- Corresponding Author:
- Ujwala Shinde
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098, India.
E-mail: ujwalas29@gmail.com
| Date of Submission | August 30, 2011 |
| Date of Revision | June 14, 2012 |
| Date of Acceptance | June 19, 2012 |
| Indian J Pharm Sci, 2012, 74 (3): 237-247 |
Abstract
Topical microemulsion systems for the antiacne agent, nadifloxacin were designed and developed to overcome the problems associated with the cutaneous delivery due to poor water solubility. The solubility of nadifloxacin in oils, surfactants and cosurfactants was evaluated to screen the components of the microemulsion. Various surfactants and cosurfactants were screened for their ability to emulsify the selected oily phase. The pseudoternary diagrams were constructed to identify the area of microemulsion existence. The influence of km (surfactant/cosurfactant) ratio on the microemulsion existence region was determined and optimum systems were designed. The systems were assessed for drugâ??loading efficiency and characterised for optical birefringence, pH and refractive index, robustness to dilution, globule size, drug content and thermodynamic stability. Optimised microemulsion systems were formulated into gel form and evaluated for viscosity, spreadability, drug content, ex vivo skin permeation and antibacterial activity. The maximum solubility of nadifloxacin in the microemulsion system was found to be 0.25%. The nadifloxacin microemulsions had a small and uniform globule size (67.3-121.23 nm). The stability results revealed that all formulations showed a stable globule size and the polydispersity index under stress conditions. Incorporation of nadifloxacin in microemulsion gel increased the ex vivo skin permeation and antibacterial activity when compared to marketed cream.
Keywords
Insulin resistance, melatonin, thiazolidinediones, type 2 diabetes
Worldwide prevalence of Type 2 diabetes is near about 246 millions in 2007, and may rise to 380 million in 2025. The socio-economical burden is great consequently; Type 2 diabetes presents a major challenge to healthcare systems around the world [1].
Glucocorticoids, as an endogenous hormones, antiinflammatory and immunosuppressive drugs, have been reported to induce Cushing’s syndrome, which is characterized by central obesity and insulin resistance. The chronic treatment with dexamethasone has been associated with hyperinsulinemia in both animals and humans [2]. The increase level of glucocorticoids via endogenous or exogenous pathways leads to increases glucose production and decrease glucose uptake in peripheral tissues. These effects lead to the insulin resistance due to the decrease in insulin sensitivity [3,4]. Long term glucocorticoid administration lead to insulin resistance by inducing post-receptor defects in the insulin actions [4] as well as inhibition of the insulin secretion from pancreatic β cells [5]. Other mechanisms by which the dexamethasone may induce peripheral insulin resistance, includes inhibition of GLUT4 translocation [6], increased lipoprotein lipase activity in the adipose tissue [7], impaired insulin signalling due to reduction in the phosphatidylinositol- 3-kinase (PI-3K) activity [8,9].
Long term administration of dexamethasone leads to generation of the free radicals which may contribute to oxidative stress [10]. High glucose concentrations induce mitochondrial ROS, which suppresses the first phase of glucose-induced insulin secretion, at least in part, through the suppression of glyceraldehydes 3-phosphate dehydrogenase activity [11]. In early stages, insulin resistance is compensated by hyperinsulinemia which characterised by the increase in insulin, FFA, and/or glucose levels, may associate with increase in ROS production and oxidative stress. This, in turn, can deteriorate both insulin secretion and action, accelerating the sequence to type 2 diabetes. Many studies support the hypothesis on the basis of in vitro as well as in vivo animal studies that; antioxidants have been shown to improve insulin sensitivity [11].
Oxidative stress induces the expression of NF-?B (Nuclear factor KB) which directly or indirectly contributes to the insulin resistance [12]. Thus, treatment with antioxidants can be used to reduce oxidative stress induced insulin resistance. Thiazolidinediones (pioglitazone and rosiglitazone) are well known agonists of the transcription-factor peroxisome proliferator activated receptor-γ (PPAR-γ) and currently used in the treatment of Type 2 diabetes [13]. Thiazolidinediones increase sensitivity to insulin by restoration of adipokines levels and increased in the expression of GLUT-4 [13]. Still, there exists concern about use of these drugs clinically, because of their uncertain cardiovascular safety and other idiosyncratic adverse events [14].
