- *Corresponding Author:
- N. J. Shah
Shri B. M. Shah College of Pharmaceutical Education and Research, Modasa-383 315, India.
E-mail: nehal9175@yahoo.co.in
| Date of Submission | 29 August 2005 |
| Date of Revision | 19 June 2006 |
| Date of Acceptance | 14 February 2007 |
| Indian J. Pharm. Sci., 2007, 69 (1): 140-142 |
Abstract
A simple, precise, accurate and rapid high performance thin layer chromatographic method has been developed and validated for the determination of cefuroxime axetil in dosage form. The stationary phase used was precoated silica gel 60F 254 . The mobile phase used was a mixture of chloroform:methanol:toluene (4:2:2 v/v). The detection of spot was carried out at 290 nm. The method was validated in terms of linearity, accuracy, precision and specificity. The calibration curve was found to be linear between 300 to 800 ng/spot for cefuroxime axetil. The limit of detection and the limit of quantification for the cefuroxime axetil were found to be 50 ng/spot and 100 ng/spot. The proposed method can be successfully used to determine the drug content of marketed formulation.
Keywords
Cefuroxime is a second-generation cephalosporin. Cefuroxime axetil is an ester prodrug of cefuroxime, which is rendered more lipophilic by esterification of carboxyl group of the molecule by the racemic 1-acetoxyethyl bromide, thus enhancing absorption. The absorbed ester is hydrolyzed in the intestinal mucosa and in portal circulation. Products of hydrolysis are active cefuroxime, acetaldehyde and acetic acid. Cefuroxime is chemically (1RS)-1-[(acetyl) oxy] ethyl-(6R, 7R)-3-(carbamoyloxy) methyl]-7-[(Z-2-furan- 2yl)-2-(methoxy imino) acetyl) amino]-8-oxo-5-thia-1-aza bicyclo-(4,2,0)-oct-2-ene-2-carboxylate [1-2]. Literature survey revealed that few HPLC methods were reported for the estimation of cefuroxime axetil in the biological fluids [3]. HPTLC method was also reported for the simultaneous estimation of cefuroxime axetil and probenecid [4]. So far no HPTLC method has been reported for the estimation of cefuroxime axetil in formulation. So authors have tried to develop accurate, precise and specific HPTLC method for the estimation of cefuroxime axetil.
Cefuroxime axetil working standard was procured as a gift sample from Zorex Pharmaceuticals Ltd., Ahmedabad. Silica gel 60F254 TLC plates (10×10 cm, layer thickness 0.2 mm, E. Merck, Mumbai) were used as a stationary phase. All chemicals and reagents used were of analytical grade. Chloroform:methanol:toluene (4:2:2 v/v) was used as mobile phase. Methanol was used as solvent. Tablets containing cefuroxime axetil (equivalent to 250 mg cefuroxime base) were purchased from local market (Altacef-250, Glenmark Pharmaceuticals Ltd. and Forcef 250, Aristo Pharmaceuticals Ltd). A Camag HPTLC system comprising of Camag Linnomate V automatic sample applicator, Hamilton syringe (100 μl), Camag TLC Scanner 3, Camag WinCATS software, Camag Twin-trough chamber (10×10 cm) and ultrasonicator were used during study.
Working standard of cefuroxime axetil (10 mg) was weighed accurately and diluted with methanol to obtain the final concentration of 100 μg/ml of drug. The content of twenty tablets was ground to fine powder. Weight equivalent to 25 mg of cefuroxime axetil was transferred to conical flask and dissolved in methanol. The solution was sonicated for 15 min. The extracts were filtered through Whatmann filter paper No. 41 and residue was washed with methanol. The extracts and washing were pooled and transferred to a 250 ml volumetric flask and volume was made with methanol. Required dilutions were made to get 100 μg/ml of cefuroxime axetil.
