- *Corresponding Author:
- P. M. Mazumder
Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Mesra-835215, Ranchi, India
E-mail: pmitramazumder@bitmesra.ac.in
| Date of Received | 08 June 2021 |
| Date of Revision | 31 October 2022 |
| Date of Acceptance | 08 March 2023 |
| Indian J Pharm Sci 2023;85(2):361-368 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Asthma is a common prolonged inflammatory disease of the upper respiratory tract, affecting millions of people in all age groups. At present, the treatment of asthma has become a challenge for practitioners because of the regular increment in air pollution worldwide. Subsequently, during the new drug development process, preclinical assessment of active compounds through a quick and robust model is urgently required. Thus, this study aimed to establish an acute asthma model using ovalbumin and formaldehyde in mice. In this experimental study, all animals were sensitized with ovalbumin on 0th and 5th d and formaldehyde on the 3rd, 4th, 6th, and 7th d followed by an ovalbumin challenge from 8th to 10th d. During induction of disease, individual groups received beclomethasone and rutin as standard and test drugs, respectively. At the end of the study, the whole lung was harvested, weighed, and assessed for lung weight index. Also, oxidative stress and inflammatory biomarkers were estimated, and lung histopathology was performed. In the results, it was observed that the lung weight index, total leukocyte count and differential leukocyte count, and malondialdehyde values, significantly (p<0.05) decreased, and superoxide dismutase, glutathione and catalase activity were markedly (p<0.05) increased in the rutin treated group as compared to the diseased group. Histopathological studies of lung tissue in the diseased group showed extensive perivascular edema. However, in the rutin-treated group, mucus gland hyperplasia was significantly decreased. Therefore, in this experimental research, an attempt was made to develop a successful acute asthma model within a short period, which reversed on repeated dosing with rutin in murine.
Keywords
Ovalbumin, formaldehyde, beclomethasone, rutin, asthma
Asthma is an allergic inflammatory condition of the lung airway[1], with symptoms comprising reversible obstruction of airflow, bronchospasm, wheezing, excessive mucus production, shortness of breath and chest tightness[2]. Ovalbumin (OVA) is the majorly used allergen to produce an asthmatic response in experimental models[3]. Asthma induced by OVA in mice is usually characterized by excess production of T- helper type II derived cytokines like Interleukin (IL) 4, IL-5, IL-13, nitric oxide, oxidative damage and accumulation of inflammatory cells[4].
Formaldehyde (FA) is an organic compound widely used in manufacturing industries as a preservative and disinfectant where a large amount of inhaled FA is absorbed in the upper respiratory tract. However, it causes sensitization in the airways at low concentrations, induces coughing, mucus formation and breathlessness, and may act as a potent asthmatic agent. Also, it suggested that the prevalent use of FA in industries considered it an allergen responsible for occupational asthma[5].
Glucocorticosteroids are the standard drugs used for asthma treatment despite restricted adequacy in reversingairwayinflammation. Usingbeclomethasone by inhalation causes many side effects like Cataracts, cushing's syndrome, anaphylactic reactions, oral candidiasis and infection in the airway. Long-term use of this drug causes mood change and adrenal insufficiency[6].
Now-a-days, the use of natural products as alternative medicine has increased in treating various diseases[7]. These flavonoids mainly possess antioxidants, scavenging free radicals, cyclo-oxygenase inhibitors, xanthine oxidase inhibitors, lipoxygenase inhibitors and immunoregulatory properties, mainly due to their high polyphenolic content[8,9].
Rutin is a flavonol class of flavonoids having a ketone group. Rutin (3, 3', 4', 5, 7-pentahydroxyflavone-3- rahmnoglucoside) also called quercetin-3-rutinoside, rutoside and sophorin. Rutin is principally found in citrus fruits like grapefruit, lemon, apple, green tea, buckwheat. It possesses powerful anti- inflammatory and antioxidant action[10] and has other pharmacological activities like anti-Alzheimer’s, anti-depressant, anti-diabetic, anti-asthmatic, anti- osteoporotic and anti-eczematous actions[11].
Materials and Methods
Male healthy Swiss albino mice inbred strain, 20 in number, weighing between (22-28 g) were obtained from the animal house with conventional facilities of BIT, Mesra (1968/PO/Re/S/17CPCSEA). Animals were accommodated in propylene cages with straw bale under a 12 h light/dark cycle at 25±5° of temperature with 60 %-70 % relative humidity and standard pellet food (Hindustan Lever Ltd., India), and water were given ad libitum. All experimental animals were sanctioned by Institutional Animal Ethics Committee (IAEC) with approval no 1972/ PH/BIT/66/18/IAEC.
Preparation of OVA:
The OVA used in this study was prepared by considering the previously discussed procedure elsewhere. In brief, 30 ml of the white portion from fresh unfertilized eggs were taken out and mixed with 100 ml and then centrifuged for 10 min at 2000 rpm. The clear supernatant portion was poured out in dried test tubes up to a specific level. These test tubes were kept in the refrigerator to freeze the content at -80° for 48 h. Freeze drying was carried out by using Heto PowerDry LL3000 Freeze Dryer. The temperature of the freeze dryer was set at least below -50°, and the test tubes content was attached to the sample connecting tubes. From 4 ml of the content, approximately 460 mg of lyophilized powdered OVA was obtained after 7 h of freeze drying[12].
