uterine fibroids are characterized by a low mitotic index, it is the extracellular matrix that contributes to tumour bulk[59]. The tumour bulk and stiffness also lead to the manifestation of clinical symptoms such as pain and abnormal bleeding[60].
Fig. 2: Mechano-transduction process of uterine fibroids.
Note: The interaction between integrins (a and ß) and collagen of matrix molecules activates FAK activates RhoA, further activating ROCK and
ERK/p38 MAPK-signaling cascade. The activation of the pathways leads to cell proliferation, diminished apoptosis and an increase in genes involved
in matrix production.
Ultra structure of extracellular matrix:
Preliminary research shows the matrix associated with uterine fibroids had increased collagen, fibronectin and proteoglycan. Collagen is the most expressed matrix protein in tissues undergoing fibrosis. Several studies suggest that collagen constitutes most of the matrix in leiomyoma with collagen type 1 being the most predominant type. Enzymes related to post-translational modification of collagen were also found to be greater in fibroids. Collagen is also associated with leiomyoma cell proliferation. Fibronectin is a dimer that binds to collagen type 1 leading to cell adhesion, cell signalling, and excessive fibrosis. It also acts as a reservoir of growth factors that can promote cell signalling upon release. Glycosaminoglycans and proteoglycans help in ligand-ligand binding, acts as receptor for growth factors, and also a reservoir for growth factors in the extracellular matrix[61]. Therefore, many studies aims in targeting the matrix components associated with fibrotic process[62] (Table 3).
| Drugs | Targets | Adverse effects |
|---|---|---|
| Vitamin D | Decreased fibronectin, collagen type 1, PAI-1, pSmad2, Wnt4, ß-catenin, mTOR, fibromodulin, biglycan, and versican | Well tolerated |
| Celecoxib | Decreased collagen A, fibronectin, PDGF, and TGF-ß | MI, stroke, and heart failure risk |
| Curcumin | Decreased fibronectin | Well tolerated |
| Resveratrol | Decreased fibronectin, collagen types I, collagen III, fibromodulin. biglycan, and MMP-9, TIMP2 | Nausea, diarrhea, and weight loss |
| Tranilast | Decreased COL1A1, fibronectin versican and activin-A | Well tolerated |
| All-trans retinoic acid | Decreased Collagen 1, collagen 4, fibronectin, versican and TGF- ß3 | Transient headache, dry skin, and mucosa, nausea and vomiting, myalgias,dyspnea and sensorineural hearing loss |
| Liarozole | Decreased COL1A1, COL4A2, versican, fibromodulin, fibronectin and TGF-ß3 | Dryness of mouth, eyes, lips, and skin disorder |
Table 3: Drugs targeting extracellular matrix components.
Conclusions and Future Perspectives
Substantial shreds of evidence state that uterine fibroids contain smooth muscle cells and matrix molecules. The matrix molecules are majorly produced from activated fibroblasts having fibroblast activation markers such as FAP and α-smooth muscle actin. These play a major role in the initiation of fibrosis during chronic inflammation. The stiff matrix also initiates mechanotransduction processes leading to tumour growth and bulk associated symptoms. As uterine leiomyoma cells possess a low mitotic index and the entire tumour bulk consists of matrix predominantly, future interventions should target the fibrotic processes and aim in decreasing matrix production or cause dissolution of matrix molecules. Despite an established fibrotic background for uterine fibroids most of the drugs are introduced as anti-proliferative and not anti-fibrotic. The available pharmacological interventions such as leuprolide, cetrorelix, asoprisnil, ulipristal mifepristone and raloxifene also seem to influence certain fibrotic mechanisms indirectly by decreasing matrix components like fibronectin, versican, collagen. But these interventions are associated with severe adverse effects due to hormonal modulation and so are indicated only as short-term pre-operative management strategy. After years of uterine fibroid discovery, hysterectomy is the only gold standard treatment for uterine fibroids. With advances in medical science, an invasive procedure like hysterectomy should be a last resort and not the first.
Fibroblast activation protein plays a major role in the activation of fibroblasts. Previously the use of FAP inhibitory traditional Chinese medicinal herbs was found very effective in the treatment of fibroids by inhibiting fibroblast activation, fibroblast proliferation, and by influencing matrix components[63]. But to date, allopathic FAP inhibitors are not introduced for the treatment of fibroids.
