- *Corresponding Author:
- Krutika Sawant
Drug Delivery Research Laboratory, TIFAC Centre of Relevance and Excellence in NDDS, G. H. Patel Building, Faculty of Pharmacy, The M. S. University of Baroda, Fatehgunj, Vadodara-390 002
E-mail: dr_krutikasawant@yahoo.co.in
| Date of Submission | 19 July 2017 |
| Date of Revision | 21 April 2018 |
| Date of Acceptance | 22 November 2018 |
| Indian J Pharm Sci 2019;81(1):71-81 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Felbamate, an antiepileptic drug is administered multiple times a day to obtain proper restorative action against seizures in childhood onset epilepsy (Lennox-Gastaut syndrome), which usually result in poor therapeutic efficacy because of fluctuating plasma levels and low patient compliance. Hence, controlled release hydroxypropyl methylcellulose matrix tablets of felbamate were formulated to overcome these drawbacks. The results of pre-formulation studies such as differential scanning calorimetry and Fourier transform infrared spectroscopy showed compatibility of drug with the selected excipients. The formulation variables were optimized using D-optimal design which can elucidate the effect of all variables simultaneously during formulation optimization. In vitro drug release at the end of 2, 8 and 20 h were taken as the response parameters for the optimization study by D-optimal design. The results enabled selection of the formulation with the desired drug release pattern approaching to zero order. The optimized batch was subjected to in vivo pharmacokinetic studies in rabbits which showed extended release of drug up to 24 h. Thus, the felbamate controlled release tablets optimized by D-optimal design have potential to reduce the dose and dosing frequency, improve therapy and patient compliance.
Keywords
Felbamate, controlled release, HPMC, D-optimal design, in vivo pharmacokinetics
Introduction
For long-term treatment, conventional drug formulations are required to be administered in multiple doses, which have several disadvantages [1]. Design of controlled release (CR) formulations that release drug over an extended period of time is desirable in chronic disease conditions. CR formulations offer many advantages such as low dose, reduced or no fluctuation of drug concentration in the blood, minimal side effects, improved patient compliance and cost effectiveness [2,3].
Matrix technologies have proven to be popular among oral controlled drug delivery technologies because of their ease of manufacturing and simplicity of formulation, high degree of reproducibility, stability of the excipients and dosage form, and ease of technology transfer during scale-up and process validation [4]. Hydrophilic matrix systems are widely used for providing CR from solid oral dosage forms. Hydroxypropyl methylcellulose (HPMC), a semisynthetic polymer derived from cellulose, shows minimal interaction problems when used in basic, acidic or other electrolytic systems due to its nonionic nature. HPMC is enzyme resistant, chemically stable over a wide pH range, has consistently high quality and regulatory approval, making it an excellent carrier material for a matrix system [5]. In matrix tablets prepared with HPMC, the polymer quickly hydrates to form a gelatinous layer when it comes in contact with water. As the outer gel layer fully hydrates and dissolves, a newer inner layer forms gel like structure to retard the water influx and control drug diffusion [6]. Hence, in the current investigation, HPMC was used to prepare CR hydrophilic matrix tablets.
Microcrystalline cellulose (MCC), which is used as a bulking agent, provides hardness with reduced lubricant requirement due to its low co-efficient of friction and decreased residual die wall pressure. Due to its plastic nature, MCC provides exceptional binding properties. However, compared to other brittle excipients, MCC is more lubricant sensitive. The presence of high levels of hydrophobic excipients, such as magnesium stearate, may result in softer tablets [7]. Therefore, it was necessary to optimize the formulation for quantities of rate-controlling polymer HPMC, binder MCC, and lubricant magnesium stearate.
Optimization of any pharmaceutical process starts with the objective to find out and evaluate the effect of independent variables on the formulation response parameters, determine them and establish their effect using best response values. During formulation development, various formulation and process variables related to effectiveness and safety should be simultaneously optimized instead of using one variable at a time approach. Statistical experimental design (design of experiments, DoE) is a well-established technique for planning and execution of informative experiments for optimization of formulation. An important type of DoE is a mixture design. In this design, the effect of change in the mixture components on the properties of the mixture is explored [8]. The characteristics of a mixture is that the sum of its components is 100 % (ΣXk=1) [9]. This means that these components (Xk), mixture factors, cannot be changed independently of one another, as their proportions lie somewhere between 0 to 1. Thus, this design considers all variables at a time in experimental work [10].
D-optimal design is a type of mixture design, which can minimize the generalized variance of the estimated regression coefficients and provides optimization of independent factors with minimum number of runs in comparison to other factorial designs [11]. The dependent response for the design is measured for each trial and then either simple linear model (Eqn. 1), or interactive model (Eqn. 2) or a quadratic model (Eqn. 3) is fitted. The model selection is carried out based on analysis of variance (ANOVA) result for each response and statistically significant terms are then identified. Eqn. 1: Y = b0+b1X1+b2X2+b3X3; Eqn. 2: Y= b0b1+b2X2+b3X3+b12X1X2+b13X1X3+b23X2X3+b123X1X2X3; Eqn. 3: Y = b0+b1X1+b2X2+b3X3+b12X1X2+ b13X1X3+b23X2X3+b12X11+b22X22+b32X33+b123X1X2X3 where, Y is estimated response; b0 is constant; b1, b2, b3 are linear coefficients; b12, b23, b13 are interaction coefficients; and b12, b22, b32 are quadratic coefficients [12].
Felbamate (2-phenyl-1,3-propanediol dicarbamate) is an antiepileptic drug used in adults for partial seizures and in children for Lennox-Gastaut syndrome [13]. Its marketed forms of tablets and suspension have to be administered multiple times a day, leading to drawbacks such as poor patient compliance and poor control over therapy due to fluctuating plasma drug levels. CR once daily formulation of felbamate can reduce the number of single doses during the day and also reduce the fluctuation of serum levels, thereby offering better therapeutic efficacy and increasing patient compliance.
