- *Corresponding Author:
- L. Mehta
Amity Institute of Pharmacy, Amity University, Noida, 201301, India
E-mail: mehta.lovekesh@gmail.com
| Date of Submission | 02 June 2020 |
| Date of Revision | 08 October 2020 |
| Date of Acceptance | 24 December 2020 |
| Indian J Pharm Sci 2020;82(6):958-966 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In present study, a novel, simple, robust, stability-indicating, highly sensitive and specific reverse phase ultra-performance liquid chromatographic method was established and validated for the determination of ibrutinib using acquity charged surface hybrid C-18 (100 mm×2.1 mm, 1.7 μm) column with a mobile phase consisting of mixture of Eluent-A phosphate buffer, 0.1 % triethylamine, (pH 6.0) and Eluent-B acetonitrile with flow rate 0.3 ml min-1. The detection was monitored at 215 nm using photodiode array detector. Linearity was observed from 25 to 250 ng ml-1 (correlation of coefficient was 0.9999) with equation, y=100.1880x-130.8966. The observed limit of detection and limit of quantitation were 25 ng ml-1 and 50 ng ml-1 respectively. Ibrutinib was subjected to stress conditions comprising hydrolytic (acid, alkali and neutral), oxidative, photolytic and thermal degradation as per International council for harmonisation Q1 guidelines. The results showed that substantial degradation were observed in acid, base and peroxide degradation condition and resistant to neutral, photolytic and thermal degradation. This is first highly sensitive stability-indicating ultra-performance liquid chromatography method capable of separating ibrutinib and its ten degradation products at nano gram (ng) level. The method was validated as stated by International council for harmonisation guidelines.
Keywords
Ibrutinib, reverse phase ultra-performance liquid chromatographic, stability-indicating, stress study, validation
Ibrutinib, a United States Food and Drug Administration approved drug, chemically known as 1-[(3R)-3 -[4- Amino-3-(4-phenoxyphenyl)-1H-pyrazolo [3, 4-d] pyrimidin-1-yl] piperidin-1-yl] prop-2-en-1-one is a white to off-white solid powder soluble in polar solvents like acetonitrile, methanol and water. It is irreversible and elective small molecule which is used in the management of patients with chronic lymphocytic leukemia by binding perpetually to a protein, Bruton’s tyrosine kinase (BTK), that inhibits B cell antigen receptor (BCR) signaling in human B cells via specific active-site occupancy [1]. Ibrutinib leads to inhibition of BTK enzymatic activity by making a covalent bond with a cysteine residue in the BTK active site [2-4]. Structure of ibrutinib is shown in fig. 1.
Ibrutinib is useful in the management of Waldenstrom’s macroglobulinemia, lymphocytic leukemia and is second-line drug for treatment of chronic graft, marginal zone lymphoma versus host disease and mantle cell lymphoma [5-7]. It decreases chronic lymphocytic cancer cell chemo taxis and prevents cellular linkage followed by stimulus at the B-cell receptor [8-10]. This anticancer drug is giving fairly good results and is currently under development by few big pharmaceutical divisions like Johnson’s & Johnson’s Jansen and Pharmacyclics Inc.
Very few High performance liquid chromatographic (HPLC) [11-16] methods are available and no ultraperformance liquid chromatographic (UPLC) methods are available till date for determination and quantification of ibrutinib in pharmaceutical dosage forms and bulk drugs. Only one HPLC method [17] is reported yet in which the authors have separated and identified six known impurities and eight genotoxic impurities, degradation products, unknown impurities, blank peaks and also placebo peaks. The method had a total run time of 85 min. A method development by HPLC is reported for determination of genotoxic impurity hydrazine hydrate using old conventional HPLC column (Inertsil ODS-3V) [18]. Recently, a highly sensitive High resolution mass spectrometry (HRMS)/ Mass spectrometry (MS)/ Time-of-flight secondary ion mass spectrometry (TOF) was reported by Lovekesh et al., for identification and characterization of ten degradation products and their degradation pathway formed during stress study [19].