Melatonin (N-acetyl-5-methoxytryptamine) is a well known endogenous biological antioxidant synthesized in the pineal gland. It acts as a buffer against oxidative stress, induced by the reactive oxygen and nitrogen species [15]. Melatonin is reported to possess pharmacological actions, other than its antioxidant activity, such as insulin resistance reducing activity [16], enhancing the glucose uptake in skeletal muscle in vitro via IRS-1/PI-3-kinase (Insulin Receptor Substrate-1/ protein kinase-3) pathway stimulates glucose transport [17] and lipid lowering activity [18].
Now in the market different combinations of the oral hypoglycemic agents are used in the treatment of Type 2 diabetes. However, effective treatment for Type 2 diabetes usually requires a combination of two or more oral agents in the longer term, often as an introduction to the insulin therapy. Issues regarding the safety, tolerability, notably weight gain limits the optimal use of the oral hypoglycaemia drugs such as sulphonylureas and thiazolidinediones in combination [19].
On the basis of the reported pharmacological activities of melatonin and thiazolidinediones, in order to have new combination approach in the treatment of Type 2 diabetes. The present study was design to evaluate effect of the combination of thiazolidinediones (pioglitazone and rosiglitazone) with melatonin in the treatment of Type 2 diabetes preclinically.
Materials and Methods
Pioglitazone, rosiglitazone, melatonin were obtained from Themis Laboratories Ltd., Mumbai; Glenmark Pharmaceuticals Ltd., Nasik and Arati Bulk Manufacturer Pvt Ltd., Mumbai, India, respectively. Other chemicals and kits for biochemical estimations were obtained from Biolab Diagnostic Pvt. Ltd., India.
Experimental animals:
Forty two Swiss albino mice weighing 25-30 g were used for study and were kept in animal house at 26±2° with relative humidity 44-56% along with light and dark cycles of 12 h respectively. Animals were provided with standard diet and water ad libitum. The food was withdrawn 18–24 h before the start of the experiment. Institution animal ethics committee has approved the experimental protocol (198/99/CPCSEA).
Experimental protocol:
All 42 mice were weighed before treatment, NC (Normal Control) group received equivalent amount of 1% Na-CMC (sodium carboxymethyl cellulose) (1 ml/kg, p.o.), and remaining 36 mice were rendered hyperglycemic by daily administration of a prestandardized dose of dexamethasone (1 mg/kg, i.m.) [20] for consecutive 7 days and then divided into six groups of six each. DC group (dexamethasone control) continued to receive only dexamethasone and 1% CMC (1 ml/kg, p.o.) for next 15 days. PIO group, received pioglitazone (10 mg/kg, p.o.) [21] along with dexamethasone respectively for 15 days. ROSI group, received rosiglitazone (5 mg/kg, p.o.) [21] along with dexamethasone for 15 days. MEL group, received melatonin (10 mg/kg, p.o.) [22] along with dexamethasone for 15 days. PM group received pioglitazone (10 mg/ kg, p.o.) plus melatonin (10 mg/kg, p.o.) along with dexamethasone respectively for 15 days. RM group received rosiglitazone (5 mg/kg, p.o.) plus melatonin (10 mg/kg, p.o.) along with dexamethasone respectively for 15 days.
Biochemical estimations:
Blood samples were collected and used for estimation of glucose and triglyceride on 1, 7 and 22 day. Biochemical estimation of serum glucose and serum triglyceride was done by GOD/POD and GPO/POD method respectively using standard diagnostic kits from Biolabs India ltd., India.
Hepatic antioxidant enzymes assay:
Liver samples were dissected out and washed immediately with ice cold saline to remove as much blood as possible. Liver homogenates (5% w/v) were prepared in cold 50 mM Tris buffer (pH 7.4) using Remi homogenizer. The unbroken cells and cell debris were removed by centrifugation at 5000 rpm for 10 min using a Remi refrigerated centrifuge. The supernatant was used for the estimation of malondialdehyde (MDA) [23], superoxide dismutase (SOD) [24], catalase [25,26] and GSH reductase levels [27].