Experimental design:
All healthy male Swiss albino inbred strain mice were randomly divided into four treatment groups with five animals each. In group 1 (Normal control) mice were sensitized with aluminum hydroxide [Al(OH)3] after being suspended in saline via intraperitoneal (i.p.) route on 0 d and 5th d and challenged with inhaled saline on 8th to 10th d for 30 min. Group 2 (Disease control) was designated as OVA+FA treated mice where they were sensitized by 10 mg OVA with 1 mg Al(OH)3 in 250 µl of saline (i.p) on 0 d and 5 d, followed by inhaled exposure of 1 % v/v FA on 3rd, 4th, 6th and 7th d for 30 min. After completion of sensitization, they were challenged with inhalational exposure of 1 % w/v OVA in saline solution on the 8th to 10th d for 30 min[13,14]. In Group 3 (Standard treated), beclomethasone (150 µg/kg) was given to the animals as standard drug treatment. It was given in the inhaled form with the flow rate of 0.25 ml/20 s daily from 0 d to 10th d, followed by the OVA+FA sensitization and challenge phase[15]. An automatic compressor nebulizer (OMRON) of 8 l/min flow rate was used for nebulization. In Group 4 (Test treated), rutin was given as a test drug. 100 mg/kg dose of rutin was provided to be administered. It was given orally daily from 0 till 10 d afterward; animals were sensitized and challenged OVA+FA[16]. Estimation of lung weight index, Bronchoalveolar lavage fluid (BALF) hematological parameter and lung oxidative stress biomarkers were done after the asthmatic animal model development.
Lung weight index determination:
The lung was excised from all the animal groups and washed with 1.15 % w/v ice-cold potassium chloride (pH 7.45). The dissected lungs were weighed and lung index was calculated[17].
Enumeration of Total Lymphocyte Count (TLC) and Differential Lymphocyte Count (DLC) in BALF:
Animals were overnight fasted after the challenge and anaesthetized with phenobarbital sodium and weighed. For BALF, 500 µl of saline was inserted through the trachea and recollected. The procedure was replicated, and up to 1000 µl of saline was collected. Then collected saline was mixed with trypan blue. The quantification of TLC and DLC found in BALF was done using a hemocytometer[18].
Lung oxidative stress biomarker determination:
Superoxide dismutase (SOD) was determined by the method of Marklund et al.[19]. Lung homogenate was centrifuged at 3000 rpm for 5 min. After the centrifugation, 500 µl of supernatant from lung homogenate was added to the 150 µl of ice-cold chloroform and 750 µl of (96 % v/v) ethanol and vortexed for 1 min. That vortexed mixture was further centrifuged at 2000 rpm for 22 min. After centrifugation, 500 µl of supernatant was pipetted out, added to the 500 µl of (0.6 mM) ethylene diamine tetra acetate (EDTA) and 1 ml of (0.1 M, pH 10.2) carbonate bicarbonate buffer. The reaction began after the adding up of 50 µl of (0.2 mM) pyrogallol. Absorbance was observed at 420 nm against blank and SOD activity was presented in unit/g.
Glutathione (GSH) activity was determined using the method by Ellman et al.[20]. 0.1-0.2 ml of supernatant was pipetted out from lung homogenate and added to the 0.1-0.2 ml of 5 % trichloroacetic acid (TCA) in a 1:1 manner. Then 2 ml (0.6 mM) DTNB (5, 5’-dithiobis-(2-nitrobenzoic acid) and 0.2 M (pH 8.0) sodium phosphate buffer was added into that and volume was made up to 3 ml. Then the absorbance was measured at 412 nm against blank.
Estimation of Malondialdehyde (MDA) was done on the method by Ohkawa et al.[21]. 0.1 ml of lung homogenate was added to the 0.2 ml of 8.1 % sodium dodecyl sulphate (SDS), 1.5 ml of 20 % acetic acid (pH 3.5), and an aqueous solution of 1.5 ml of 0.8 % thiobarbituric acid (TBA). After that mixture was heated at 95° for 60 min. After heating, the mixture was allowed to cool for 4 min, and then the mixture of (15:1) n-butane:pyridine was added into it. Absorbance was measured at 532 nm.
Catalase (CAT) activity was determined by the method by Aebi et al.[22]. To 10 ml of lung homogenate, 10 ml of phosphate buffer, 40 µl of 30 % v/v hydrogen peroxide, and 1 % v/v triton X- 100 was added and centrifuge at 4° at 13 000 rpm for 20 min. Absorbance was measured at 240 nm.
Lung histopathological examination:
The right lobe of the lung was excised and kept into 10 % v/v formalin for fixing. Then the lung was cut into small slices, dehydrated by ethanol (70 %-100 %), cleared by xylol, and surrounded by paraffin wax. The slices were cut into a 5 µm section for hematoxylin and eosin (H and E) staining. The H and E staining was done to assess thickness of epithelial wall, perivascular edema, bronchial mucous gland hyperplasia, acute perivascular-peribronchial inflammation, and bronchiolar epithelium histopathological changes[23,24].