HPLC is now an outdated technology in terms of sensitivity and cost effectiveness. The present study aims at development and validation of accurate, rapid, highly sensitive and economical reverse phase ultra-performance liquid chromatographic (RP-UPLC) method as per International council for harmonisation (ICH) guidelines [20]. The study is also aimed at forced degradation studies of ibrutinib to generate degradation products (DPs) under vigorous stressed conditions.
Materials and Methods
Chemicals and reagents:
Pharmaceutical grade ibrutinib standard was procured from MSN Laboratories limited, Hyderabad, as gift sample. Acetonitrile (99.9 %) was procured from JT Baker. Potassium dihydrogen o-phosphate, orthophosphoric acid (OPA) purity 85 %, sodium hydroxide (NaOH), hydrogen peroxide (30 %) and hydrochloric acid were purchased from Merck life sciences. The solutions used in the study were filtered through 0.22 μm filter paper (Millipore, India). Purification of water used for preparation of solution was done by Milli-Q-Plus system.
Equipment:
The Waters acquity H Class UPLC system coupled with quaternary solvent manager (QSM), sample manager and photo diode array (PDA) detector was used. All weighing operations were carried out on analytical balance (Mettler Toledo, Switzerland). Other instruments used in the study were Photo stability chamber (thermo lab scientific instruments, India), sonicator (Equitron, 5 L), hot air oven (Medline Scientific, India) and digital pH meter (Metrohm, India). Empower PRO 2.0 software was used for monitoring and integration of chromatographic peaks.
UPLC chromatographic conditions:
Various parameters were taken into consideration while developing and optimizing the chromatographic separation conditions including mobile phase, stationary phase, flow rate and detector wavelength. Chromatographic separation was done using reverse phase gradient mode of elution with ACQUITY CSH C-18 (100 mm×2.1 mm, 1.7 μm) column using mobile phase that contains phosphate buffer, triethylamine (pH-6) and acetonitrile at flow rate of 0.3 ml min-1 and column temperature was 45°. Mobile phase was prepared freshly and was sonicated and filtered through 0.22 μm filter paper. PDA was used and detection was performed at 215 nm with injection volume 5 μl. The optimized chromatographic conditions are shown in Table 1.
| Column | ACQUITY UPLC CSH C-18 (100 mm×2.1 mm, 1.7 µm) |
|---|---|
| Eluent A | 20 mm potassium dihydrogen phosphate, 0.1 % v/v triethylamine, pH adjusted to 6.0 with dilute Orthophosphoric acid solution |
| Eluent B | Acetonitrile |
| Elution type | Gradient |
| Injection volume | 5 µl |
| Flow Rate | 0.3 ml min-1 |
| Detector, Wavelength | PDA, 215 nm |
| Column Temperature | 45° |
| Auto sampler Temperature | 10° |
| Run time | 15 min |
| Diluent | Water: Acetonitrile (50:50) v/v |
| Nominal concentration | 100 µg ml-1 |
PDA is photo diode array detector
Table 1: Chromatographic Conditions for Optimized Method
Diluent preparation:
Water and acetonitrile in equal proportion (50:50) v/v were used as diluent throughout the study. All the solutions prepared were sonicated and filtered through 0.22 μm filter paper prior to injection in UPLC.
Sample solution:
A stock solution of strength 1 mg ml-1 was prepared with diluent. Ten milligram of drug substance transferred in 10 ml volumetric flask and it was dissolved in diluent. This stock solution was then diluted further in order to get nominal concentration of 100 μg mL-1. This solution is further diluted in order to perform various parameters like precision, limit of detection (LOD), linearity, limit of quantitation (LOQ).