Effect on glucose uptake in isolated mice hemidiaphragm:
Glucose uptake in mice hemi-diaphragm was estimated by the method described by Ghaisas et al. [20]. Fourteen sets, containing graduated test tubes (n=6) each, were used for study of non-insulin assisted and insulin assisted glucose uptake. The diaphragms were taken out quickly avoiding traumas and divided into two halves. The hemi-diaphragms were rinsed in cold Tyrode solution (without glucose) to remove any blood clots. In non-insulin assisted glucose uptake study, one hemidiaphragm of each animal from all groups was exposed to 2 ml Tyrode solution with glucose (2000 mg/l) in respective graduated test tubes. In insulin assisted glucose uptake study, the remaining hemidiaphragm of each animal from all was exposed to 2 ml Tyrode solution with glucose (2000 mg/l) + Insulin (0.25 IU/ml) in respective graduated test tubes. All the graduated test tubes were incubated for 30 min at 37ο in an atmosphere of 95% O2 - 5% CO2 with shaking at 140 cycles per min. Following incubation, the hemidiaphragm were taken out and weighed. The glucose content of the incubated medium was measured by GOD/POD, enzymatic method. Glucose uptake was calculated as the difference between the initial and final glucose content in the incubation medium.
Statistical analysis:
The results were expressed as Mean±SEM and statistically analyzed by ANOVA followed by Dunnett test, with level of significance set at P<0.05.
Results
Effect on the body weight and biochemical parameters:
Mice treated with dexamethasone alone showed statistically significant (P<0.05) decrease in the percentage of change in the body weight than normal mice (Table 1), but showed statistically significant (P<0.05) increased in the serum glucose and triglyceride levels on day 7 and day 22 than normal mice (Tables 2 and 3). Pioglitazone plus melatonin and rosiglitazone plus melatonin treated mice showed significant (P<0.05) increase in the percentage of change in the body weight than dexamethasone alone treated mice as well as melatonin treated mice, also showed statistically significant (P<0.05) decrease in the serum glucose and triglyceride levels on day 22 than dexamethasone as well as melatonin alone treated mice.
| Group | Change in Body weight (%) |
|---|---|
| NC | +0.94±0.16 |
| DC | –2.09±0.06## |
| PIO | +0.72±0.09** |
| ROSI | +0.60±0.07** |
| MEL | –1.06±0.07** |
| PM | +1.11±0.04**acc |
| RM | +0.98±0.06**bcc |
Table 1: Effect Of Combination Of Thiazolidinediones With Melatonin On Percentage Change In Body Weight In Dexamethasone-Induced Insulin Resistance In Mice
| Group | Serum glucose (mg/dl) on | ||
|---|---|---|---|
| Day 1 | Day 7 | Day 22 | |
| NC | 67.31±3.78 | 69.12±4.69 | 68.43±5.88 |
| DC | 69.76±5.65 | 166.57±6.05## | 184.00±6.49## |
| PIO | 64.34±5.19 | 159.31±5.89 | 83.43±5.67** |
| ROSI | 67.32±3.03 | 157.94±8.17 | 90.84±6.45** |
| MEL | 64.25±5.89 | 163.45±6.53 | 173.65 ±5.23 |
| PM | 65.84±6.66 | 154.11±4.69 | 81.68±4.56**cc |
| RM | 60.99±5.69 | 153.58±3.30 | 84.90±7.79**cc |
Table 2: Effect Of Combination Of Thiazolidinediones With Melatonin On Serum Glucose In Dexamethasone-Induced Insulin Resistance In Mice
| Group | Serum triglyceride (mg/dl) on | ||
|---|---|---|---|
| Day- 1 | Day- 07 | Day- 22 | |
| NC | 86.45±5.78 | 87.12±5.90 | 84.55±6.43 |
| DC | 84.34±6.65 | 136.38±6.44* | 154.79±4.33* |
| PIO | 87.64±7.56 | 139.31±8.48 | 113.56±4.30# |
| ROSI | 89.89±5.43 | 137.94±7.57 | 119.36±6.32# |
| MEL | 82.53±3.34 | 134.4±4.64 | 126.98±5.55# |
| PM | 86.43±7.34 | 124.11±3.34 | 89.68±4.46#,a,c |
| RM | 84.22±4.98 | 130.58±7.73 | 98.11±6.54#,b,c |
Mel-Melatonin, PM-Pioglitazone plus melatonin, RM-Rosiglitazone
plus melatonin. Results are presented as mean ± SEM, (n=6). ANOVA followed
by Dunnett test. *P <0.05 when compared with normal control. #P<0.05
when compared with dexamethasone control. aP<0.05 when compared with
pioglitazone treated group, bP<0.05 when compared with rosiglitazone treated
group, cP<0.05 when compared with melatonin treated group.