TLC plates were prewashed with methanol. Activation of plates was done in an oven at 50° for 5 min. The chromatographic conditions maintained were precoated silica gel 60F254 aluminum sheets (10×10 cm) as stationary phase, chloroform:methanol:toluene (4:2:2 v/v) as mobile phase, chamber and plate saturation time of 30 min, migration distance allowed was 72 mm, wavelength scanning was done at 290 nm keeping the slit dimention at 5×0.45 mm. A deuterium lamp provided the source of radiation. Five microlitres of the standard solution of cefuroxime axetil was spotted and developed at constant temperature. Photometric measurements were performed at 290 nm in reflectance mode with Camag TLC scanner 3 using Win CATS software.
Aliquots of 3, 4, 5, 6, 7 and 8 μl of standard solution of cefuroxime axetil were applied on the TLC plate (100 μg/ml of drug). TLC plate was dried, developed and analyzed photometrically as described earlier. The standard calibration curve was generated using regression analysis with Microsoft Excel.
The developed method was validated [5-10] in terms of linearity, accuracy, specificity, limit of detection, limit of quantification, intra-day and inter-day precision and repeatability of measurement as well as repeatability of sample application. Five microlitres of the filtered solutions of the marketed formulation was spotted on to the same plate followed by development scanning [11,12]. The analysis was repeated in triplicate. The content of the drug was calculated from the peak areas recorded.
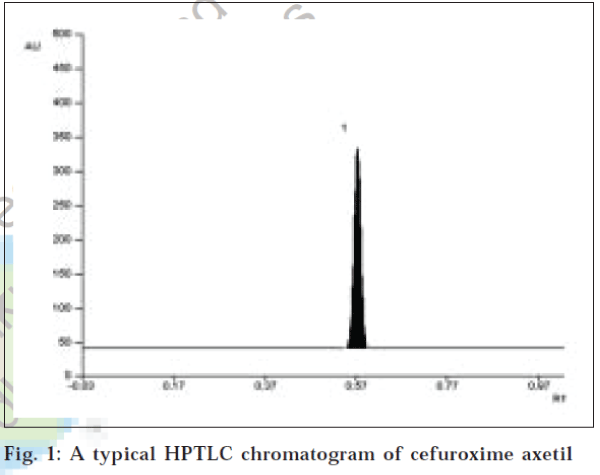
A solvent system that would give dense and compact spot with appropriate a significantly Rf values was desired for quantification of cefuroxime axetil in pharmaceutical formulations. The mobile phase consisting of chloroform: methanol: toluene (4:2:2 v/v) gave Rf values of 0.57 (±0.03) cefuroxime (fig. 1). The linear regression data (n=5, Table 1) showed a good linear relationship over a concentration range of 300-800 ng/spot for cefuroxime axetil. The limit of detection and limit of quantification were found to be 50 ng/spot and 100 ng/spot, respectively.
| Parameters | Result |
|---|---|
| Linearity range (ng/spot) | 300-800 |
| Correlation coefficient (r) | 0.995 |
| Regression equation (y=mx+c) | |
| Slope (m) | 4.46 |
| Intercept (c) | 69.67 |
| Limit of detection (LOD) | 50 ng/spot |
| Limit of quantification (LOQ) | 150 ng/spot |
| Precision (% CV) | |
| Repeatability of application (n=5) | 0.14 |
| Repeatability of measurement (n=5) | 0.59 |
Table 1: Method validation parameters
The intra-day precision was determined by analyzing standard solutions in the concentration range of 400 ng/ spot to 700 ng/spot of each drug for 3 times on the same day while inter-day precision was determined by analyzing corresponding standards daily for 3 day over a period of one week. The intra-day and inter-day coefficients of variation are in range of 0.41 to 1.0 and
0.82 to 1.52, respectively.
Repeatability of sample application was assessed by spotting 5 μl of drug solution 5 times on a TLC plate followed by development of plate and recording the peak area for 5 spot. The % RSD for peak area values of cefuroxime axetil was found to be 0.014.
Repeatability of measurement of peak area was determined by spotting 5 μl of cefuroxime axetil solution on a TLC plate and developing the plate. The separated spot was scanned five times without changing the position of the plate and % RSD for measurement of peak area of cefuroxime axetil was 0.59. To confirm the specificity of the proposed method, the solution of the formulation was spotted on the TLC plate, developed and scanned. It was observed that the excipients present in the formulation did not interfere with the peaks of cefuroxime axetil.