Acknowledgements:
The authors would like to thank the Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure in Universities and Higher educational institutions (DST-FIST), New Delhi for their infrastructure support to the Pharmacology Department of JSS college of pharmacy.
Conflict of interest:
The authors declare that the contents in this article have no conflict of interest.
References
- Guo XC, Segars JH. The impact and management of fibroids for fertility: An evidence-based approach. Obstet Gynecol Clin North Am 2012;39(4):521-33.
[Google Scholar] [PubMed] [Crossref]
- Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling pathways in Leiomyoma: Understanding pathobiology and implications for therapy. Mol Med 2015;21(1):242-56.
[Google Scholar] [PubMed] [Crossref]
- McWilliams MM, Chennathukuzhi VM. Recent advances in uterine fibroid etiology. Semin Reprod Med 2017;35(2):181-9.
[Google Scholar] [PubMed] [Crossref]
- Wilde S, Scott-Barrett S. Radiological appearances of uterine fibroids. Indian J Radiol Imaging 2009;19(3):222-31.
[Google Scholar] [PubMed] [Crossref]
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: Burden and unmet medical need. Semin Reprod Med 2017;35(6):473-80.
[Google Scholar] [PubMed] [Crossref]
- Parsons BL. Many different tumor types have polyclonal tumor origin: Evidence and implications. Mutat Res 2008;659(3):232-47.
[Google Scholar] [PubMed] [Crossref]
- Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Hum Reprod 2014;20(3):250-9.
[Google Scholar] [PubMed] [Crossref]
- Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: Developmental origin, pathogenesis, and treatment. Endocr Rev 2022;43(4):678-719.
[Google Scholar] [PubMed] [Crossref]
- Wise LA, Laughlin TSK. Epidemiology of uterine fibroids: From menarche to menopause. Clin Obstet Gynecol 2016;59(1):2-24.
[Google Scholar] [PubMed] [Crossref]
- Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med 2013;31(5):370-9.
[Google Scholar] [PubMed] [Crossref]
- Gao M, Wang H. Frequent milk and soybean consumption are high risks for uterine leiomyoma: A prospective cohort study. Medicine (Baltimore) 2018;97(41):e12009.
[Google Scholar] [PubMed] [Crossref]
- Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans: A review. Med Sci Monit 2009;15(6):137-45.
[Google Scholar] [PubMed]
- Shen Y, Ren ML, Feng X, Cai YL, Gao YX, Xu Q. An evidence in vitro for the influence of bisphenol A on uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol 2014;178:80-3.
[Google Scholar] [PubMed] [Crossref]
- Glass AR. Endocrine aspects of obesity. Med Clin North Am 1989;73(1):139-60.
[Google Scholar] [PubMed] [Crossref]
- Schneider J, Bradlow HL, Strain G, Levin J, Anderson K, Fishman J. Effects of obesity on estradiol metabolism: decreased formation of nonuterotropic metabolites. J Clin Endocrinol Metab 1983;56(5):973-8.
[Google Scholar] [PubMed] [Crossref]
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ Health Perspect 2003;111(8):1037-54.
[Google Scholar] [PubMed] [Crossref]
- Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med 2010;28(3):204-17.
[Google Scholar] [PubMed] [Crossref]
- Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: Reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed) 1986;293(6543):359-62.
[Google Scholar] [PubMed] [Crossref]
- Kubínová K, Mára M, Horák P, Kuzel D. Genetic factors in etiology of uterine fibroids. Ceska Gynekol 2012;77(1):58-60.
[Google Scholar] [PubMed]
- Holzmann C, Markowski DN, Bartnitzke S, Koczan D, Helmke BM, Bullerdiek J. A rare coincidence of different types of driver mutations among uterine leiomyomas. Mol Cytogenet 2015;8(1):1-7.
[Google Scholar] [PubMed] [Crossref]
- Tochie JN, Badjang GT, Ayissi G, Dohbit JS. Physiopathology and management of uterine fibroids. InFibroids 2020:97-109.