Forced degradation studies:
Studies on forced degradation were performed in order to understand the chemical stability, degradation pathways and DPs of drug substance and drug products. It involves exposure of drug substance under hydrolytic, photolytic, oxidative and thermal degradation conditions. These studies depict the specificity and stability-indicating ability of method. Ibrutinib was exposed to different stress conditions as stated by International Conference on Harmonization (ICH) guidelines [21-22]. PDA detector was used to ensure peak purity of ibrutinib and its DPs produced during stress study. Stress studies were done by initially preparing 1 mg ml-1 stock solution of drug substance. Sample of acid hydrolysis was neutralized with sodium hydroxide and sample of alkaline hydrolysis was neutralized with hydrochloric acid. Before performing analysis, these solutions were filtered through 0.22 μm filter paper.
Hydrolytic degradation:
Hydrolytic degradation covers wide range of pH (acidic, basic and neutral). Functional groups that may undergo hydrolysis are esters, amides, alcohol, aryl amines, carbamates etc. The analyte was exposed separately with 5 ml of 1 M HCl, 5 ml of 1 M NaOH and water maintained at 80° for 8 h. After desired time, treated sample were allowed to attain the room temperature and then neutralized with acid and base. Further diluted with diluent in order to achieve final analyte concentration of 100 μg ml-1.
Oxidative degradation:
Though many agents that oxidize such as radical initiators (e.g., AIBN, azobisisobutyronitrile) and metal ions can be used to perform studies on degradation but hydrogen peroxide is most widely used for oxidation degradation. The oxidative degradation of drug substance includes an electron transfer mechanism to form reactive cations and anions. Sulfides, phenols and amines are liable to electron transfer oxidation to give sulfones, sulphoxide, hydroxylamine and N-oxides. Sample was treated with hydrogen peroxide (10 % H2O2) and kept at room temperature for 8 h. The stressed sample was further diluted with diluent in order to achieve a final concentration of 100 μg ml-1.
Degradation due to photolysis:
Photolytic stability was done to study the influence of light when drug substance is exposed to ultraviolet (UV) or fluorescent conditions. Functional groups like carbonyl, N-oxide, aryl chloride, nitro aromatic, weak O-H and C-H bonds, polyenes and sulfides are likely to introduce drug photosensitivity. Adequate amount of drug was kept in petri-dish and exposed to an overall illumination of 1.2 million lx h of fluorescent and a combined near UV energy of 200 W h/m2 light in photo stability chamber. After desired time period, sample was taken out from chamber to prepare a final concentration of 100 μg ml-1 with diluent.
Degradation due to thermal conditions:
Thermal degradation studies were carried as per ICH Q1A guidelines suggested at accelerated conditions. Arrhenius equation is used to study the consequence of temperature on thermal degradation of a drug substance. The eqn. is k=Ae-Ea/RT where A is frequency factor, k is specific reaction rate, R is gas constant (1.987 cal/deg mole), Ea is energy of activation and T is absolute temperature.
Sufficient amount of drug substance was kept in petridish and exposed to dry oven at temperature 105 ° for 24 h. After desired time period, sample was taken out to prepare the final concentration of 100 μg ml-1 with diluent.
Method validation:
The developed method was validated as per the guidelines of ICH Q2 (R1). Various parameters like selectivity/specificity, accuracy/recovery, linearity, precision, detection limit, quantitation limit, robustness and stability of analyte in solution were performed for the method validation. All the validation parameter and their acceptance criteria as stated by ICH guidelines are shown in Table 2.
| Parameter | Experiment | Acceptance criteria |
|---|---|---|
| System precision | Six replicate injections of ibrutinib (100 µg ml-1) standard solution | RSD should not more than 2.0 % |
| Method precision | Sample at three different concentration (QL, 100 % and 150 %) prepared and analyzed in triplicate as per developed method | RSD should not more than 2.0 % |
| Limit of detection and quantitation | Determination of LOD and LOQ based on slope of linearity | Correlation coefficient (r2) should not be less than 0.999 |
| Accuracy/Recovery | Control sample in triplicate and spiked at QL, 100 % and 150 % concentration in triplicate | Percentage Recovery should be in between 98-102 % |
| Robustness | By changing flow rate By changing column temperature By changing Percentage B composition |
USP resolution between closely eluted impurities should not less than 2.0 |
| Solution stability | Solution stability at different interval 0, 12 and 24 h | RSD should not more than 2.0 % |
DL is detection limit, QL is quantitation limit and RSD id relative standard deviation.