Statistical analysis:
Results were illustrated as mean±SD. The statistical analysis for comparison between the different treatment groups was done by one-way Analysis of Variance (ANOVA) followed by Tukey Kramer comparison post hoc test along with the help of Graph pad prism (v 9) (serial number: ACTGP-A5B7841C- 6D0E2497-A8306AD4-7C038CFA). (p<0.05) was used as statistical significant value.
Results and Discussion
This study investigated the acute asthmatic effects with short-duration exposure of OVA+FA in mice. OVA-induced model is used for induction of asthma because it imitates human asthmatic attack[25]. This model gives the basis for functional and structural features of asthma[2,26]. FA has been utilized as an air pollutant, indoor-outdoor allergen, and occupational sensitizer to investigate the role of such ubiquitous allergen on the expansion of asthma. The concentration for exposure of FA used in the present study was based on previously examined values, which do not show mortality in experimental animals[27]. Besides, beclomethasone is an approved treatment against asthmatic conditions and is here used as a standard drug to compare with the test, i.e., rutin. In a previous study, it was proven that beclomethasone showed a protective effect against OVA induced model[15]. It was also clinically proven that beclomethasone has protective action in case of asthma[28,29]. In general, steroidal medicines presented good efficacy in acute cases of inflammation, but they are not recommended for chronic conditions as they produce a serious adverse effect on prolonged use[30]. Some of the serious adverse effects typically shown by inhalation of beclomethasone are adrenal insufficiency, Cushing's syndrome, cataract, and throat infection[6]. Rutin is a bioflavonoid that reflects potent anti-inflammatory effects in earlier preclinical experiments. Similarly, in this study, rutin was found profoundly decreased vascular edema, inhibited leukocytes infiltration, also decreased oxidative stress as well as assisted in maintaining alveolar architecture.
As the outcomes of the current study, induction with OVA+FA showed remarkable structural and pathophysiological changes in the lung tissues within a short period of time. Biochemical data, including TLC and DLC counts, lung weight index, and MDA values were significantly increased. In contrast, the GSH level, SOD, and CAT activities significantly decreased, reflecting the development of the inflammatory condition and disturbance in oxidant/antioxidant homeostasis within the lungs.
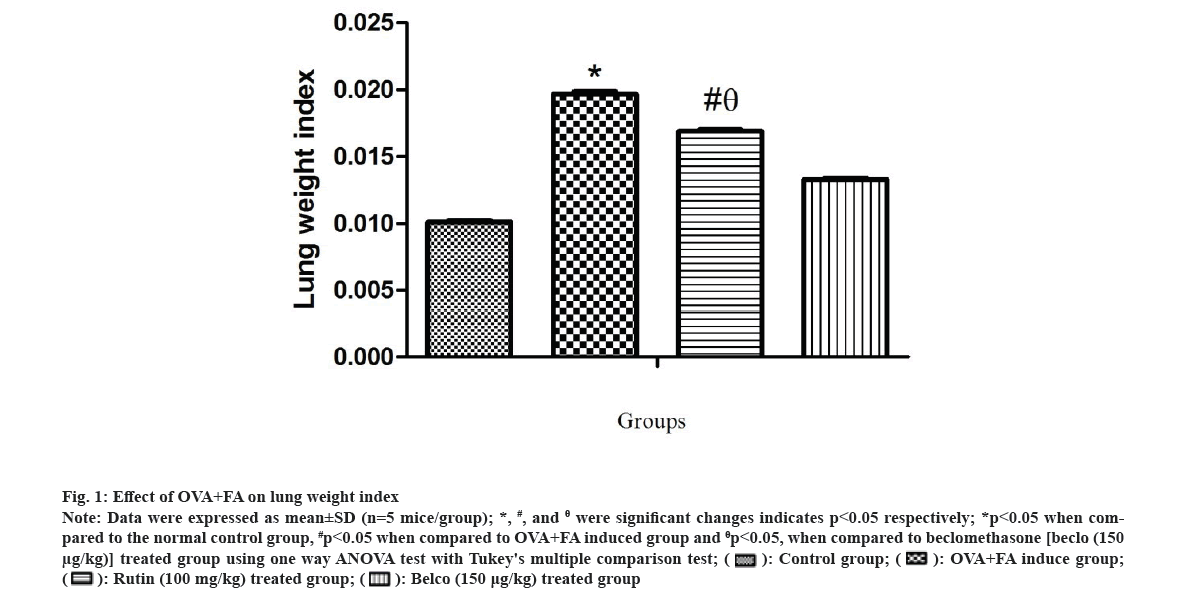
The lung weight index was estimated for all the treatment groups, and the data have been shown in fig. 1. A significant *(p<0.05) increase in lung weight index was observed in the OVA+FA induced group compared to the normal control. However, the rutin-treated group showed a significant #(p<0.05) decrease in lung weight index as compared to the OVA+FA induced group and showed a significantly θ(p<0.05) high value of lung weight index when compared to the beclomethasone-treated group. Previously, it was proven that in acute and sub-acute asthma models, airway inflammation and edema were both found to be elevated[31,32]. Also, the lung weight index reflected vascular permeability and edema, which is considered an important marker during lung inflammation. Moreover, OVA-treated asthmatic animals showed increased lung edema due to increased microvascular leakage[33,34].