The purpose of the present study was to design oral CR tablets of felbamate using HPMC as the release retardant polymer. The effect of HPMC K4M, magnesium stearate and MCC on the release kinetics of felbamate from the matrix tablet was optimized by D-optimal design.
Materials and Methods
Felbamate was received as a gift sample from Zydus Research Center, Ahmedabad, India. HPMC K4M and polyvinylpyrrolidone K 30 (PVP K 30) were received as gift sample from Kaptab Pharmaceuticals, Vadodara, India. MCC (Avicel®PH101) was obtained as gift sample from Signet Chem Pvt., Ltd., Mumbai, India. Magnesium stearate and Aerosil were purchased from S. D. Fine Chem Ltd., Mumbai, India. Isopropyl alcohol (IPA, assay ≥99.5 %, certified ACS Reagent Grade) was purchased from Ghanshyam Traders, Vadodara, India. All other chemicals and reagents used were of analytical reagent grade. JMP-SAS version 11.1.1 (SAS Institute Inc., Cary, NC, USA) was used for experimental design and data analysis.
Drug-excipient compatibility study
In order to identify drug-excipient incompatibility, if any, compatibility study was performed by Fouriertransform infrared spectroscopy (FTIR) spectroscopy and differential scanning calorimetry (DSC). The sample (drug alone or a 1:1 drug and excipient mixture) was dispersed uniformly in KBr by triturating it in a mortar and compressed to form a pellet at pressure of 5 tons for 5 min in a hydraulic press. The IR spectra was recorded in the region of 4000 to 400 cm-1 using FTIR spectrophotometer, Bruker Optik GmbH, Germany [14]. DSC was performed using DSC-41 (Shimadzu, Japan) to study the compatibility of various excipients with felbamate. Solid admixture was prepared by dry mixing of drug with each excipient in 1:1 ratio and was sealed in aluminium pan by applying external pressure. The aluminum pans were heated from 25° to 300° under nitrogen atmosphere at a scanning rate of 10°/min.
Formulation of felbamate CR matrix tablets
CR HPMC K4M matrix tablets of felbamate were formulated by non-aqueous wet granulation method. Based on the results of the initial trials, the levels of felbamate (40 %), Aerosil (1 %) and PVP K 30 (2 %) were kept constant. Therefore, the experimental range for D-optimal design lay between 0 and 57 % (w/w) for the three variables, HPMC K4M, MCC and magnesium stearate. The target weight of the final tablet was kept 1000 mg. All the ingredients except magnesium stearate were passed through 40# sieve while magnesium stearate was passed through 60# sieve. The uniformly mixed drug, polymer and MCC blend was granulated with PVP in IPA (5 % w/v). The wet mix was passed from 20# sieve. The granules were dried in a tray drier at 40° till the loss on drying was less than 2 %. The dried granules were passed through 40# sieve and blended with Aerosil and magnesium stearate. The granules were compressed on 8 station rotary tablet press (General Machinery, India) using round, flat faced 16 mm punch at a compression force required to produce hardness of about 8 kg/cm2.
In vitro drug release studies
In vitro drug release was studied up to 24 h using USP type 2 (paddle) dissolution apparatus (Electrolab, India), in 900 ml of 0.5 % sodium lauryl sulphate in water at 37.5±0.5°. The stirring speed was set at 50 rpm. Five millilitre sample was withdrawn at intervals of 2 h and replaced with fresh dissolution medium. After appropriate dilution, the samples were analysed by the in-house developed and validated UV spectrophotometric method at 257 nm using spectrophotometer (UV-1700, Shimadzu, Japan) [15].
Experimental design
A 18-run, three factors, two-level D-optimal mixture design having 3 centre points and 5 replicate runs was employed to study the effect of formulation (independent) variables on % drug release (dependent variable). Preliminary trials were performed to select the discrete levels of the independent variables. Table 1 summarizes the independent and dependent variables evaluated for formulation optimization and the constraints that were placed on the responses. The constraints were applied to % drug release so as to provide extended release upto 24 h.
| Independent variables | Low level | High level | |||
|---|---|---|---|---|---|
| HPMC K4M (X1) | 0.3508 (200 mg) | 0.8770 (500 mg) | |||
| MCC (X2) | 0.1140 (65 mg) | 0.6402 (365 mg) | |||
| Magnesium stearate (X3) | 0.0087 (5 mg) | 0.0175 (10 mg) | |||
| Dependent variables | Low limit | High limit | Goal | ||
| % drug released in 2 h (Y1) | 5 | 20 | Minimize | ||
| % drug released in 8 h (Y2) | 20 | 60 | Minimize | ||
| % drug released in 20 h (Y2) | 80 | 90 | Is target=90 | ||
Table 1: Variables of the D-Optimal Mixture Design for Optimization
Felbamate matrix CR tablets were prepared according to the design generated using the statistical software JMP SAS version 11.0 (Statistical Discovery, SAS, Malaysia). Polynomial models were generated for the responses Y1 (% drug released in 2 h), Y2 (% drug released in 8 h) and Y3 (% drug released in 20 h). Linear model was chosen based on the p value for lack of fit and F value. The model was used to predict the composition of the formulation, which would provide desired drug release profile.
Analysis of release kinetics
The release mechanism and kinetics of the release profiles were inferred based on the correlation coefficient values obtained from the plots of the Korsmeyer-Peppas model [16]. Korsmeyer-Peppas’ Eqn., Mt/M∞ = ktn, where, Mt/M∞ is fraction of drug released at any time t; k is release constant; n is diffusional exponent, which indicates mechanism of drug release. In case of tablets (cylindrical shape), a value of n=0.45 suggests Fickian (case I) release; 0.45<n<0.89 non- Fickian (anomalous) release; n=0.89 zero order drug release; and n>0.89 specifies super case II release.
Mean dissolution time (MDT) was calculated for each formulation matrix from the arithmetic mean value of the dissolution profile. The MDT value is used to depict drug release rate from a dosage form and it also indicate the drug release retarding efficiency of the polymer. Higher the value, more will be the drug release retarding ability of the polymer [17].
