- *Corresponding Author:
- U. K. Chhalotiya
Indukaka Ipcowala College of Pharmacy, P.O. Box No.53, P.O.Vithal Udhyog Nagar, New Vallabh, Vidyanagar - 388 121
E-mail: usmangani84@gmail.com
| Date of Submission | 8 February 2010 |
| Date of Revision | 28 August 2010 |
| Date of Acceptance | 20 November 2010 |
| Indian J. Pharm. Sci. 2010, 72 (6): 814-818 |
Abstract
A simple, specific, accurate, and precise ultra performance liquid chromatographic method was developed and validated for the estimation of venlafaxine hydrochloride in tablet dosage forms. A acquity TM BEH column having C18, 100Χ2.1 mm i.d. in isocratic mode, with mobile phase containing dipotassium hydrogen phosphate: Acetonitrile (30:70 v/v; pH 7.00 with dilute o-phosphoric acid) was used. The flow rate was 0.75 ml/min and effluents were monitored at 227 nm. The retention time of venlafaxine hydrochloride was 0.548 min. The method was validated for specificity, linearity, accuracy, precision, limit of quantification, limit of detection, robustness and solution stability. Limit of detection and limit of quantification for estimation of venlafaxine hydrochloride were found to be 6.11 μg/ml and 20.33 μg/ml, respectively. Recoveries of venlafaxine hydrochloride in tablet formulations were found to be in the range of 99.3-99.5%. Proposed method was successfully applied for the quantitative determination of venlafaxine hydrochloride in tablet dosage forms.
Keywords
UPLC, validation, venlafaxine hydrochloride
Venlafaxine is a bicyclic antidepressant, and is usually categorized as a serotonin-norepinephrine reuptake inhibitor (SNRI), but it has also been referred to as a serotonin-norepinephrine-dopamine reuptake inhibitor. Venlafaxine hydrochloride chemically is (R/S)-1- [2-(dimethylamino)-1-(4- methoxyphenyl) ethyl] cyclohexanol hydrochloride or (±)-1- [a [α-(dimethylamino) methyl] p-methoxybenzyl] cyclohexanol hydrochloride salt and has the empirical formula of C17H27NO2.HCL.
Various methods have been reported for estimation of venlafaxine hydrochloride in biological matrices such as plasma, which includes the use of LC with UV detection [1], LC with electrospray ionization mass spectrometry [2], LC with coulometric detection [3], LC with fluorimetric detection [4,5], LC with diode array detection [6,7], GC-MS [8], LC-MS-MS [9] and for estimation of it in serum by use of LC [10]. Stability indicating methods have also been reported for its in vitro determination in gastric and intestinal fluids [11] and pharmaceutical formulations [12]. RP-HPLC method is also reported for estimation of it in tablet dosage form [13].
The reported stability indicating methods and RP-HPLC uses acetonitrile and buffer in various proportions for quantification of venlafaxine hydrochloride. Present study involves development of UPLC method using simple mobile phase containing acetonitrile and buffer for quantitative estimation of venlafaxine hydrochloride in capsule dosage forms which is sensitive and requires shorter analysis time. The developed method was validated as per ICH guidelines [14,15].
An Acquity TM UPLC Instrument Control Option Pack Version 1.23b system (waters, equipped with a diode array detector, isocratic pump, column oven) rhenodyne valve with 5 μl fixed loop, and acquity TM BEH column having C18 (100×2.1 mm id, 0.2 μm particle size) was used.
Analytically pure powder venlafaxine hydrochloride was procured as gratis samples from Cadila Healthcare Limited, Moraiya, India. HPLC grade acetonitrile and orthophosphoric acid were purchased from E. Merck (Mumbai, India) and were of analytical grade. HPLC grade water was purchased from Milli Q. The marketed formulation containing 150 mg of venlaflaxine (Efflexor-XR, Zydus-Cadila Healthcare Ltd., Ahmedabad, India) were purchased form pharmacy.
The Acquity TM BEH column C18 was used at ambient temperature. The mobile phase consisted of dipotassium hydrogen phosphate: Acetonitrile (30:70 v/v; pH 7.00 with dilute o-phosphoric acid) was used. The flow rate was 0.75 ml/min and effluents were monitored at 227 nm. The mobile phase was passed through HVLP 0.22 μm membrane filter and degassed before use. The elution was monitored at 227 nm, and the injection volume was 5 μl.