Table 3: Effect Of Combination Of Thiazolidinediones With Antioxidant On Serum Triglyceride Level In Dexamethasone-Induced Insulin Resistance In Mice.
Pioglitazone and rosiglitazone alone treated mice showed significant (P<0.05) decrease in the serum glucose level on day 22 than dexamethasone alone treated mice. Pioglitazone, rosiglitazone and melatonin alone treated mice showed significant (P<0.05) increase in the percentage of change in the body weight than dexamethasone alone treated mice, also showed significant (P<0.05) decrease in the serum glucose level on day 22 than dexamethasone alone treated mice.
The combination of pioglitazone plus melatonin and rosiglitazone plus melatonin also showed statistically significant (P<0.05) increased in the body weight than individual pioglitazone and rosiglitazone treated groups (Table 1).
Effect on the glucose uptake in isolated hemidiaphragm of mice:
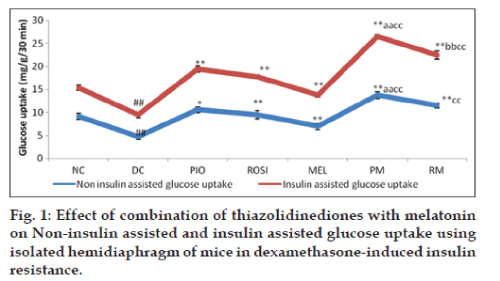
The glucose uptake in the isolated hemidiaphragm of mice in the insulin stimulated as well as non insulin stimulated glucose uptake was statistically significant (P<0.05) decrease in the dexamethasone alone treated group mice than normal group mice (fig. 1). The Pioglitazone plus melatonin and rosiglitazone plus melatonin treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in both insulin as well as non insulin assisted than dexamethasone alone treated mice and melatonin treated mice. Pioglitazone plus melatonin and rosiglitazone plus melatonin treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in insulin assisted than pioglitazone, rosiglitazone treated mice respectively.
Figure 1:Effect of combination of thiazolidinediones with melatonin on Non-insulin assisted and insulin assisted glucose uptake using isolated hemidiaphragm of mice in dexamethasone-induced insulin resistance.
Rosiglitazone plus melatonin treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in insulin assisted than melatonin treated mice also. Pioglitazone and rosiglitazone alone treated mice showed statistically significant (P<0.05) increase in the glucose uptake in the isolated hemidiaphragm in insulin assisted and non-insulin assisted than dexamethasone alone treated mice. Melatonin alone treated mice did not showed any change in the glucose uptake in the isolated hemidiaphragm in insulin assisted and non-insulin assisted than dexamethasone alone treated mice.
Effect on the oxidative stress parameters in the liver:
Mice treated with dexamethasone alone showed statistically significant decrease (P<0.05) in the hepatic levels of GSH, SOD, Catalase while statistically significant increase in MDA (P<0.05) level than normal mice (Table 4). Pioglitazone plus melatonin treated mice showed statistically significant increase (P<0.05) in hepatic levels of GSH, SOD, catalase, while statistically significant decrease (P<0.05) in LPO level when compared with dexamethasone alone treated mice, pioglitazone treated and melatonin treated mice. Rosiglitazone plus melatonin treated mice showed statistically significant increase (P<0.05) in hepatic levels of GSH, SOD and Catalase while statistically significant decrease (P<0.05) in LPO level than dexamethasone alone mice, but when compared with rosiglitazone treated mice, rosiglitazone plus melatonin treated mice showed statistically significant increase (P<0.05) in GSH, SOD only and statistically significant decrease in LPO levels. Also rosiglitazone plus melatonin treated mice when compared with melatonin treated group showed statistically significant increase (P<0.05) in the SOD level.