The MDT values were calculated using the following Eqn.: MDT = Σnj=1ΔMj/Σnj=1ṫjΔMj, where, j is the sample number, n is the number of dissolution sample times, ṫj is the time at midpoint between tj and tj−1, and ΔMj is the additional quantity of drug released between tj and tj−1. The model selection criterion (MSC) is a statistical tool for model selection and for evaluation of goodness of fit [18]. It is a modified reciprocal form of the Akaike information criterion and is normalized to render it independent of the scaling of the data points. It is defined as: MSC = In [Σni=1wi(yiobs–ӯobs)2/Σn i=1wi(yiobs–ӯi pre)2–2p/n –2p/n], where wi is the weighting factor, which value is chosen 1 for fitting dissolution data, yiobs is the ith observed y value, ӯi pre is the ith predicted y value, ӯobs is mean of all observed y data points, n is number of data points and p is number of parameters.
In vivo pharmacokinetic study in rabbits
The in vivo pharmacokinetic study was performed on male New Zealand white rabbits (weighing around 1.8- 2.0 kg) obtained from the Animal Vaccine Institute, Gandhinagar, India. The rabbits were housed in cages in an air conditioned room (25±2°, 30-65 % RH), 1 animal per cage, with free access to pelleted food (Pranav Agro Foods Pvt. Ltd., Vadodara, India) and water. The preclinical study protocol was approved by the Institutional Animal Ethics Committee, Pharmacy Department, The M. S. University of Baroda, Vadodara, India. All the experimental procedures were carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India.
Study protocol
Overnight fasted rabbits were divided into two groups (n=4) for cross over pharmacokinetic study. The specially compressed small (5 mm) tablets were orally administered at a dose of 20.55 mg/kg to both the groups as shown in Table 2. The dose given to the rabbits was calculated by the following Eqn. 4 [19], rabbit dose (mg/kg) = human equivalent dose (mg/kg)×37/12. The tablet was put in the diastema for easy access to the oral cavity and to avoid destruction due to biting and chewing.
| Group | Phase I | Wash out period | Phase II(cross over phase) |
|---|---|---|---|
| I | IR felbamate Tablet | 2 weeks | CR felbamate Tablet |
| II | CR felbamate Tablet | 2 weeks | IR felbamate Tablet |
Animal grouping and oral treatment (20.55 mg/kg) with immediate release (IR) and controlled release (CR) tablets of felbamate
Table 2: Cross Over Pharmacokinetic Study Design
The rabbit’s ear was swabbed with 70 % IPA in water with cotton and 1.0 ml blood samples were collected from the marginal ear vein at 1, 3, 5, 8, 12, 24, 31, 48 and 55 h post dose with a 22# gauge needle in microcentrifuge tubes. The blood was immediately centrifuged using cooling centrifuge (C-24, Remi Equipment’s Pvt. Ltd., Mumbai, India) at 4000 rpm for 10 min at 4° [20]. The serum was separated and stored at –72° until analysis by high performance liquid chromatography (HPLC) method. Different pharmacokinetic parameters such as Cmax, tmax, half life, area under curve (AUC), mean residence (MRT) and elimination rate constant (Ke) were calculated using Kinetica 5.0 pharmacokinetic data analysis software (Thermo Scientific™).
Analysis of drug in serum
Quantitative estimation of felbamate in serum was done by HPLC method as described by Paw et al. [21]. The HPLC system (Shimadzu LC- 20 AD) with a UV detector and Chromacil™ C-18 column (4.6 mm× 25 cm, 5-μm packing) was used. A degassed mixture of acetonitrile and water (1:4 v/v) was used as the mobile phase. The injection volume was 20 μl. The retention time was 6.52 min at the flow rate of 1 ml/min, when detected at 210 nm.
Results and Discussion
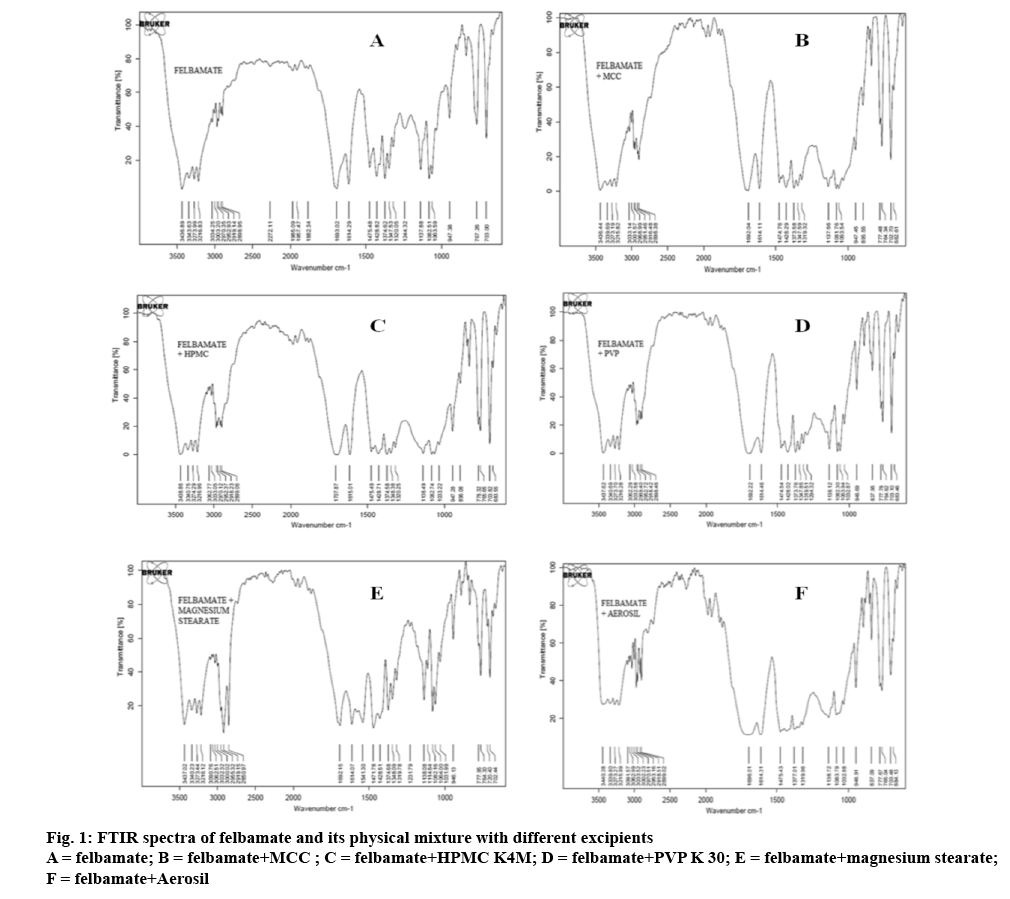
The possible interaction between felbamate and selected excipients was studied by FTIR spectroscopy and DSC. The FTIR spectra of felbamate and its physical mixture with excipients are shown in figure 1. Pure felbamate showed major peaks at 764, 1693, 1614 and 3400 cm-1 corresponding to C-H (out of plane) stretch of aromatic structure, C=O stretch, N-H bend and N-H stretch of 1° amine (figure 1A) [14]. The spectra showed no considerable changes in FTIR peaks of felbamate when mixed with excipients suggesting its compatibility with the selected excipients (figure 1B-F).