- Donnez J, Dolmans MM. Uterine fibroid management: From the present to the future. Hum Reprod Update 2016;22(6):665-86.
[Google Scholar] [PubMed] [Crossref]
- Munro MG, Critchley HOD, Broder MS, Fraser IS. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 2011;113(1):3-13.
[Google Scholar] [PubMed] [Crossref]
- Marsh EE, Al-Hendy A, Kappus D, Galitsky A, Stewart EA, Kerolous M. Burden, Prevalence, and treatment of uterine fibroids: A survey of US women. J Womens Health 2018;27(11):1359-67.
[Google Scholar] [PubMed] [Crossref]
- Wallach EE, Buttram Jr VC, Reiter RC. Uterine leiomyomata: Etiology, symptomatology, and management. Fertil Steril 1981;36(4):433-45.
[Google Scholar] [PubMed] [Crossref]
- Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids.
Best Pract Res Clin Obstet Gynaecol 2008;22(4):615-26.[Google Scholar] [PubMed] [Crossref]
- Vollenhoven BJ, Lawrence AS, Healy DL. Uterine fibroids: A clinical review. Br J Obstet Gynaecol 1990;97(4):285-98.
- Muram D, Gillieson M, Walters JH. Myomas of the uterus in pregnancy: Ultrasonographic follow-up. Am J Obstet Gynecol 1980;138(1):16-9.
[Google Scholar] [PubMed] [Crossref]
- Mas A, Tarazona M, Dasí Carrasco J, Estaca G, Cristóbal I, Monleón J. Updated approaches for management of uterine fibroids. Int J Womens Health 2017;9:607-17.
[Google Scholar] [PubMed] [Crossref]
- Farris M, Bastianelli C, Rosato E, Brosens I, Benagiano G. Uterine fibroids: An update on current and emerging medical treatment options. Ther Clin Risk Manag 2019;15:157-78.
[Google Scholar] [PubMed] [Crossref]
- Simms SD, Fletcher H. Counselling patients with uterine fibroids: A review of the management and complications. Obstet Gynecol Int 2012;2012:539365.
[Google Scholar] [PubMed] [Crossref]
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol 2005;105(4):877-81.
[Google Scholar] [PubMed] [Crossref]
- Wan AY, Shin JH, Yoon HK, Ko GY, Park S, Seong NJ, et al. Post-operative hemorrhage after myomectomy: Safety and efficacy of transcatheter uterine artery embolization. Korean J Radiol 2014;15(3):356-63.
[Google Scholar] [PubMed] [Crossref]
- Singh SS, Belland L. Contemporary management of uterine fibroids: Focus on emerging medical treatments. Curr Med Res Opin 2015;3(1):1-12.
[Google Scholar] [PubMed] [Crossref]
- Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci 2012;19(4):339-53.
[Google Scholar] [PubMed] [Crossref]
- Mohammad EP, Bahia NJ, Mayram PN. Medical management of uterine fibroids. Curr Obstet Gynecol Rep 2012;1(2):81-88.
[Crossref]
- Lewis TD, Malik M, Britten J, San PAM, Catherino WH. A Comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res Int 2018;2018:2414609.
[Google Scholar] [PubMed] [Crossref]
- Sabry M, Al-Hendy A. Innovative oral treatments of uterine leiomyoma. Obstet Gynecol Int 2012;2012:943635.
[Google Scholar] [PubMed] [Crossref]
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ Health Perspect 2003;111(8):1037-54.
[Google Scholar] [PubMed] [Crossref]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214(2):199-210.
[Google Scholar] [PubMed] [Crossref]
- Klingberg F, Hinz B, White ES. The myofibroblast matrix: Implications for tissue repair and fibrosis. J Pathol 2013;229(2):298-309.
[Google Scholar] [PubMed] [Crossref]
- Vallée A, Lecarpentier Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci 2019;9(1):1-15.
[Google Scholar] [PubMed] [Crossref]
- Protic O, Toti P, Islam MS, Occhini R, Giannubilo SR, Catherino WH, et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res 2016 ;364(2):415-27.
[Google Scholar] [PubMed] [Crossref]
- Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol 2006;195(2):415-20.