| Groups | GSH (µg of GSH/g of tissue) | SOD (units/mg of tissue) | Catalase (µM of H2O2/g of tissue/min) | LPO (nM of MDA/ g of tissue) |
|---|---|---|---|---|
| NC | 24.42±0.33 | 68.43±0.45 | 7.17±0.13 | 10.58±0.53 |
| DC | 14.98±0.23# | 23.56±0.75# | 3.12±0.21# | 26.22±0.14# |
| PIO | 16.89±0.52* | 29.43±0.51* | 5.19±0.35* | 21.45±0.69* |
| ROSI | 16.56±0.35* | 28.76±0.43* | 4.89±0.41* | 23.32±0.54 |
| MEL | 19.34±0.42* | 38.98±0.46* | 6.11±0.21* | 17.44±0.55* |
| PM | 22.76±0.29*ac | 57. 89±0.87*ac | 6.87±0.34*a | 12.54±0.38*ac |
| RM | 20.67±0.37*b | 45.13±0.37*bc | 6.43±0.86* | 16.79±0.53*b |
Table 4: Effect Of Combination Of Thiazolidinediones With Melatonin On Hepatic Levels Of Gsh, Sod, Catalase, And Lpo In Dexamethasone-Induced Insulin Resistance In Mice.
Pioglitazone, rosiglitazone and melatonin alone treated mice showed statistically significant increase (P<0.05) in hepatic levels of GSH, SOD, catalase, while statistically significant decrease (P<0.05) in LPO level when compared with dexamethasone alone treated mice.
Discussion
The present investigation focussed mainly on the adverse effects induced by the long-term administration of dexamethasone such as muscle catabolism, hyperphagia, increased adiposity and induction of insulin resistance [28]. A dexamethasoneinduced decrease in IRS-1 expression has been suggested to be also mechanism for insulin resistance [29-31]. It has been also proved that, dexamethasone treatment for longer period may leads to a decrease in insulin binding and that this was attributed to a reduction in the number of available cell-surface insulin receptors [32]. This could contribute to insensitivity to insulin but probably not to the reduction in maximal insulin response in glucose uptake, since fat cells have a large proportion of spare receptors [33].
Dexamethasone is a synthetic glucocorticoid which has a 50-fold greater affinity for the glucocorticoid receptor relative to cortisol. When administered in excess, dexamethasone induces adverse effects such as muscle catabolism, hyperphagia, increased adiposity, and increased insulin resistance [28]. Insulin antagonistic hormone, glucocorticoids, is under control of hypothalamic-pituitary–adrenal axis [34,35]. The insulin resistance by the glucocorticoid is via suppresses hepatic glucose production, stimulate peripheral glucose utilization and a direct inhibitory effect on glucose-induced insulin release in the b-cells [36].
In the present study, dexamethasone administration resulted in significant increase in the serum glucose and triglyceride levels. The combinations pioglitazone plus melatonin and rosiglitazone plus melatonin, decrease the elevated blood glucose level may be via increase in the glucose uptake though translocation of GLUT-4 by melatonin [17] and thiazolidinediones along with the increased insulin sensitivity [28,37]. The decrease in the serum triglyceride level in combination groups, pioglitazone plus melatonin and rosiglitazone plus melatonin might be due to increase in the lipoprotein lipase activity which causes uptake of triglyceride in fat cells [13] by thiazolidinediones and melatonin may decreases Δ-5 desaturase activity in liver microsomes which may help in decrease in hyperlipidemia in the combination treated group [18].
As the insulin resistance is characterized by decrease in the glucose uptake in muscles, hence, the glucose uptake in the isolated hemidiaphragm was evaluated. The glucose uptake decreased in isolated hemidiaphragm in dexamethasone alone treated mice, which indicates the development of insulin resistance. The insulin stimulated glucose uptake in skeletal muscle and fat cells is by the facilitative glucose transporter isoform GLUT4 [38-40]. Most of GLUT4 (90%) is in the basal state sequestered in an intracellular pool, and insulin stimulates glucose uptake mainly by recruiting this intracellular GLUT4 pool to the plasma membrane [40-42]. Dexamethasone may interact with the intrinsic activity of GLUT4 with support to this it has been reported that, the translocation process of GLUT4 from intracellular compartments to the plasma membrane in response to insulin is reduced by dexamethasone [43,44]. The probable mechanisms behind the increase in glucose uptake in combination treated mice may be due to enhanced insulin sensitivity and GLUT- 4 expression in the muscle by thiazolidinediones [28] and melatonin [16,17]. The results of pioglitazone plus melatonin and rosiglitazone plus melatonin groups were more statistically significant than individual pioglitazone, rosiglitazone and melatonin treated groups which indicated that the combination treated groups showed better improvement in reducing insulin resistance than alone treated group of thiazolidinediones and melatonin.