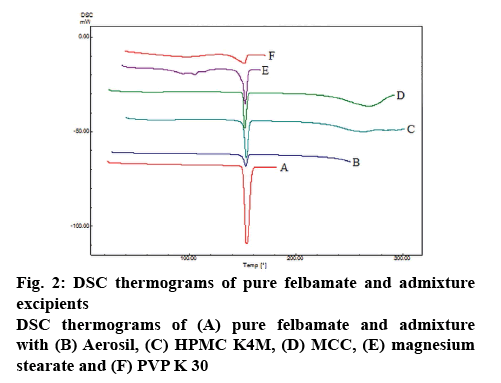
The compatibility between the drug and excipients was further supported by DSC data. The DSC thermogram of pure felbamate showed a sharp melting endothermic peak at 153.78° as shown in figure 2A. There was no change in its endothermic peak in presence of the selected excipients indicating their compatibility (figure 2B-F).
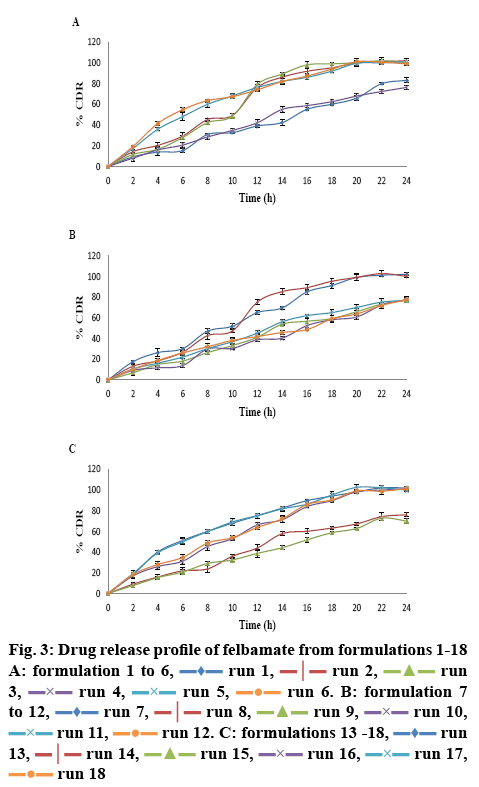
In vitro drug release studies were carried out for the batches prepared as per the experimental designs to correlate the effect of formulation variables on the drug release at different time points. The wide variation in drug release indicated that the factor combinations resulted in different drug release rates. The experimental runs and the observed responses are given in Table 3. Dissolution profiles of the 18 formulations are shown in figure 3.
| Run | X1 (HPMC K4M)* | X2 (MCC)* | X3 (Magnesium Stearate)* | Y1 (% Drug released in 2 h) | Y2 (% Drug released in 8 h) | Y3 (% Drug released in 20 h) |
|---|---|---|---|---|---|---|
| 1 | 0.8770 | 0.1143 | 0.0087 | 9.23±1.76 | 30.87±2.23 | 66.12±2.48 |
| 2 | 0.3508 | 0.6317 | 0.0175 | 14.23±2.19 | 45.32±3.30 | 98.98±5.37 |
| 3 | 0.3508 | 0.6317 | 0.0175 | 11.52±1.18 | 42.37±4.43 | 98.18±4.85 |
| 4 | 0.8685 | 0.1140 | 0.0175 | 7.94±0.96 | 28.46±2.69 | 68.01±1.38 |
| 5 | 0.3511 | 0.6402 | 0.0087 | 17.35±1.69 | 60.04±3.86 | 99.75±2.45 |
| 6 | 0.3511 | 0.6402 | 0.0087 | 19.26±1.36 | 63.63±4.54 | 101.23±2.46 |
| 7 | 0.6118 | 0.3750 | 0.0130 | 17.56±1.38 | 47.14±2.73 | 99.12±2.35 |
| 8 | 0.3508 | 0.6317 | 0.0175 | 13.25±1.48 | 42.84±3.58 | 99.12±2.57 |
| 9 | 0.8685 | 0.1140 | 0.0175 | 6.98±0.45 | 26.43±1.34 | 65.98±2.84 |
| 10 | 0.8770 | 0.1143 | 0.0087 | 9.24±0.56 | 29.89±1.27 | 60.93±2.38 |
| 11 | 0.8685 | 0.1140 | 0.0175 | 10.53±1.36 | 30.24±2.36 | 70.12±2.48 |
| 12 | 0.8770 | 0.1143 | 0.0087 | 10.25±1.34 | 32.09±3.58 | 63.86±2.84 |
| 13 | 0.3511 | 0.6402 | 0.0087 | 18.23±2.52 | 59.98±2.80 | 98.12±1.35 |
| 14 | 0.8685 | 0.1140 | 0.0175 | 8.98±1.37 | 23.76±3.27 | 67.26±1.46 |
| 15 | 0.8770 | 0.1143 | 0.0087 | 7.65±0.93 | 28.97±2.47 | 62.90±1.78 |
| 16 | 0.6118 | 0.3750 | 0.0130 | 16.98±1.35 | 45.34±3.58 | 98.32±1.47 |
| 17 | 0.3511 | 0.6402 | 0.0087 | 19.23±2.27 | 59.87±1.76 | 102.56±2.57 |
| 18 | 0.6118 | 0.3750 | 0.0130 | 18.23±1.27 | 48.73±2.46 | 99.12±3.27 |
* X1, X2 and X3 are coded values
Table 3: 3 Component D-Optimal Design for Optimization of Felbamate Cr Tablets
Drug release at 2 h (response Y1), 8 h (response Y2) and 20 h (response Y3) were considered as the primary responses and were analysed by JMP-SAS software. The percent drug release in 2 h (response Y1) ranged from 6.98±0.45 % (formulation 9) to 19.26± 1.36 % (formulation 6), that in 8 h (response Y2) ranged from 23.76±3.27 % (formulation 14) to 63.63±4.54 % (formulation 6) while for 20 h (response Y3), it ranged from 60.93±2.38 % (formulation 10) to 102.56± 2.57 % (formulation 17). A good reproducibility in tablet preparation and dissolution analysis was exemplified by the good agreement between the four test replicates (formulations 1, 10, 12 and 15).
