- *Corresponding Author:
- N. J. Shah
Shri B. M. Shah College of Pharmaceutical Education & Research, Modasa - 383 315, India.
E-mail: nehal9175@yahoo.co.in
| Date of Submission | 13 February 2006 |
| Date of Decision | 3 October 2006 |
| Date of Acceptance | 24 April 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 331-333 |
Abstract
A simple, precise, accurate and rapid high performance thin layer chromatographic method has been developed and validated for the determination of cefpodoxime proxetil in dosage form. The stationary phase used was precoated silica gel 60F254. The mobile phase used was a mixture of chloroform: methanol: toluene (4:2:4 v/v/v). The detection of spot was carried out at 289.0 nm. The method was validated in terms of linearity, accuracy, precision and specificity. The calibration curve was found to be linear between 100 to 700 ng/spot for cefpodoxime proxetil. The limit of detection and the limit of quantification for the cefpodoxime proxetil were found to be 30 ng/spot and 90 ng/spot, respectively. The proposed method can be successfully used to determine the drug content in marketed formulation.
Cefpodoxime is a third-generation cephalosporin. Cefpodoxime proxetil is chemically (RS)1(isopropoxycarbonyloxy)ethyl(+)-(6R,7R)-7-[2-(2- amino-4-thiazoly)-2-{(Z)methoxyimino} acetamido]-3- methoxymethyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- carboxylate [1]. Literature survey revealed that only a few HPLC methods were reported for the estimation of cefpodoxime proxetil in the biological fluids [2-6]. So far no HPTLC method has been reported for the estimation of cefpodoxime proxetil in formulation. So authors have tried to develop accurate, precise and specific HPTLC method for the estimation of cefpodoxime proxetil.
Cefpodoxime proxetil working standard was procured as a gift sample from Necter Life Sciences, Chandigarh. Silica gel 60F254 TLC plates (10 × 10 cm, layer thickness 0.2 mm, E. Merck, Mumbai) were used as a stationary phase. All chemicals and reagents used were of analytical grade. Chloroform:methanol:toluene (4:2:4 v/v/v) was used as mobile phase. Methanol was used as solvent. Tablets containing cefpodoxime proxetil (equivalent to 200 mg cefpodoxime base) were purchased from local pharmacy (Cepodem-200, Ranbaxy Pharmaceuticals, New Delhi and Cefoprox-200, Cipla Pharmaceuticals, Ltd., Mumbai). A Camag HPTLC system comprising of Camag Linnomate V automatic sample applicator, Hamilton syringe (100 μl), Camag TLC Scanner 3, Camag Win CATS software, Camag Twin-trough chamber (10 × 10 cm) and ultrasonicator were used during study.
Cefpodoxime proxetil (10 mg) was weighed accurately and transferred to a 10 ml volumetric flask. It was dissolved in and diluted up to mark with methanol. The solution (1 ml) was diluted further to 10 ml with methanol. The final solution contained 100 μg of cefpodoxime proxetil per ml of the solution. The content of twenty tablets were grounded to fine powder. The tablet powder equivalent to 25 mg of cefpodoxime proxetil was transferred to volumetric flask and dissolved in methanol. The solution was sonicated for 15 min. The extracts were filtered through Whatmann filter paper No. 41 and residue was washed with methanol. The extracts and washing were pooled and transferred to a 250 ml volumetric flask and volume was made up to 250 ml with methanol to get 100 μg/ml of cefpodoxime proxetil. TLC plates were prewashed with methanol. Activation of plates were done in an oven at 500 for 30 min. The chromatographic conditions maintained were precoated silica gel 60F254 aluminum sheets (10×10 cm) as stationary phase, chloroform: methanol: toluene (4:2:4 v/v/v) as mobile phase, chamber and plate saturation time of 30 min, migration distance allowed was 72 mm, wavelength scanning was done at 289.0 nm keeping the slit dimension at 5×0.45 mm. A deuterium lamp provided the source of radiation. Cefpodoxime proxetil (5 μl standard solution) was spotted and developed at constant temperature. Photometric measurements were performed at 289.0 nm in reflectance mode with Camag TLC scanner 3 using Win CATS software.
Aliquots of 1, 2, 3, 4, 5, 6, and 7 μl of standard solution of cefpodoxime proxetil were applied on the TLC plate (100 μg/ml of drug). TLC plate was dried, developed and analyzed photometrically as described earlier. The standard calibration curve was generated using regression analysis.
The developed method was validated in terms of linearity, accuracy, specificity, limit of detection, limit of quantification, intra-day and inter-day precision and repeatability of measurement as well as repeatability of sample application.
Four microlitres of the filtered solutions of the marketed formulation was spotted on to the same plate followed by development scanning. The analysis was repeated in triplicate. The content of the drug was calculated from the peak areas recorded.