UPLC method depends upon the nature of the sample (ionic or ionizable or neutral molecule), its molecular weight and solubility. UPLC was selected for the initial separation because of its simplicity and suitability. To optimize the chromatographic conditions the effect of chromatographic variables such as mobile phase, pH, flow rate and solvent ratio were studied. The resulting chromatograms were recorded and the chromatographic parameters such as capacity factor, asymmetric factor, and resolution and column efficiency were calculated. The condition that gave the best resolution, symmetry and capacity factor was selected for estimation.
Accurately weighed 56 mg of venlafaxine HCl was transferred to a 50 ml volumetric flask and dissolved in 20 ml of the solvent. The flask was sonicated for 15 min. and volume was made up to the mark with the solvent. From this stock solution working standard solution was prepared by transferring further 5.0 ml to 50 ml volumetric flask and the solvent was added up to the mark to give a solution containing 112 μg/ ml venlafaxine HCl.
Average weight of the capsule pallets was computed by accurately weighing 20 capsule pallets. Powder equivalent to 100 mg of venlafaxine HCl was weighted accurately and transferred to a 200 ml volumetric flask. After addition of 100 ml of the solvent flask was sonicated for 30 min. Solution was filtered through 0.22 μm HVLP filter 5.0 ml of filtrate was pipette out to 25 ml volumetric flask and solvent was added up to mark to get final concentration of 100 μg/ml of venlafaxine HCl. The prepared sample solutions were chromatographed for 5 min using mobile phase at a flow rate of 0.75 ml/min.
Appropriate volumes of aliquots from standard venlafaxine HCl stock solution were transferred to different volumetric flasks. The volume was adjusted to the mark with the solvent to obtain the concentration of 28, 56, 90, 112, 135, 168 and 196 μg/ml. Area of each solution were measured at 227 nm and the plot of absorbance v/s concentration was plotted. The straight-line equation was determined from calibration curve.
Accuracy is the closeness of the test results obtained by the method to the true value. Accuracy is performed at three levels 50, 100 and 150%. Percentage recovery and low relative standard deviation value show's accuracy of the UPLC method. The precision of analytical method is the degree of agreement among individual test results when the method is applied repeatedly to multiple samplings of homogeneous samples. The relative standard deviation for six replicates of sample solution was less than 2.0%, which met the acceptance criteria established for UPLC method. Ruggedness test was determined between two different days, analysts and instruments. The value of RSD found to be below 2.0% showed ruggedness of developed UPLC method. The linearity of analytical method is its ability to elicit test results that are directly proportional to the concentration of analyte in sample within the given range. The range of the analytical method is the interval between the upper and lower levels of analyte that have been demonstrated to be determined within a suitable level of accuracy and linearity using the method as written.
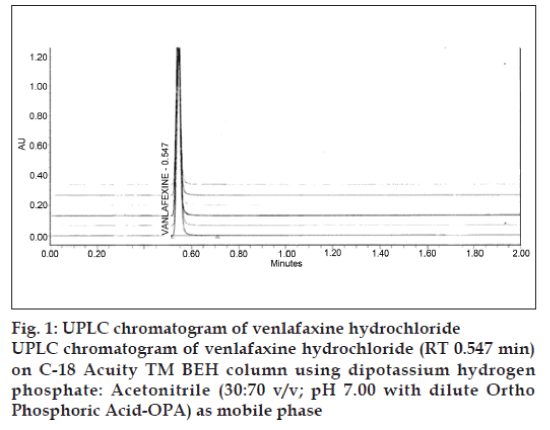
Specificity is a procedure to detect quantitatively the analyte in the presence of components that may be expected to be present in the sample matrix. Selectivity is a procedure to detect qualitatively the analyte in presence of components that may be expected to be present in the sample matrix. Commonly used excipients in capsule preparation were spiked in a preweighed quantity of drug and then absorbance was measured and calculations done to determine the quantity of the drug. The method was found to be robust, as small but deliberate changes in the method parameters have no detrimental effect on the method performance as shown in. Low value of relative standard deviation was indicating that the method was robust. Standard and sample solution stability was evaluated at room temperature for 48 h. The relative standard deviation was found below 2.0%. It showed that both standard and sample solution were stable up to 48 h at room temperature. Several mobile phases were tried to resolve venlafaxine HCl but the resolution was not satisfactory. So modification was made in the above mobile phase. Finally the system containing acetonitrile: Buffer (70:30, pH=7.0) was found to be satisfactory and gave well resolved peak for venlafaxine HCl. The retention time for venlafaxine HCl was 0.548 min (fig. 1). For the selection of detection wavelength, the spectrum of 112 ppm venlafaxine HCl revealed that, at 227 nm the drug possess significant absorbance. So considering above fact, 227 nm was selected as a detection wavelength for estimation of venlafaxine HCl using UPLC. For selection of appropriate column and flow rate, zorbex column and various flow rates were tried but the satisfactory resolution was obtained with columnaquity and flow rate 0.75 ml/min. The retention time for venlafaxine HCl was found to be 0.548 min and theoretical plates observed were 5705. From the peak area obtained in the chromatogram, the amount of the drug was calculated as 99.3±0.2 % w/w.