The release kinetics for all the 18 formulations were analysed by Korsmeyer-Peppas model. The n values ranged from 0.53 to 1.07 (Table 4). Ideally, values of n from 0.53 to 0.88 suggest anomalous (non-Fickian) transport whereas greater than 0.89 suggests super case- II release. Hence, the release kinetics of felbamate from the CR tablets may involve more than one mechanisms.
| Runs | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Korsmeyer - Peppas | R2 | 0.9888 | 0.9570 | 0.9320 | 0.9912 | 0.9864 | 0.9834 | 0.9872 | 0.9539 | 0.9915 |
| n | 1.07 | 0.79 | 0.81 | 0.90 | 0.57 | 0.53 | 0.79 | 0.82 | 0.94 | |
| MDT | (h) | 12.31 | 9.10 | 8.72 | 10.77 | 7.86 | 7.13 | 9.58 | 9.10 | 11.33 |
| MSC | 3.6325 | 2.4866 | 2.6258 | 4.2152 | 4.1192 | 2.6143 | 3.3622 | 1.8072 | 3.8536 | |
| Runs | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
| Korsmeyer - Peppas | R2 | 0.9881 | 0.9900 | 0.9917 | 0.9885 | 0.9819 | 0.9936 | 0.9889 | 0.9863 | 0.9894 |
| n | 1.03 | 0.85 | 0.81 | 0.56 | 0.88 | 0.90 | 0.77 | 0.56 | 0.74 | |
| MDT | (h) | 12.25 | 10.26 | 11.21 | 7.72 | 10.57 | 10.61 | 9.49 | 7.38 | 9.29 |
| MSC | 3.3410 | 4.3329 | 4.2413 | 3.1444 | 3.2784 | 5.4833 | 3.3821 | 3.8700 | 4.0426 | |
Table 4: Release Kinetics by Korsmeyer - Peppas Model
As suggested by the n values of Korsmeyer-Peppas model ranging from 0.90 to 1.07 and R2 values ranging from 0.9881 to 0.9936, the batches 1, 4, 9, 10 and 15 followed super case-II drug release, which is the drug transport mechanism associated with state-transition and stresses in hydrophilic glassy polymers, swelled in water or biological fluids [22]. This transport indicates that the dominant mechanism of drug release is polymer relaxation due to swelling of the HPMC gel. For the remaining batches, n values ranged from 0.53 to 0.88, indicating anomalous non-Fickian drug release. It can be inferred that the release was dependent on both, diffusion and polymer relaxation.
In general, the mechanism of drug release from hydrophilic polymeric matrices like HPMC is described by swelling and disentanglement of the polymer. As the solvent molecules move within the HPMC matrix like a solvent front at a uniform speed, the thickness of the area increases with time in the opposite direction at the same time due to swelling [23]. The disentanglement and erosion theory of drug release from HPMC involves penetration of water into the dry matrix of tablet followed by hydration and swelling of HPMC and diffusion of the drug dissolved in the matrix. The drug release rate was faster in batches with low concentration of HPMC owing to lesser polymer entanglement and poor gel strength as compared to formulations containing high proportion of HPMC. Due to low gel strength, the effective molecular diffusional area is high, which enhances drug release at low polymer concentration. As the polymer proportion increases, the gel layer thickness increases, which ultimately increases the gel strength and forms longer diffusional path-length that hinders movement of drug molecules and leads to decrease in its release rate [16]. Detailed ANOVA for the selected responses is summarized in Table 5. The ANOVA results show low values of prob>F, which indicates that lack of fit for the suggested model is non-significant, i.e. the model selected for the design (linear) fits well to the data.