Table 2: Method Validation Parameters And Acceptance Criteria
System suitability:
System suitability testing is a vital part of many analytical procedures. Standard analyte solution of ibrutinib at concentration of 150 ng ml-1 was injecting six times. System suitability was tested by calculating the parameter like USP tailing factor, theoretical plate count and percentage relative standard deviation (Percentage RSD). The percentage RSD of area from six replicate injections was calculated.
Specificity and selectivity:
The ability of analytical method to determine the response of pure drug substance in the presence of potential impurities and DPs formed during stress study is called specificity. The specificity was examined by analyzing mixtures of the acidic, basic and oxidative degradation products with known concentrations of ibrutinib. Specificity parameter was performed by establishing the desired USP resolution between the ibrutinib peak and nearest DP peak formed during the degradation study and establish the resolution of all DPs from each other. PDA detector was used to check the peak purity of ibrutinib peak and DPs peak in order to check the method selectivity.
Linearity:
The calibration curve was prepared by plotting response of ibrutinib against their respective concentration of analyte. Linearity of the method was calculated by analyzing standard solutions at seven different concentration levels covering the range of 25-250 ng ml-1 keeping the injection volume constant in all injections. The correlation coefficient (r2), y-intercept and slope of ibrutinib were calculated.
Precision:
System precision was assessed in terms of inter-day precision (reproducibility) and intra-day (repeatability). Precision study was done by injecting three different concentrations (50, 150 and 250 ng ml-1) in triplicate in RP-UPLC on same day and next day. The value of percentage RSD and standard deviation (SD) was assessed for both intra-day and inter-day precision.
Accuracy/Recovery:
The accuracy of method is the closeness of test results obtained by that method when compared with the true values. Method accuracy was assessed by performing recovery studies for the pure drug by standard addition process. Known concentration of drug substance (150 ng ml-1) was added to three different concentration level (QL, 100 and 150 %). Control sample and recovery samples were injected in triplicate and percentage recovery were calculated at each level.
Limits of detection (LOD) and limit of quantification (LOQ):
LOD and LOQ capability determine the sensitivity of method. Calibration curve method was used to establish LOD and LOQ. Series of dilute solution having known concentration were injected to determine the LOD and LOQ values. Signal to noise ratio was calculated for LOD and LOQ. Percentage RSD of the peak area was calculated at QL concentration level.
LOD= (3×standard deviation of y-intercept)/(slope of calibration curve)
LOQ= (10×standard deviation of y-intercept)/(slope of calibration curve)
Robustness:
The measurement of capacity of method of analysis to remain unaffected by little but deliberate changes in developed experimental conditions is called robustness. This parameter provides an indication that developed method is robust and rugged. Different variables like flow rate, temperature of column and change in proportion of mobile phase were evaluated in robustness study. Specificity solution containing ibrutinib drug substance and its DPs was injected in actual condition as well as in all variable condition to check the robustness of developed method conditions. Effect on system suitability parameter like United States Pharmacopeia (USP) resolution between closely related compounds and USP tailing were observed in initial condition and in each deliberate variation.
Solution stability:
Solution stability was established at room temperature. These solutions were injected serially at respective time intervals and the stability of these solutions was evaluated till 24 h. The comparison of these solutions was evaluated against the freshly prepared sample solution.
Results and Discussion
The main purpose of stability-indicating method development was to achieve the separation and quantification of ibrutinib and its impurities (process related or degradation impurities). The absorbance maximum of the drug substance and its DPs is 215 nm. Therefore, detection was performed out at wavelength 215 nm. Depending upon the physicochemical properties, solubility, molecular weight and structure of ibrutinib, different systematic trials such as selection of stationary phase, buffer selection, organic modifier and different pH conditions were taken for development of robust, linear, precise and accurate UPLC method for estimation of ibrutinib.