The calibration curve for venlafaxine HCl was prepared by plotting area v/s concentration. The linearity plot of venlafaxine HCl is found linear with the linear equation y= 1.324e004x+1.24e004 and correlation coefficient 0.9993 for venlafaxine HCl. This indicates that the method is linear in the specified range for the analysis of venlafaxine HCl in dosage form. The LOD for venlafaxine HCl was found to be 6.11 μg/ml. The method was found to be accurate with % recovery 99.3 – 99.5% and was found within the acceptance criteria (i.e. 98.0 to 102.0%) with acceptable %RSD of not more than 2% at each level (Table 1).
| Levels | Sets | Area (n=3) |
mg Added |
mg Added (Actual) |
mg Recovered |
% Recovery | Mean % Recovery | % RSD |
|---|---|---|---|---|---|---|---|---|
| 50% | 1 | 680927 | 50 | 49.75 | 49.36 | 99.2 | 99.3 | 0.4 |
| 2 | 682129 | 50.2 | 49.95 | 49.45 | 99 | |||
| 3 | 683725 | 49.9 | 49.65 | 49.57 | 99.8 | |||
| 100% | 1 | 1357842 | 100.2 | 98.92 | 98.84 | 99.7 | 99.5 | 0.3 |
| 2 | 1358191 | 100 | 99.5 | 98.46 | 99 | |||
| 3 | 1354847 | 100 | 99.508 | 98.22 | 98.7 | |||
| 150% | 1 | 1975626 | 145.1 | 145.1 | 143.22 | 99.2 | 99.3 | 0.1 |
| 2 | 1984493 | 145.6 | 145.6 | 143.86 | 99.3 | |||
| 3 | 1982236 | 145.4 | 145.4 | 143.7 | 99.3 |
Table 1: Data derived from accuracy of venlafaxine hydrochloride the proposed UPLCmethods.
Precision was calculated as repeatability and intraday and interday variation for venlafaxine HCl. The method was found to be precise with CV=0.6 for intraday (n=3) and CV =0.7 for interday (n=3) for venlafaxine HCl. The low CV (i.e. NMT 2%) has observed for the six assay results (Table 2) hence it concluded that the method is precise for the analysis of venlafaxine HCl in their dosage form.
| Paramete | Venlafaxine hydrochloride |
|---|---|
| Linearity (µg/ml) | 28-196 |
| Correlation co –efficient (r) | 0.99952 |
| Residual std. Regression (σ) | 26926.18519 |
| Slope of Regression (S) | 13241.03169 |
| LOD (µg/ml)a | 6.11 |
| LOQ (µg/ml)b n=5 | 20.33 |
| Accuracy, % | 99.3 – 99.5 |
| Repeatability, (% Assay, n = 6) | 99.2 - 100 |
| Precision (% Assay) | |
| Interday (n = 3) | 101.2–101.8 (% RSD = 0.7) |
| Intraday (n = 3) | 100.3–101.4 (% RSD = 0.6) |
aLOD=Limit of detection, bLOQ= Limit of quantitation
Table 2: Summary of validation parameters.For venlafaxine hydrochloride the proposed UPLC methods.
Ruggedness test was determined between two different days, analysts and instruments. The value of RSD was to be found 0.9 which is within acceptance criteria of below 2.0% showed ruggedness of developed UPLC method. There is no interference of mobile phase, solvent and placebo with the analyte peak and also the peak purity of analyte peak is greater than 990 which indicate that the method is specific for the analysis of venlafaxine HCl in their dosage form.
The method was found to be robust, as small but deliberate changes in the method parameters have no detrimental effect on the method performance as shown in Table 3. The low value of relative standard deviation was indicating that the method was robust. Standard and sample solution stability was evaluated at room temperature for 48 h. The relative standard deviation was found below 2.0%. It showed that both standard and sample solution were stable up to 48 h at room temperature.
| Parameters | Normal condition | Change in condition | Change in % RSD |
|---|---|---|---|
| Temprature | 45° | 43° | 0 |
| 47° | 0.007 | ||
| Flow Rate | 0.75 ml/min | 0.5 ml/min | 0.22 |
| 1 ml/min | 0.00 | ||
| pH | 7.0 | 6.5 | 0.00 |
| 7.5 | 1.74 | ||
| Mobile phase ratio | 30:70 | 25:75 | 0.18 |
| 35:65 | ------ |
Table 3: Data derived from robustness of the proposed UPLC methods.