Recovery studies were carried out to assess accuracy parameters. These studies were carried out at three levels. Sample stock solution from tablet formulation of 100 μg/ml was prepared. Dilutions were made and recovery studies were performed. % recovery was found to be within the limits as listed in Table 2. The assay value for the marketed formulation was found to be within the limits as listed in Table 2. The low RSD value indicated the suitability of the method for routine analysis of cefuroxime axetil in pharmaceutical dosage forms.
| Label claim (mg / tablet) | Amount (added%) | Total amount added (mg) | Amount recovered* (mg) ± SD | % Recovery± SD | % Assay± SD |
|---|---|---|---|---|---|
| Cefuroxime | 33.33 | 83.32 | 81.95±3.75 | 98.45±3.7 | 100.51 |
| Axetil 250 | 66.66 | 166.65 | 167.01±0.15 | 100.22±0.16 | |
| (Altacef) | 100 | 250 | 248.67±0.07 | 99.47±0.07 | |
| Cefuroxime | 33.33 | 83.32 | 84.89±0.93 | 101.89±0.95 | 102.94 |
| axetil 250 | 66.66 | 166.65 | 166.58±2.28 | 99.96±2.28 | |
| (Forcef) | 100 | 250 | 251.77±1.34 | 100.71±1.35 |
*Each value is a mean±standard deviation of three determinations. Altacef is a brand of Glenmark Pharmaceuticals Ltd and Forcef is a brand of Aristo Pharmaceuticals Ltd.
Table 2: Recovery studies of cefuroxime axetil*
The developed HPTLC technique is simple, precise, specific and accurate and the statistical analysis proved that method is reproducible and selective for the analysis of cefuroxime axetil in bulk drug and tablet formulations.
Acknowledgements
Author thanks the Zorex Pharmaceuticals Ltd., Ahmedabad for the gift sample of cefuroxime axetil. Authors are thankful to Dr. J. O. Shah, Kirti Coporation and Bhakti medical, Modasa for providing financial assistance.
References
- United States Pharmacopoeia (XXIV) and National Formulary (XIX), Asian Edn., US Pharmacopoeial Convention, Inc., Rockville,MD, 2000, 335
- Insel, P.A., In; Hardman, J.G., Limbard, L.E., Molinoff, P.B., Ruddon, W.R. and A.G., Eds., Goodman and Gilman’s The PharmacologicalBasis of Therapeutics, 9th Edn, McGraw-Hill, New York, 1996, 617.
- Rosseel, M.T., Peleman, R.S., Van, H.H. and Pouwels, R.A., J. Chromatogr. Biomed. Sci. Appl., 1997, 689, 438.
- Sireesha, K.R., Mhaske, D.V., Kadam, S.S. and Dhaneshwar, S.R., Indian J. Pharm. Sci., 2004, 66, 278.
- El-Gindy, A., El-Waily, A.F.M. and Bedari, M.F., J. Pharm. Biomed. Anal., 2000, 23, 341.
- United States Pharmacopoeia (XXIV) and National Formulary (XIX), Asian Edn., US Pharmacopoeia Convention, Inc., Rockville.MD, 2000, 1393.
- Sethi, P.D., Eds, In; HPTLC Quantitative Analysis of PharmaceuticalFormulations 1st Edn., CBS Publishers and Distributors, New Delhi,1996,download3.
- Validation of Analytical Procedures, Methodology, ICH harmonizedtripartitePublicationsguidelines, 1996, 1.
- Thoppil, S.O., Cardoza, R.M. and Amin, P.D., J. Pharm. Biomed. Anal., 2001, 25, 15.
- Eric-Jovanovic, S., Agbada, D., Zivanor-Stakic, D. and Vladimirov, S.,J. Pharm. Biomed. Anal.,1998, 18, 893.
- Pillai, A.D., Ravishankar, M.N. and Handral, R.D., Indian J. Pharm. Sci., 2001, 63, 362.
- Patel, C.N., Shah, S.A., Rathod, I.S. and Save, S.S., Indian J.Pharm. Sci., 2002, 64, 362.