Various UPLC columns (stationary phase) were tried for separation of ibrutinib peak and its DPs such as AQUITY HSS C-18 (2.1 mm×100 mm, 1.8 μm), AQUITY BEH C-18 (2.1 mm×100 mm, 1.7 μm), AQUITY CSH C-18 (2.1 mm×100 mm, 1.7 μm), AQUITY BEH phenyl (2.1 mm×100 mm, 1.7 μm) and AQUITY CSH phenyl hexyl (2.1 mm×100 mm, 1.7 μm). Based on various parameters like USP resolution between closely related compounds (DP-III and DP-IV), USP tailing and theoretical plate count, AQUITY CSH C-18 (2.1 mm×100 mm, 1.7 μm) was selected as a suitable column.
Many different buffers like formate, phosphate, acetate, ammonia covering pH having range from acidic to basic were tried based on the pKa value. It was observed that in acidic pH of around 2 to 3, DPs were merged with each other using different buffers and in basic pH 8 to 10, peak shape was distorted. Best separation was observed at at acidic pH (pH-6) with phosphate buffer but tailing observed due to free silanol effect. Tailing of peak was improved after addition of triethylamine in buffer. Therefore, potassium dihydrogen phosphate buffer along with triethylamine was selected as buffer for analysis. Both acetonitrile and methanol were evaluated as organic solvents for mobile phase. The study was observed better resolution and peak shapes with acetonitrile when compared with methanol. Therefore, acetonitrile was utilized as an organic modifier for the analysis.
Various gradient trials were taken in order to achieve best peak shape and separation of all DPs with ibrutinib in very short run time. The detection was carried out at 215 nm wavelength based on absorbance maxima of ibrutinib. Different column temperature and flow rate trials were also taken in order to get desired peak shape and resolution. The optimized columns temperature was 45 °C with flow rate of 0.3 ml min-1 in order to achieve good peak shape and separation between closely eluted peaks. The final optimized UPLC method using The typical retention times of DP-I, DP-II, DP-III, DP-IV, DP-V, DP-VI, DP-VII, DP-VIII, DP-IX and DP-X were about 4.11, 4.58, 5.45, 5.70, 6.00, 6.48, 8.00, 8.57, 9.63 and 10.42 min. respectively. The optimized method was capable of separating all DPs with ibrutinib and for estimation of ibrutinib at very low level.
The average theoretical plate count of six injections was 86870 and average USP tailing of six injections was 0.9 (<1.8) and percent relative standard deviation (Percentage RSD) of area of ibrutinib peak in six replicate injection of system suitability solution was 0.47 (<5.0). Method was suitable for use as all the parameters were within limit. The results of system suitability parameter were summarized in Table 3.
| Sr. No | Peak Name | Retention Time (min) | Area | USP plate count |
USP tailing | |
|---|---|---|---|---|---|---|
| 1 | Ibrutinib | 7.55 | 15020 | 84771 | 0.9 | |
| 2 | Ibrutinib | 7.54 | 14861 | 85221 | 0.9 | |
| 3 | Ibrutinib | 7.54 | 14974 | 83998 | 0.9 | |
| 4 | Ibrutinib | 7.54 | 14894 | 87250 | 0.9 | |
| 5 | Ibrutinib | 7.53 | 15031 | 91499 | 0.9 | |
| 6 | Ibrutinib | 7.54 | 14908 | 88480 | 0.9 | |
| Mean | 7.54 | 14948 | 86870 | 0.9 | ||
| Std. Deviation | 0.01 | 70.48 | 2812.03 | <0.01 | ||
| Percentage RSD | 0.08 | 0.47 | 3.24 | <0.01 |