Dexamethasone administration in mice showed reduction in the body weight when compared with normal control mice, which may be due to complex metabolic changes such as hyperleptinemia, decreased food consumption, weight loss etc. induced by increased ob gene expression [21,45]. The combination groups, pioglitazone plus melatonin and rosiglitazone plus melatonin tends to restore the body weight loss in mice, the mechanism behind restoration of body weight in the combination groups may be due to decreased leptin expression and protein catabolism by the thiazolidinediones and melatonin [13,46].
In hyperglycemic conditions, there is reduction in the endogenous antioxidant levels and increase in the free radical generation [47] which may directly or indirectly contributes to the insulin resistance. Insulin resistance induces release of cytokines like TNF-α (Tumor necrosis factor alpha), IL-8 (Interleukine-8) which leads to further development of the oxidative stress in liver by reducing endogenous mitochondrial levels of Cu/ Zn SOD, glutathione and production H2O2 radicals [48].
Mice treated with dexamethasone alone showed decrease in the levels of antioxidant enzymes SOD, catalase, GSH and increased lipid peroxidation (MDA) in the liver homogenate. The pioglitazone plus melatonin and rosiglitazone plus melatonin showed increase levels of antioxidant enzymes such as GSH, SOD catalase in the liver and decrease in lipid peroxidation in liver homogenates. The antioxidant potential exhibited by combination of pioglitazone plus melatonin and rosiglitazone plus melatonin, might be due to potent antioxidant effect of melatonin which scavenges a variety of reactive oxygen and nitrogen species including hydroxyl radical, hydrogen peroxide, singlet oxygen, nitric oxide and peroxynitirite anion and also ability to reduce lipid peroxidation [49] as well as oxidative stress reducing effect of thiazolidinediones by decreased hyperglycemia [50,51].
In conclusion, the combination of thiazolidinediones with melatonin showed greater improvement in reducing the insulin resistance via antioxidant as well as hypoglycemia effects of melatonin and thiazolidinediones than individual agent’s monotherapy and warrants further investigation.
References
- Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: Targets and new therapies. PharmacolTher 2010;125:328-61.
- Qi D, Rodrigues B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am J PhysiolEndocrinolMetab 2007;292:654-67.
- Gholap S, Kar A. Gymnemic acids from Gymnemasylvestre. Potentially regulates dexamethasone-induced hyperglycemia in mice. Pharm Biol 2005;43:192-5.
- Thomas DM, Udagawa N, Hards DK, Gidsii J, Resret K, Injytt A, et al. Insulin receptor expression in primary and cultured oseoclast like cells. Bone 1998;23:181-6.
- Lambillotte C, Gilon P, Henquin J. Direct Glucocorticoid Inhibition of Insulin Secretion. J Clin Invest 1999;99:414-23.
- Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, Krause U, et al. Effects of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in rat skeletal muscle. Biochem J 2007;321:707-12.
- Ong JM, Simsolo RB, Saffari B, Kern PA. The regulation of lipoprotein lipase gene expression by dexamethasone in isolated rat adipocytes. Endocrinol 1992;130:2310-6.
- Rask E, Olsson T, Soderberg S, Andrew RW, Livingstone DE, Johnson O. Tissue-specific dysregulation of cortisol metabolism in human obesity. J ClinEndocrinolMetab 2001;86:1418-21.
- Buren J, Lai YC, Lundgren M, Eriksson J W, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch BiochemBiophys 2008;474:91-101.
- Bjelakovic G, Beninati S, Pavlovic D, Kocic G, Jevtovic T, Kamenov B, et al. Dexamethasone and oxidative stress. J Basic ClinPhysiolPharmacol 2007;18:115-27.
- Ceriello A, Motz E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. ArteriosclerThrombVascBiol 2004;24:816-823.
- Ogihara T, Asano T, Katagiri H, Sakoda H, Anai M, Shojima N, et al. Oxidative stress induces insulin resistance by activating the nuclear