Fig 1: Effect of OVA+FA on lung weight index
Note: Data were expressed as mean±SD (n=5 mice/group); *, #, and θ were significant changes indicates p<0.05 respectively; *p<0.05 when compared to the normal control group, #p<0.05 when compared to OVA+FA induced group and θp<0.05, when compared to beclomethasone [beclo (150 µg/kg)] treated group using one way ANOVA test with Tukey's multiple comparison test;
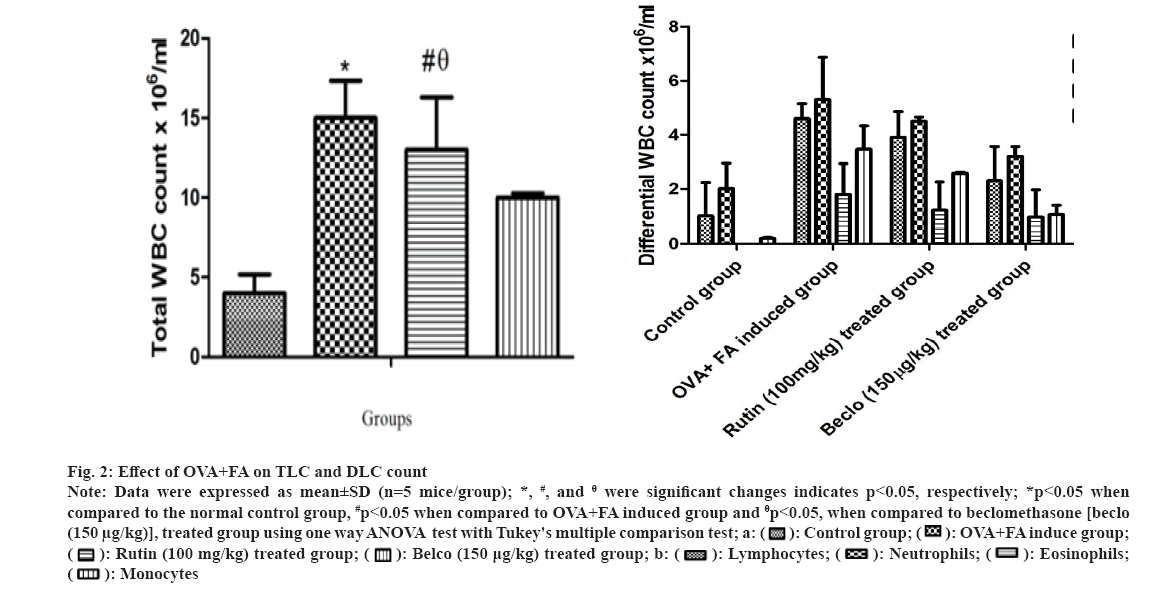
The TLC and DLC counts were estimated for all the treatment groups, and the results are presented in fig. 2. A significant *(p<0.05) increase in TLC and DLC count was observed in the OVA+FA induced group compared to normal control. However, the rutin-treated group showed a significant #(p<0.05) decrease in TLC and DLC count when compared to OVA+FA induced group and a considerable θ(p<0.05) increase in TLC and DLC counts when compared to the beclomethasone-treated group. Similarly, it is evident that T-Helper 2 (Th2) cells stimulate the accumulation of eosinophils by antigen presentation in BALF, which is considered an important phenomenon during asthmatic conditions[35]. Additionally, a previous study also reflected that OVA-treated asthmatic animals showed a raised value of TLC and DLC counts[36].