[Google Scholar] [PubMed] [Crossref]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer 2004;40(3):204-17.
[Google Scholar] [PubMed] [Crossref]
- Carrino DA, Mesiano S, Barker NM, Hurd WW, Caplan AI. Proteoglycans of uterine fibroids and keloid scars: similarity in their proteoglycan composition. Biochem J 2012;443(2):361-8.
[Google Scholar] [PubMed] [Crossref]
- Moore AB, Yu L, Swartz CD, Zheng X, Wang L, Castro L, et al. Human uterine leiomyoma-derived fibroblasts stimulate uterine leiomyoma cell proliferation and collagen type I production, and activate RTKs and TGF beta receptor signaling in coculture. Cell Commun Signal 2010;8(1):1-12.
[Google Scholar] [PubMed] [Crossref]
- Puré E, Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018;37(32):4343-57.
[Google Scholar] [PubMed] [Crossref]
- Lin W, Ma S, Wang L. Study on the correlation of MLCK and FAP expression with uterine fibroid cell proliferation and invasion. J Hainan Med Univ 2017;23(12):79-82.
- Luo N, Guan Q, Zheng L, Qu X, Dai H, Cheng Z. Estrogen-mediated activation of fibroblasts and its effects on the fibroid cell proliferation. Transl Res 2014;163(3):232-41.
[Google Scholar] [PubMed] [Crossref]
- Lee BS, Margolin SB, Nowak RA. Pirfenidone: A novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab 1998;83(1):219-23.
[Google Scholar] [PubMed] [Crossref]
- Taylor DK, Leppert PC. Treatment for uterine fibroids: Searching for effective drug therapies. Drug Discov Today Ther Strateg 2012;9(1):e41-9.
[Crossref] [Google Scholar] [PubMed]
- Grudzien MM, Low PS, Manning PC, Arredondo M, Belton Jr RJ, Nowak RA. The antifibrotic drug halofuginone inhibits proliferation and collagen production by human leiomyoma and myometrial smooth muscle cells. Fertil Steril 2010;93(4):1290-8.
[Crossref] [Google Scholar] [PubMed]
- Koohestani F, Qiang W, MacNeill AL, Druschitz SA, Serna VA, Adur M, et al. Halofuginone suppresses growth of human uterine leiomyoma cells in a mouse xenograft model. Human Reprod 2016;31(7):1540-51.
[Crossref] [Google Scholar] [PubMed]
- Jayes FL, Liu B, Moutos FT, Kuchibhatla M, Guilak F, Leppert PC. Loss of stiffness in collagen-rich uterine fibroids after digestion with purified collagenase Clostridium histolyticum. Am J Obstetr Gynecol 2016;215(5):596-e1.
[Crossref] [Google Scholar] [PubMed]
- Brunengraber LN, Jayes FL, Leppert PC. Injectable Clostridium histolyticum collagenase as a potential treatment for uterine fibroids. Reprod Sci 2014;21(12):1452-9.
[Crossref] [Google Scholar] [PubMed]
- Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol 2012;31(1):57-65.
[Crossref] [Google Scholar] [PubMed]
- Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int 2014;2014:e783289.
[Crossref] [Google Scholar] [PubMed]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005;308(5728):1589-92.
[Crossref] [Google Scholar] [PubMed]
- Jamaluddin MF, Nahar P, Tanwar PS. Proteomic characterization of the extracellular matrix of human uterine fibroids. Endocrinology 2018;159(7):2656-69.
[Crossref] [Google Scholar] [PubMed]
- Fujisawa C, Castellot JJ. Matrix production and remodeling as therapeutic targets for uterine leiomyoma. J Cell Commun Signal 2014;8:179-94.
[Crossref] [Google Scholar] [PubMed]
- Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum Reprod Update 2018;24(1):59-85.
[Crossref] [Google Scholar] [PubMed]
- Feng Y, Zhao Y, Li Y, Peng T, Kuang Y, Shi X, et al. Inhibition of fibroblast activation by components of Rhizoma curcumae and Rhizoma sparganii in a rat model of uterine leiomyoma. Front Public Health 2021;9:650022.
[Crossref] [Google Scholar] [PubMed]