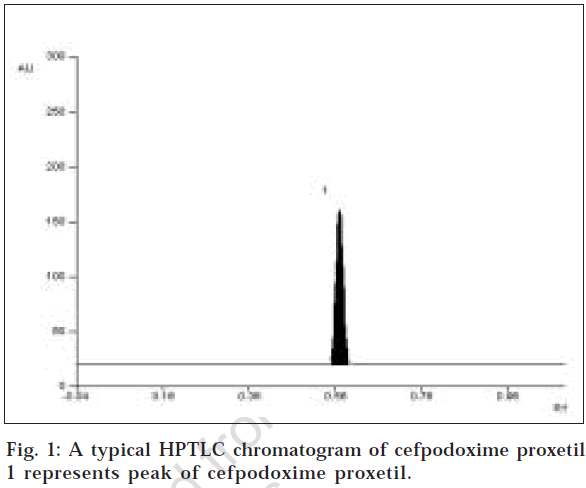
The mobile phase consisting of chloroform: methanol: toluene (4:2:4 v/v/v) gave Rf values of 0.56 (±0.03) cefpodoxime proxetil (fig.1). The linear regression data (n=5) showed a good linear relationship over a concentration range of 100-700 ng/spot for cefpodoxime proxetil. The limit of detection and limit of quantification were found to be 30 ng/spot and 90 ng/spot, respectively.
The intra-day precision was determined by analyzing standard solutions in the concentration range of 200 ng/ spot to 600 ng/spot of each drug for 3 times on the same day while inter-day precision was determined by analyzing corresponding standards daily for 3 day over a period of one week. Repeatability of sample application was assessed by spotting 4 μl of drug solution 3 times on a TLC plate followed by development of plate and recording the peak area for 5 spot. The % RSD for peak area values of cefpodoxime proxetil was found to be 0.45. Repeatability of measurement of peak area was by spotting 4 μl of cefpodoxime proxetil solution on a TLC plate and developing the plate. The separated spot was scanned five times without changing the position of the plate and % RSD for measurement of peak area of cefpodoxime proxetil was 0.74. To confirm the specificity of the proposed method, the solution of the formulation was spotted on the TLC plate, developed and scanned. It was observed that the excipients present in the formulation did not interfere with the peaks of cefpodoxime proxetil.
Recovery studies of the drugs were carried out for the accuracy parameters. These studies were carried out at three levels i.e. multiple level recovery studies. Sample stock solution from tablet formulation of 100 μg/ml of was prepared. To the above prepared solutions, 50%, 100% and 150% of the standard drug solutions were added. Dilutions were made and recovery studies were performed. Percentage recovery was found to be within the limits as listed in Table 1. The assay value for the marketed formulation was found to be within the limits as listed in Table 1. The low RSD value indicated the suitability of the method for routine analysis of cefpodoxime proxetil in pharmaceutical dosage forms. The developed HPTLC technique is simple, precise, specific and accurate and the statistical analysis proved that method is reproducible and selective for the analysis of cefpodoxime proxetil in bulk drug and tablet formulations.
| Label claim mg/tablet | Amount added % | Amount recovered* (mg)±SD | Recovery % ±SD | % Assay* |
|---|---|---|---|---|
| Cefpodoxime | 50 | 97.46±1.09 | 97.46±1.11 | |
| proxetil 200 | 100 | 196.8±1.93 | 98.4±0.98 | 99.26±1.55 |
| (cepodem) | 150 | 278.88±1.58 | 99.6±0.52 | |
| Cefpodoxime | 50 | 42.56±0.41 | 98.04±0.41 | |
| proxetil 200 | 100 | 92.31±0.35 | 100.21±0.33 | 101.38±1.45 |
| (cefoprox) | 150 | 138.64±0.15 | 98.94±0.18 |
*Each value is a mean±standard deviation of three determinations. Cepodem-200 is a brand of Ranbaxy Pharmaceuticals, New Delhi and Cefoprox-200 is a brand of Cipla pharmaceuticals, Ltd., Mumbai.
Table 1: Recovery Studies and Assay of Cefpodoxime Proxetil
Acknowledgements
Author thanks the Necter Life Sciences, Chandigarh for the gift sample of cefpodoxime proxetil.
References
- Lester AM. Antibiotics and antimicrobial agents. In: David AM,Thomas LL. Foye’s Principle of Medicinal Chemistry. 5thed, DaviT.Philadelphia: Lippncott Williams and Wilkins; 2002; 840-852.
- Lee KY, Nurok D, Zlatkis A. Determination of cefpodoxime proxetil by High Performance Liquid Chromatography. J Chromatogr A 1997; 174: 187.
- Kakumanu VK, Arora VK, Bansal AK. Development and validation of isomer specific RP-HPLC method for quantification of cefpodoxime proxetil extraction method coupled with HPLC-UV. J Chromatogr B 2006;835:16.
- Naoto F, Yuichi S, Takao K, Koichi S, Hiroyuki N. LC/MS/MS method for the determination of trace amounts of cefmetazole and cefpodoxime proxetil contaminants in pharmaceutical manufacturing environments. J Pharm Biomed Anal 2006; 41: 1243.
- Demetra K, Helen G, Zoe A, Nickolas V, Stavroula P, Thomas G, Garyphalia P, Agissilaos H, George K Cefpodoxime-Proxetil versus Trimethoprim-Sulfamethoxazole for Short Term Therapy of Uncomplicated Acute Cystitis in Women. Antimicrob Agents Chemother 2003; 47: 897-900.
- Aleksic M, Ilic M, Kapetanovic V. Adsorptive properties of cefpodoxime proxetil as a tool for a new method of its determination in Urine.J Pharm Biomed Anal 2004; 36(4): 899-903.