| Response | Source | Degree of freedom | Sum of squares | Mean square | F ratio | Prob>F |
|---|---|---|---|---|---|---|
| Y1 (% Drug release 2 h) | Model | 2 | 233.3427 | 116.671 | 16.3651 | 0.0002* |
| Error | 15 | 106.9395 | 7.129 | |||
| Corrected total | 17 | 340.2822 | ||||
| Y2 (% Drug release 8 h) | Model | 2 | 2708.9609 | 1354.48 | 71.746 | <0.0001* |
| Error | 15 | 283.1817 | 18.88 | |||
| Corrected total | 17 | 2992.1426 | ||||
| Y3 (% Drug release 20 h) | Model | 2 | 4561.6443 | 2280.82 | 49.9529 | <0.001* |
| Error | 15 | 684.8921 | 45.66 | |||
| Corrected total | 17 | 5246.5364 |
*Tested against reduced model: Y = mean
Table 5: Analysis of Variance for Linear Model for the Responses
Whether the effect of individual factor on drug release is significant or non-significant can be concluded from leverage plot. The whole model graph shows combined effect of the factors on the drug release at the selected stages of drug release as shown in the figure 4. In the leverage plot for HPMC and MCC, the line of fit (dark line) along with confidence interval line (5 %, dashed line) crosses the horizontal line, suggesting their significant effect on drug release. For magnesium stearate, although the line of fit (dark line) crosses the horizontal line, the confidence interval line (5 %, dashed line) is asymptotic to the horizontal line, suggesting non-significant effect on drug release. The leverage plot was be explained in terms of p value and F ratio as shown in Table 6. The probability value for determination of statistical significance was set at 0.05, which indicates that the null hypothesis would be rejected if the calculated p value was less than 0.05 in favour of the alternate hypothesis. The results showed that the F ratio was higher and the p value was lower for the factors X1 (HPMC) and X2 (MCC), suggesting their significant effect on each response Y1, Y2 and Y3, i.e drug release at different time points. As suggested by the p value and F ratio, the efffect of magnesium stearate was not significant on drug release, which was also evident from the contour plots shown in fig. 5A and B.
| X | Y | Count | p Value | F Ratio |
|---|---|---|---|---|
| HPMC | Y1 (2 h) | 18 | 0.0002 | 24.29 |
| MCC | 18 | 0.0001 | 25.05 | |
| Magnesium stearate | 18 | 0.1739 | 2.03 | |
| HPMC | Y2 (8 h) | 18 | 5.26*10-7 | 64.52 |
| MCC | 18 | 3.54*10-7 | 68.56 | |
| Magnesium stearate | 18 | 0.1228 | 2.65 | |
| HPMC | Y3 (20 h) | 18 | 2.14*10-8 | 103.66 |
| MCC | 18 | 2.41*10-8 | 101.90 | |
| Magnesium stearate | 18 | 0.9961 | 2.44*10-5 |
Table 6: P Value and F Ratio for Each Variable (X) for Response (Y)
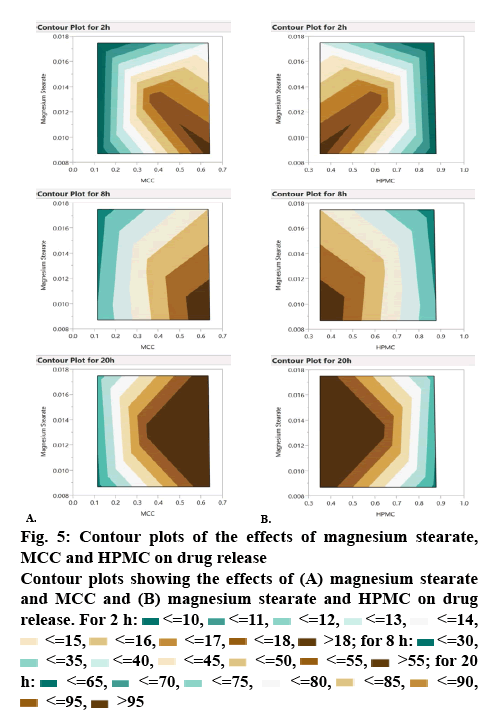
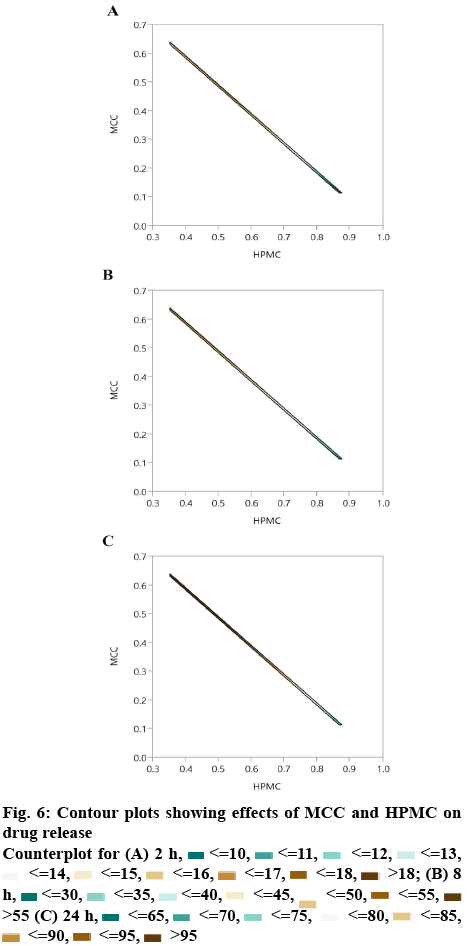
The quantitative relationship between the factors and responses are evident from the contour plots. The effect of HPMC and MCC were reciprocal to each other (figure 6). By increasing the proportion of HPMC, the drug release was sustained whereas by increasing the proportion of MCC, the drug release was increased. This supports the results of the drug release study of different formulations prepared as per D-optimal design as shown in Table 3. The contour plots showed that by increasing the proportion of magnesium stearate, the drug release decreased whereas by increasing MCC concentration, the drug release was increased (figure 5A). HPMC and magnesium stearate showed similar effect on drug release, i.e. by increasing their proportion, the drug release decreased (figure 5B). It could be concluded from the contour plots that HPMC and MCC showed profound effect on drug release, whereas magnesium stearate had negligible effect on the same.