Fig 2: Effect of OVA+FA on TLC and DLC count
Note: Data were expressed as mean±SD (n=5 mice/group); *, #, and θ were significant changes indicates p<0.05, respectively; *p<0.05 when compared to the normal control group, #p<0.05 when compared to OVA+FA induced group and θp<0.05, when compared to beclomethasone [beclo (150 µg/kg)], treated group using one way ANOVA test with Tukey's multiple comparison test; a: 
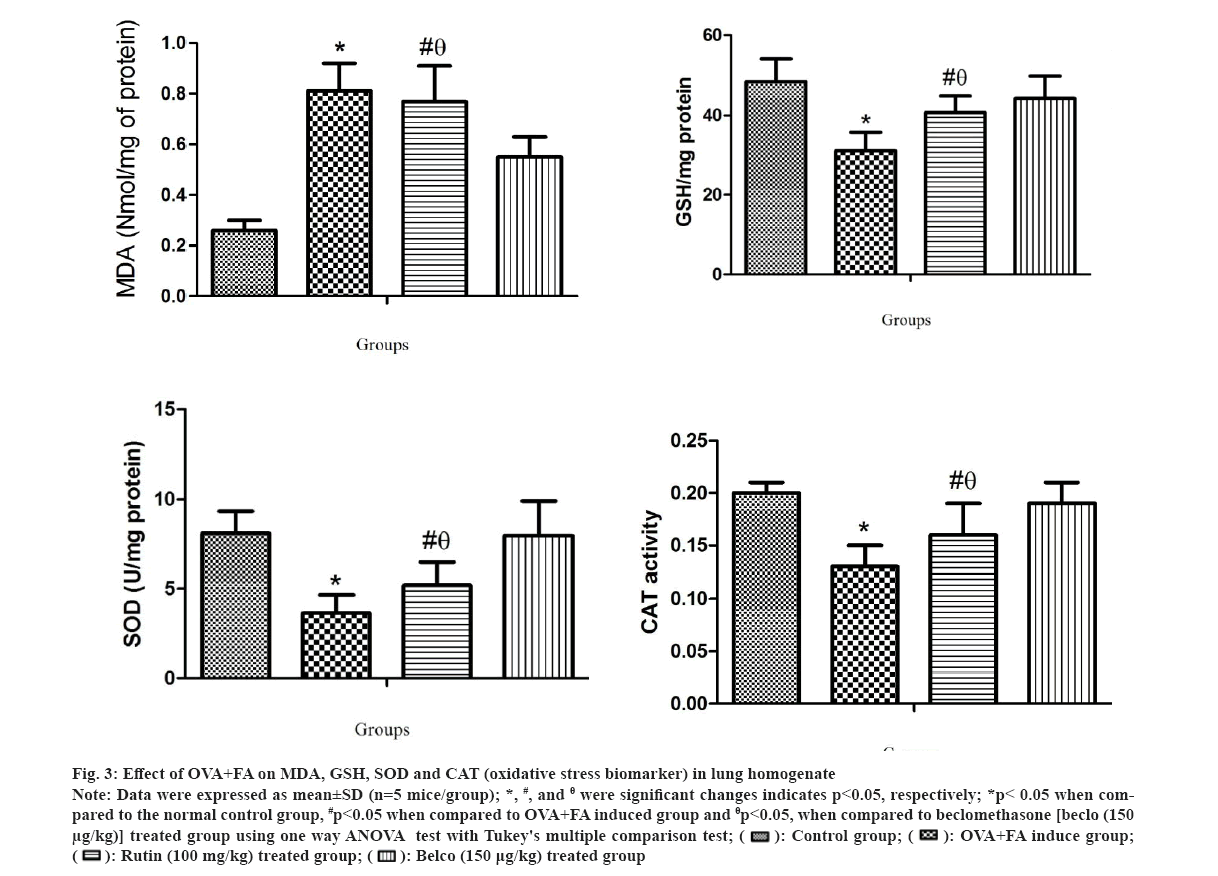
The oxidative stress biomarkers in lung homogenate were also estimated for all the treatment groups and the results are shown in fig. 3. In this study, results showed a significant *(p<0.05) increase in MDA level (fig. 3a) and a significant *(p<0.05) decrease in GSH, SOD and CAT levels (fig. 3b-fig. 3d) in OVA+FA induced group when compared to normal control. However, the rutin-treated groups showed a significant #(p<0.05) decrease in MDA level and a significant #(p<0.05) increase in GSH, SOD and CAT level when compared to OVA+FA induced group also considerable θ(p<0.05) decrease in MDA level and a markedly θ(p<0.05) decrease in GSH, SOD and CAT level when compared to the beclomethasone- treated group. Similarly, in earlier studies, it was demonstrated that Reactive Oxygen Species (ROS) generation in respiratory organs are closely related to asthma[37]. Where enhanced damage to genetic material and protein, lipid peroxidation due to ROS activities were observed. Also, oxidative damage targets membrane lipids because they cause signal transduction for various inflammatory responses[38].
Fig 3: Effect of OVA+FA on MDA, GSH, SOD and CAT (oxidative stress biomarker) in lung homogenate
Note: Data were expressed as mean±SD (n=5 mice/group); *, #, and θ were significant changes indicates p<0.05, respectively; *p< 0.05 when compared to the normal control group, #p<0.05 when compared to OVA+FA induced group and θp<0.05, when compared to beclomethasone [beclo (150 µg/kg)] treated group using one way ANOVA test with Tukey's multiple comparison test;
MDA is lipid peroxide and causes multiple damages by activating reactive oxygen species[39]. It also suggested that asthmatic conditions showed elevated MDA levels in BALF samples collected from different individual patients[40]. GSH protects against oxidative stress because it is an intracellular redox agent which detoxifies lipid hydroperoxide by linking with other antioxidants[41]. Superoxide Dismutase (SOD) produces hydrogen peroxide from the superoxide anion[42]. CAT is an important antioxidant enzyme that decomposes hydrogen peroxide into water and oxygen to protect from oxidative damage caused by ROS[43]. Also, a previous experimental study shows that GSH, SOD and CAT levels decreased significantly in OVA induced group in contrast to normal control[44]. The histopathological differences in lung architecture were estimated for the different treatment groups and the results are shown in fig. 4.