RSD is relative standard deviation. Six injections at concentration level of 150 ng mL-1 were injected and Percentage RSD of area calculated
Table 3: System Suitability Evaluation
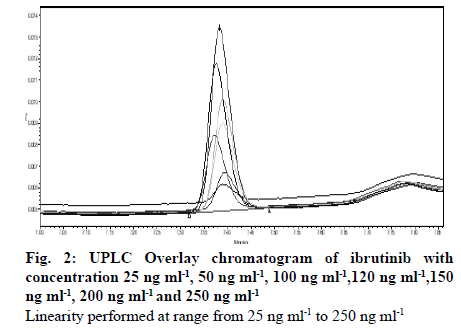
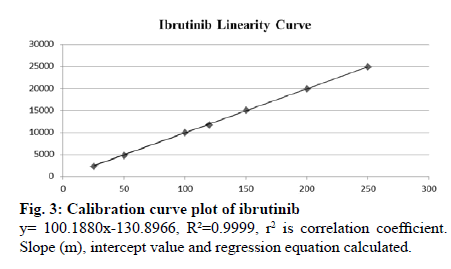
Seven different concentration of ibrutinib standard were used to analyze the linearity of the method covering the range of 25 to 250 ng ml-1. Slope, coefficient of correlation (r2) and intercept for ibrutinib were 100.1880, 0.9999, and -130.8966 respectively. Linearity data are shown in Table 4. Overlay chromatogram and calibration curve of ibrutinib are shown in fig. 2 and 3 respectively. Chromatograms of linearity study are given in supplementary fig. S1- S7. Percentage RSD values for intra-day and inter-day precision studies were and 0.40-0.81 % and 0.38 %-0.79 % respectively. Intraand inter-day precision of UPLC method of ibrutinib is presented in Table 5. The percentage recovery was found to be in the range of 99.9 %-101.8 %. The recovery results indicate that the method is precise and also found that there was no intervention due to the presence of DPs. The results are shown in Table 6.
| Concentration (ng ml-1) | Peak Area | Slope (m) | Intercept Value (b) | Correlation coefficient (R2) |
|---|---|---|---|---|
| 25 | 2351 | 100.1880 | -130.8966 | 0.9999 |
| 50 | 4835 | |||
| 100 | 9969 | |||
| 120 | 11795 | |||
| 150 | 15058 | |||
| 200 | 19870 | |||
| 25 | 24874 |
Seven different concentrations were used in order to get the calibration curve plot of ibrutinib
Table 4: Linearity Data For Ibrutinib
| Sample No | Concentration (ng ml-1) | Intra-day Precision | Inter-day Precision | ||
|---|---|---|---|---|---|
| Mean±SD | RSD (%) | Meana±SD | RSD (%) | ||
| 1 | 50 | 4812±32.25 | 0.67 | 4534±36.60 | 0.81 |
| 2 | 150 | 14848±55.8 | 0.38 | 14000±81.51 | 0.58 |
| 3 | 250 | 24658±193.63 | 0.79 | 23327±93.72 | 0.40 |
aMean of three replicate
Triplicate injection at three different concentrations were injected for three consecutive
Days and percentage RSD calculated
Table 5: Intra-Day And Inter-Day Precision Study Of Developed Method Of Ibrutinib
| Spiked Concentration (ng mL-1) | Found Concentration (ng ml-1, Meana ± SD) | RSD (%) | Recovery (%) |
|---|---|---|---|
| 50 (QL) | 49.95±30.39 | 0.20 | 99.9 |
| 150 (100 %) | 152.70±67.28 | 0.20 | 101.8 |
| 250 (150 %) | 253.5±32.62 | 0.10 | 101.4 |
aMean of three replicate
Three different concentrations with triplicate injections
Table 6: Recovery Data Of Ibrutinib (N=3)
Robustness study was performed by creating small, deliberate variations in the temperature conditions (±5°), flow rate (±0.02 ml min-1) and change in initial buffer composition in gradient and found to be unaffected upon these deliberate variations in parameters. Results are tabulated in Table 7. LOD and LOQ of ibrutinib were found to be 25 ng ml-1 and 50 ng ml-1, respectively. Signal to noise ratio for LOD was more than 3 and for LOQ was more than 10. Results of LOD and LOQ are given in Table 8 and Table 9 respectively. Percent RSD of ibrutinib was found less than 1.0 % and there was no significant change observed in peak area indicates solution and mobile phase stability up to 24 h.