Figure 5: Contour plots of the effects of magnesium stearate, MCC and HPMC on drug release
Contour plots showing the effects of (A) magnesium stearate and MCC and (B) magnesium stearate and HPMC on drug release. For 2 h:  <=10,
<=10,  <=11,
<=11,  <=12,
<=12,  <=13, <=14,
<=13, <=14,  <=15,
<=15,  <=16,
<=16,  <=17,
<=17,  <=18,
<=18,  ˃18; for 8 h:
˃18; for 8 h:  <=30,
<=30,  <=35,
<=35,  <=40,
<=40,  <=45,
<=45,  <=50,
<=50,  <=55,
<=55,  ˃55; for 20 h:
˃55; for 20 h:  <=65,
<=65,  <=70,
<=70,  <=75,
<=75,  <=80,
<=80,  <=85,
<=85,  <=90,
<=90,  <=95,
<=95,  ˃95
˃95
Based on the desirability criteria (maximum desirability=1), the formulation with highest desirability was chosen for confirmation and final optimization of the felbamate CR tablet. This optimized batch showed drug release similar to the predicted drug release of 16.91 % at 2 h, 46.21 % at 8 h and 90.00 % at 20 h as shown in Table 7. The t-test was performed to confirm the concordance between the experimental and predicted values. As the obtained tstat value (0.3662) was much lower than the tstd (2.9199), it was concluded that there was no significant difference between experimental value and observed value.
| Response | HPMC | MCC | Magnesium stearate | 2h | 8h | 20h | Desirability |
|---|---|---|---|---|---|---|---|
| Predicted | 0.702 | 0.289 | 0.009 | 16.91 | 46.21 | 90.00 | 1.00 |
| Observed | 0.702 | 0.289 | 0.009 | 13.05±2.03 | 48.84±1.34 | 95.49±4.61 | - |
Table 7: Optimization of Drug Release From Felbamate Cr Tablet
The in vivo pharmacokinetic investigation data for IR and CR tablets are tabulated in Table 8. The absorption was rapid for IR tablet as indicated by low tmax value (3 h); whereas the CR tablet exhibited delayed absorption as indicated by its high tmax value (8 h). This may be due to the release retarding effect of HPMC K4M, which is a hydrophilic swellable polymer. The Cmax and t1/2 for the IR tablet were low as compared to the CR tablet. The rapid elimination of felbamate from IR tablet was further supported by the high value of elimination rate constant. On the other hand, high values of Cmax, half life and low values of elimination rate constant for CR tablet indicated that the drug remained in the body for a prolonged time. This was further supported by higvalues of MRT in comparison with IR tablet. The low value of AUC observed for IR tablet may be due to its rapid absorption and elimination from the body; on the contrary, the CR tablet showed higher AUC values indicating increased bioavailability of felbamate.
| Parameters measured | Immediate release tablet | Controlled release tablet |
|---|---|---|
| Cmax (μg/ml) | 3.11±0.41 | 12.13±2.24 |
| tmax (h) | 3 | 8 |
| Half life (h) | 10.23±1.34 | 13.71±1.11 |
| AUC 0-t (μg/ml×h) | 58.44±4.23 | 215.38±12.12 |
| MRT (h) | 18.53±2.54 | 24.40±1.43 |
| Ke (h-1) | 0.067±0.005 | 0.050±0.003 |
Cmax = maximum serum concentration, tmax = time for maximum plasma concentration, AUC = area under the curve, MRT = mean residence time, Ke = elimination rate constant
Table 8: Pharmacokinetic parameters for felbamate IR and CR tablets
The present investigation demonstrated successful formulation development of CR tablets of the antiepileptic drug felbamate using HPMC as the release retarding polymer. From the preliminary trials it was clear that the amount of HPMC and MCC drastically affected drug release. Hence, optimization by D-optimal approach was employed to obtain zero order drug release up to 24 h. In vitro drug release analysis by different mathematical parameters confirmed zero order drug release up to 24 h. In vivo pharmacokinetic studies in rabbits suggested MRT of 24.40±1.43 h indicating sustained release from the developed CR tablets. Even though the optimized CR tablets of felbamate can overcome the drawbacks associated with conventional felbamate tablet, pharmacokinetics in humans are required to evaluate the efficacy of the formulation.
Acknowledgements
The authors thank the All India Council for Technical Education, New Delhi, India for providing post graduate fellowship to the first author. The authors also thank Zydus Research Centre, Ahmedabad, India for generous gift sample of felbamate, Kaptab Pharmaceuticals, Baroda, India for providing HPMC K4M and Signet Chem Pvt. Ltd., Mumbai, India for providing Avicel®PH101 as gift sample.
Conflict of interest
Authors report no declaration of interest.
References
- Ravi PR, Kotreka UK, Saha RN. Controlled Release Matrix Tablets of Zidovudine: Effect of Formulation Variables on the In vitro Drug Release Kinetics. AAPS PharmSciTech 2008;9(1):302-13.
- Altaf SA, Friend DR. MASRx and COSRx sustained-release technology. In: Rathbone M, Hadgraft J, Roberts MS, editors. Modified Release Drug Delivery Technology. New York: Marcel Dekker; 2002. p. 21-33.
- Hiremath PS, Saha RN. Oral Controlled Release Formulations of Rifampicin. Part II: Effect of formulation variables and process parameters on In vitro Release. Drug Deliv 2008;15(3):159-68.
- Varma MVS, Kaushal AM, Garg A, Garg S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am J Drug Deliv 2004;2(1):43-57.
- Hiremath PS, Saha RN. Controlled release hydrophilic matrix tablet formulations of isoniazid: design and in vitro studies. AAPS PharmSciTech 2008;9(4):1171-8.
- Colombo P. Swelling-controlled release in hydrogel matrices for oral route. Adv Drug Deliv Rev 1993;11(1-2):37-57.
- Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int J Pharm 2014;473(1):64-72.
- Martinello T, Kaneko TM, Velasco MV, Taqueda ME, Consiglieri VO. Optimization of poorly compactable drug tablets manufactured by direct compression using the mixture experimental design. Int J Pharm 2006;322(1):87-95.
- Nahata T, Saini TR. D-Optimal Designing and Optimization of Long Acting Microsphere-Based Injectable Formulation of Aripiprazole. Drug Dev Ind Pharm 2008;34(7):668-75.
- Eriksson L, Johansson E, Wikström C. Mixture design-design generation, PLS analysis, and model usage. Chemom Intell Lab Syst 1998;43(1):1-24.