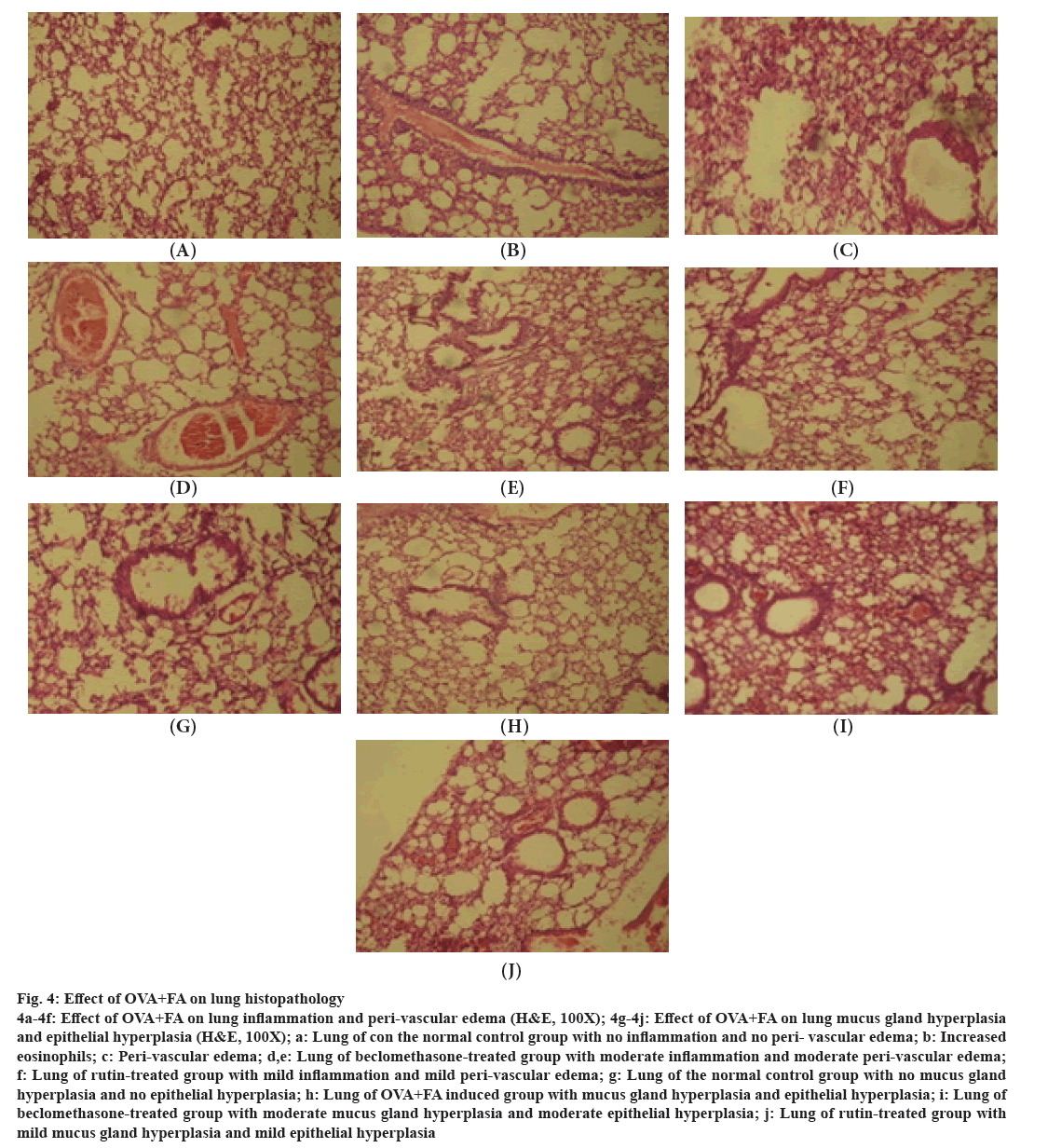
Fig 4: Effect of OVA+FA on lung histopathology
4a-4f: Effect of OVA+FA on lung inflammation and peri-vascular edema (H&E, 100X); 4g-4j: Effect of OVA+FA on lung mucus gland hyperplasia and epithelial hyperplasia (H&E, 100X); a: Lung of con the normal control group with no inflammation and no peri- vascular edema; b: Increased eosinophils; c: Peri-vascular edema; d,e: Lung of beclomethasone-treated group with moderate inflammation and moderate peri-vascular edema; f: Lung of rutin-treated group with mild inflammation and mild peri-vascular edema; g: Lung of the normal control group with no mucus gland hyperplasia and no epithelial hyperplasia; h: Lung of OVA+FA induced group with mucus gland hyperplasia and epithelial hyperplasia; i: Lung of beclomethasone-treated group with moderate mucus gland hyperplasia and moderate epithelial hyperplasia; j: Lung of rutin-treated group with mild mucus gland hyperplasia and mild epithelial hyperplasia
The normal control group showed the ideal condition of lung architecture. There was no indication of mucus gland hyperplasia, no epithelial thickness, and no inflammation. In the diseased induced group (OVA+FA), numerous eosinophils, bronchial and peribronchial inflammation, epithelial and mucus gland hyperplasia, and perivascular edema were identified. Within OVA-treated asthma-induced animals, similar results were also reported in the previous study[45]. However, the beclomethasone- treated group showed a decrease in epithelial and mucus gland hyperplasia, mild perivascular edema, and peribronchial inflammation. But, the rutin-treated group showed a moderate decrease in epithelial and mucus gland hyperplasia and a marked reduction in perivascular edema and peribronchial inflammation in the lungs.