Proposed study describes new UPLC method using simple mobile phase for the estimation of venlafaxine hydrochloride in capsule formulations. The method was validated and found to be simple, sensitive, accurate and precise. Percentage of recovery shows that the method is free from interference of the excipients used in the formulation. Therefore the proposed method can be used for routine analysis for estimation of venlafaxine hydrochloride in its capsule formulations.
Acknowledgements
We are grateful to Cadila Healthcare Limited, Moraiya, Gujarat, India for the gratis samples of pure venlafaxine hydrochloride and for providing instrumental facility. We are also thankful to SICART at Vallabh Vidyanagar for providing other instrumental facility.
References
- Raut BB, Kolte BL, Deo AA, Bagool MA, Shinde DB. A rapid and sensitive high performance liquid chromatographic method for determination of venlafaxine and o-desmethyl venlafaxine in human plasma with UV detection. J LiqChromatogr Tech 2003;26:1297-313.
- Juan H, Zhiling Z, Huande L. Simultaneous determination of fluoxetine,citalopram, paroxetine, venlafaxine in plasma by high performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-MS/ESI). J Chromatogra Bio SciAppl 2005;820:33-9.
- Clement EM, Odontiadis J, Franklin M. Simultaneous measurement of venlafaxine and its major metabolite, O-desmethylvenlafaxine, in human plasma by high-performance liquid chromatography with coulometricdetection and utilisation of solid-phase extraction. J Chromatogra Bio SciAppl 1998;705:303-8.
- Vu RL, Helmeste D, Albers L, Reist C. Rapid determination of venlafaxine and O-desmethylvenlafaxine in human plasma by highperformanceliquid chromatography with fluorimetric detection. J Chromatogra Bio SciAppl 1997;703:195-201.
- Li HD, Ding DR, Zhang BK, Yuan HY. Studies on determination of new drug venlafaxine in human plasma by high performance liquid chromatography with fluorimetric detection.YaowuFenxiZazhi2001;21:240-2.
- Titier K, Castaing N, Scotto-Gomez E, Molimard M. High-performance liquid chromatographic method with diode array detection for identification and quantification of the eight new antidepressants and five of their active metabolites in plasma after overdose. Ther Drug Monit 2003;25:581-7.
- Duverneuil C, de la Grandmaison GL, de Mazancourt P, Alvarez JC. A high-performance liquid chromatography method with photodiodearrayUV detection for therapeutic drug monitoring of the nontricyclicantidepressant drugs.Ther Drug Monit 2003;25:567-73.
- Sarah MR, Lambert EE. Development of a solid phase extraction for 13 'new' generation antidepressants and their active metabolites for gas chromatographic-mass spectrometric analysis. J Chromatogr A 2005;1098:19-29.
- Bhatt J, Jangid A, Venkatesh G, Subbaiah G, Singh S. Liquid chromatography-tandem mass spectrometry (LC-MS-MS) method for simultaneous determination of venlafaxine and its active metabolite O -desmethyl venlafaxine in human plasma. J Chromatogr Bio SciAppl2005;829:75-81.
- Frahnert C, Rao ML, Grasmader K. Analysis of eighteen antidepressants, four atypical antipsychotics and active metabolites in serum by liquid chromatography: A simple tool for therapeutic drug monitoring. J Chromatogr Bio SciAppl 2003;794:35-47.
- Asafu-Adjaye EB, Faustino PJ, Tawakkul MA, Anderson LW, Yu LX, Kwon H, et al. Validation and application of a stability-indicating HPLC method for the invitro determination of gastric and intestinal stability of venlafaxine. J Pharm Biomed Anal 2007;43:1854-9.
- Makhija SN, Vavia PR. Stability indicating LC method for the estimation of venlafaxine in pharmaceutical formulations. J Pharm Biomed Anal 2002;28:1055-9.
- Baldania SL, Bhatt KK, Mehta RS, Shah DA, Gandhi TR. RP-HPLC estimation of venlafaxine hydrochloride in tablet dosage forms. Indian J Pharm Sci 2008;70:124-8.
- ICH, Q2A Text on validation of analytical procedures, international conference on harmonization, Oct, 1994.
- ICH, Q3B validation of analytical procedures: Methodology, international conference on harmonization, Nov, 1996.