- Wojcik PJ, Pereira L, Martins R, Fortunato E. Statistical Mixture Design and Multivariate Analysis of Inkjet Printed a-WO3/TiO2/WOX Electrochromic Films. ACS Comb Sci 2014;16(1):5-16.
- Eriksson L, Johansson E, Kettaneh-Wold N, Wikström C, Wold S. Design of experiments. Principles and Applications. Stockholm: Umetrics Academy; 2000.
- USP, United States Pharmacopoeia. 36th ed. Rockville, MD: United States Pharmacopoeia Convention, Inc.; 2013. p. 3533-35.
- Sparagana SP, Strand WR, Adams RC. Felbamate Urolithiasis. Epilepsia 2001;42(5):682-5.
- Parikh K, Mundada P, Sawant K. Development of new, rapid and validated UV-spectrophotometric method for the estimation of felbamate in bulk and tablets. Indian Drugs 2006;53(3):47-51.
- Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Revi 2001;48(2):139-57.
- Podczeck F. Comparison of in vitro dissolution profiles by calculating mean dissolution time (MDT) or mean residence time (MRT). Int J Pharm 1993;97(1):93-100.
- Vora C, Patadia R, Mittal K, Mashru R. Risk based approach for design and optimization of stomach specific delivery of rifampicin. Int J Pharm 2013;455(1):169-81.
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. The FASEB J 2007;22(3):659-61.
- Parmar C, Parikh K, Mundada P, Bhavsar D, Sawant K. Formulation and optimization of enteric coated bilayer tablets of mesalamine by RSM: In vitro-in vivo investigations and roentogenographic study. J Drug Deliv Sci Technol 2018;44:388-98.
- Paw B, Misztal G, Skibinski R. Rapid and simple high-performance liquid chromatographic determination of felbamate in serum. Acta Pol Pharm 2003;60:339-42.
- Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm 1989;57(2):169-72.
- Hogan JE. Hydroxypropylmethylcellulose sustained release technology. Drug Dev Ind Pharm 1989;15(6-7):975-99.
 run 1,
run 1,  run 2,
run 2,  run 3,
run 3,  run 4,
run 4,  run 5,
run 5,  run 6. B: formulation 7 to 12,
run 6. B: formulation 7 to 12,  run 7,
run 7,  run 8,
run 8,  run 9,
run 9,  run 10,
run 10,  run 11,
run 11,  run 12. C: formulations 13 -18,
run 12. C: formulations 13 -18,  run 13,
run 13,  run 14,
run 14,  run 15,
run 15,  run 16,
run 16, run 17,
run 17,  run 18
run 18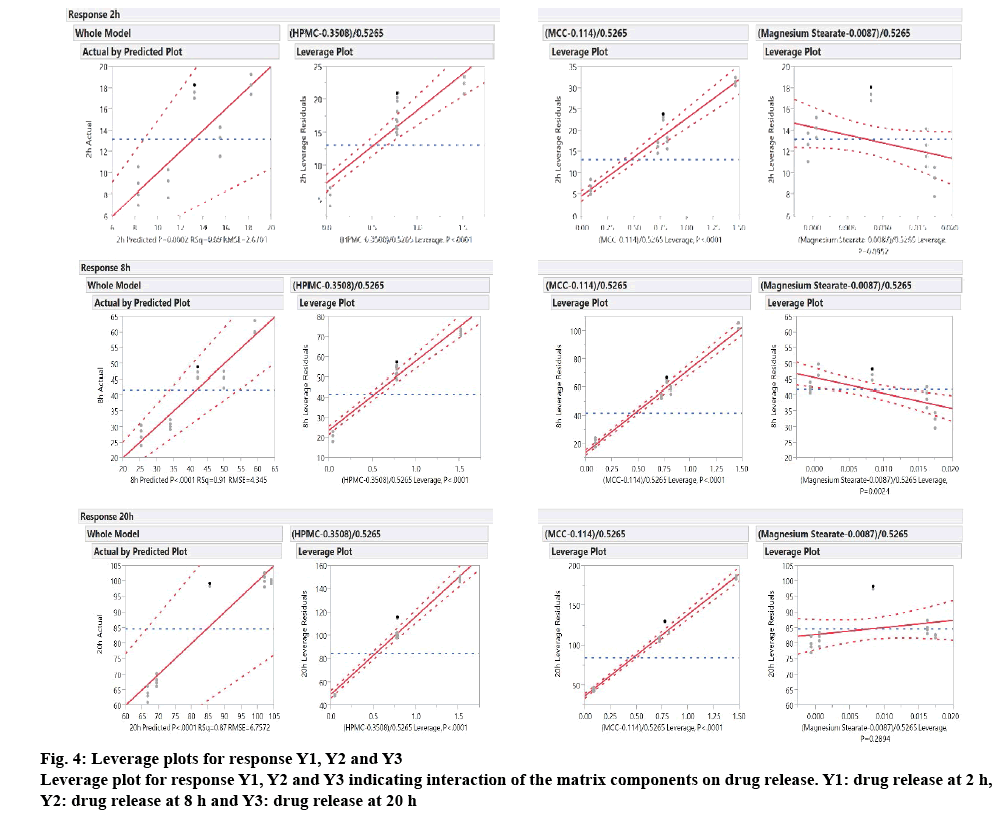
 <=10,
<=10,  <=11,
<=11,  <=12,
<=12,  <=13,
<=13,  <=14,
<=14,  <=15,
<=15,  <=16,
<=16,  <=17,
<=17,  <=18,
<=18,  ˃18; (B) 8 h,
˃18; (B) 8 h,  <=30,
<=30,  <=35,
<=35,  <=40,
<=40,  <=45,
<=45,  <=50,
<=50,  <=55,
<=55,  ˃55 (C) 24 h,
˃55 (C) 24 h,  <=65,
<=65,  <=70,
<=70,  <=75,
<=75,  <=80,
<=80,  <=85,
<=85,  <=90,
<=90,  <=95,
<=95,  ˃95
˃95