This study proposed an OVA+FA induced asthmatic model in mice, which could be developed within a short duration (10 d), and provides remarkable biochemical and structural changes in asthmatic lungs. In this acute model, physiological and biochemical alterations in all measured parameters were comparable to the previously reported other OVsA-induced asthma models in rodents. Also, treatment with standard (beclomethasone) and test (rutin) drugs presented marked reversal in oxidative, inflammatory, and histopathological changes in the lungs without significant difference compared to normal control. This reflects their potential against asthma. Thus, rutin may also be considered as a potent therapeutic agent for the treatment of asthma.
Acknowledgements:
All authors are greatly thankful to the Department of Pharmaceutical Sciences and Technology, BIT Mesra, for providing laboratory assistance for research. The first author is truly obliged to IRF (Institute Research Fellow) for grant financial aid.
Conflict of interest:
The authors declared no conflict of interests.
References
- Manuyakorn W, Howarth PH, Holgate ST. Airway remodelling in asthma and novel therapy. Asian Pac J Allergy Immunol 2013;31(1):3.
[Google Scholar] [PubMed]
- Martinez FD. Genes, environments, development and asthma: A reappraisal. Eur Respir J 2007;29(1):179-84.
[Crossref] [Google Scholar] [PubMed]
- Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: A meta-analysis. J Allergy Clin Immunol 2007;120(3):638-46.
[Crossref] [Google Scholar] [PubMed]
- Mine Y, Yang M. Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives. J Agri Food Chem 2008;56(13):4874-900.
[Crossref] [Google Scholar] [PubMed]
- Baccioglu A, Kalpaklioglu AF. An unusual form of formaldehyde induced lung disease. Allergol Immunopathol 2007;35(3):110-2.
[Crossref] [Google Scholar] [PubMed]
- Salzman GA, Pyszczynski DR. Oropharyngeal candidiasis in patients treated with beclomethasone dipropionate delivered by metered-dose inhaler alone and with Aerochamber. J Allergy Clin Immunol 1988;81(2):424-8.
[Crossref] [Google Scholar] [PubMed]
- Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, et al. Efficacy and tolerability of antiasthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol 2005;116(3):517-24.
[Crossref] [Google Scholar] [PubMed]
- Rogers J, Perkins I, Olphen AV, Burdash N, Klein TW, Friedman H. Epigallocatechin gallate modulates cytokine production by bone marrow-derived dendritic cells stimulated with lipopolysaccharide or muramyldipeptide, or infected with Legionella pneumophila. Exp Biol Med 2005;230(9):645-51.
[Crossref] [Google Scholar] [PubMed]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutri Sci 2016;5:e47.
[Crossref] [Google Scholar] [PubMed]
- Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001;56(9):683-7.
[Crossref] [Google Scholar] [PubMed]
- Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J 2017;25(2):149-64.
[Crossref] [Google Scholar] [PubMed]
- Kekwick RA, Cannan RK. The hydrogen ion dissociation curve of the crystalline albumin of the hen's egg. Biochem J 1936;30(2):227-34.
[Crossref] [Google Scholar] [PubMed]
- Abdеlaziz RR, kh Еlmahdy M, Suddеk GM. Flavocoxid attenuates airway inflammation in ovalbumin-induced mouse asthma model. Chem Biol Interact 2018;292:15-23.
[Crossref] [Google Scholar] [PubMed]
- Hsu DZ, Liu CT, Chu PY, Li YH, Periasamy S, Liu MY. Sesame oil attenuates ovalbumin-induced pulmonary edema and bronchial neutrophilic inflammation in mice. BioMed Res Int 2013;2013.
[Crossref] [Google Scholar] [PubMed]
- Hrvačić B, Bošnjak B, Tudja M, Mesić M, Merćep M. Applicability of an ultrasonic nebulization system for the airways delivery of beclomethasone dipropionate in a murine model of asthma. Pharm Res 2006;23:1765-75.
[Crossref] [Google Scholar] [PubMed]
- Lv HY, Chen J, Wang T. Rutin has anti-asthmatic effects in an ovalbumin-induced asthmatic mouse model. Tropical J Pharm Res 2017;16(6):1337-47.
- Pearce ML, Yamashita JO, Beazell J. Measurement of pulmonary edema. Circ Res 1965;16(5):482-8.
- Dhami R, He X, Gordon RE, Schuchman EH. Analysis of the lung pathology and alveolar macrophage function in the acid sphingomyelinase–deficient mouse model of Niemann-Pick disease. Lab Invest 2001;81(7):987-99.
[Crossref] [Google Scholar] [PubMed]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974;47(3):469-74.
[Crossref] [Google Scholar] [PubMed]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70-7.
[Crossref] [Google Scholar] [PubMed]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
[Crossref] [Google Scholar] [PubMed]
- York S. Aebi H.(1974). Catalase. In: Methods of enzymatic analysis, Bergmeyer HU (editor), Academic press, New York, 1974; pp. 673-84.
- Zeldin DC, Wohlford-Lenane C, Chulada P, Alyce Bradbury J, Scarborough PE, Roggli V, et al. Airway inflammation and responsiveness in prostaglandin H synthase–deficient mice exposed to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol 2001;25(4):457-65.
[Crossref] [Google Scholar] [PubMed]
- Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol 2013;50(6):1007-15.
[Crossref] [Google Scholar] [PubMed]
- Ma Y, Ge A, Zhu W, Liu YN, Ji NF, Zha WJ, et al. Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxid Med Cell Longev 2016;2016:5843672.
[Crossref] [Google Scholar] [PubMed]
- Busse WW, Calhoun WF, Sedgwick JD. Mechanisms of airway inflammation in asthma. Am Rev Respir Dis 1993;147:S20-4.
[Crossref] [Google Scholar] [PubMed]
- Lino-dos-Santos-Franco A, Domingos HV, de Oliveira AP, Breithaupt-Faloppa AC, Peron JP, Bolonheis S, et al. Differential effects of formaldehyde exposure on the cell influx and vascular permeability in a rat model of allergic lung inflammation. Toxicol Lett 2010;197(3):211-8.
[Crossref] [Google Scholar] [PubMed]
- Wilcox JB, Avery GS. Beclomethasone dipropionate corticosteroid inhaler: A preliminary report of its pharmacological properties and therapeutic efficacy in asthma. Drugs 1973;6(2):84-93.
[Crossref] [Google Scholar] [PubMed]
- Duddridge M, Ward C, Hendrick DJ, Walters EH. Changes in bronchoalveolar lavage inflammatory cells in asthmatic patients treated with high dose inhaled beclomethasone dipropionate. Eur Respir J 1993;6(4):489-97.
[Crossref] [Google Scholar] [PubMed]
- Bitto A, Squadrito F, Irrera N, Pizzino G, Pallio G, Mecchio A, et al. Flavocoxid, a nutraceutical approach to blunt inflammatory conditions. Mediators Inflamm 2014;2014:790851.
[Crossref] [Google Scholar] [PubMed]
- Abdel Aziz RR, Helaly NY, Zalata KR, Gameil NM. Influence of inhaled beclomethasone and montelukast on airway remodeling in mice. Inflammopharmacology 2013;21:55-66.
[Crossref] [Google Scholar] [PubMed]
- Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax 1998;53(10):849-56.
[Crossref] [Google Scholar] [PubMed]
- Tillie-Leblond I, Gosset P, Le Berre R, Janin A, Prangère T, Tonnel AB, et al. Keratinocyte growth factor improves alterations of lung permeability and bronchial epithelium in allergic rats. Eur Respir J 2007;30(1):31-9.
[Crossref] [Google Scholar] [PubMed]
- Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: Role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol 1997;17(4):519-28.
[Crossref] [Google Scholar] [PubMed]
- Bousquet J, Chanez P, Lacoste JY, Barnéon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990;323(15):1033-9.
[Crossref] [Google Scholar] [PubMed]
- Wei Y, Abduwaki M, Li M, Luo Q, Sun J, Lv Y, et al. Loki zupa (Luooukezupa) decoction reduced airway inflammation in an OVA-induced asthma mouse model. Chin Med 2016;11(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Kongerud J, Crissman K, Hatch G, Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhal Toxicol 2003;15(2):101-10.
[Crossref] [Google Scholar] [PubMed]
- Louhelainen N, Myllärniemi M, Rahman I, Kinnula VL. Airway biomarkers of the oxidant burden in asthma and chronic obstructive pulmonary disease: Current and future perspectives. Int J Chron Obstruct Pulmon Dis 2008;3(4):585-603.
[Crossref] [Google Scholar] [PubMed]
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438.
[Crossref] [Google Scholar] [PubMed]
- Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, Poppa M, et al. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur Respir J 2004;24(6):1011-7.
[Crossref] [Google Scholar] [PubMed]
- Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J Gen Med 2011;4:105-13.
[Crossref] [Google Scholar] [PubMed]
- Larkin EK, Gao YT, Gebretsadik T, Hartman TJ, Wu P, Wen W, et al. New risk factors for adult-onset incident asthma. A nested case–control study of host antioxidant defense. Am J Respir Critic Care Med 2015;191(1):45-53.
[Crossref] [Google Scholar] [PubMed]
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci 2004;61:192-208.
[Crossref] [Google Scholar] [PubMed]
- El-Naa MM, El-Refaei MF, Nasif WA, Abduljawad SH, El-Brairy AI, El-Readi MZ. In vivo antioxidant and anti-inflammatory activity of rosiglitazone, a peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists in animal model of bronchial asthma. J Pharm Pharmacol 2015;67(10):1421-30.
[Crossref] [Google Scholar] [PubMed]
- Yimam M, Zhao Y, Ma W, Jia Q, Do SG, Shin JH. 90-day oral toxicity study of UP446, a combination of defined extracts of Scutellaria baicalensis and Acacia catechu, in rats. Food Chem Toxicol 2010;48(5):1202-9.
[Crossref] [Google Scholar] [PubMed]