| Parameter | Value | Rt (min) | Rs | Tc |
|---|---|---|---|---|
| Flow rate (mL/min) | 0.28 | 7.64 | 3.1 | 1.3 |
| 0.30 | 7.40 | 3.4 | 1.3 | |
| 0.32 | 7.17 | 3.5 | 1.3 | |
| Column temperature (°) | 40 | 7.45 | 3.5 | 1.3 |
| 45 | 7.40 | 3.4 | 1.3 | |
| 50 | 7.37 | 3.2 | 1.3 | |
| Percentage Eluent B initial composition | 22 | 8.05 | 3.2 | 1.3 |
| 25 | 7.40 | 3.4 | 1.3 | |
| 28 | 6.93 | 3.5 | 1.3 |
cRt=retention time of ibrutinib peak, Rs = resolution between closely
related degradation products (DP-III and DP-IV), Tc = tailing factor
of ibrutinib peak
Concentration level used for robustness study was 100 μg mL-1.
Three factors were
slightly changed (Flow rate, Column temperature and Percentage B
initial composition)
Table 7: Robustness Data Of Ibrutinib And Its Degradation Products
| Sr. No. | Peak Name | Retention time (min.) | Area | S/N |
|---|---|---|---|---|
| 1 | Ibrutinib | 7.40 | 2350 | 6 |
| 2 | Ibrutinib | 7.40 | 2339 | 6 |
| 3 | Ibrutinib | 7.39 | 2351 | 6 |
| Mean | 7.40 | 2347 | 6 | |
| Std. Deviation | 0.01 | 6.66 | ||
| percentage RSD | 0.08 | 0.28 |
S/N is signal to noise ratio
Table 8: Limit of Detection (LOD) Evaluation
| Sr. No | Peak Name | Retention time (min) | Area | S/N |
|---|---|---|---|---|
| 1 | Ibrutinib | 7.40 | 4782 | 33 |
| 2 | Ibrutinib | 7.39 | 4807 | 30 |
| 3 | Ibrutinib | 7.39 | 4846 | 33 |
| 4 | Ibrutinib | 7.40 | 4840 | 30 |
| 5 | Ibrutinib | 7.38 | 4796 | 28 |
| 6 | Ibrutinib | 7.39 | 4835 | 28 |
| Mean | 7.39 | 4818 | 30 | |
| Std. Deviation | 0.01 | 26.30 | ||
| Percentage RSD | 0.10 | 0.55 |
S/N is signal to noise ratio
Table 9: Limit of Quantitation (Loq) Evaluation
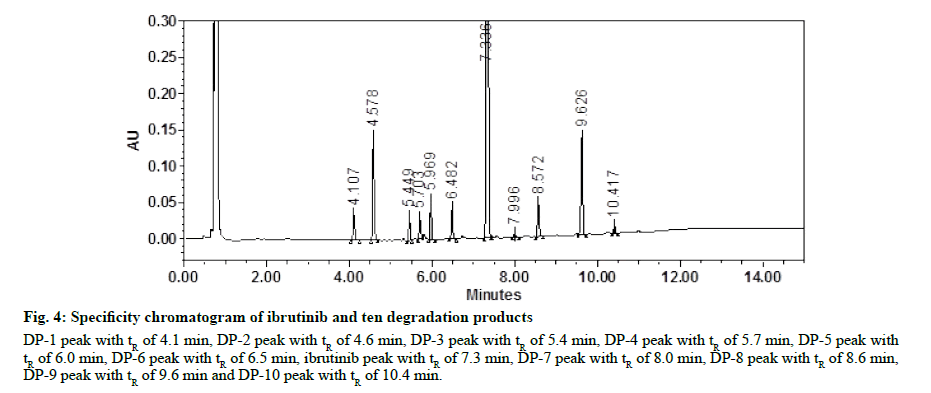
In optimized UPLC methods, all DPs formed were well separated with drug substance indicates that method is specific. PDA was used to ensure the peak purity. Purity angle of each DP including ibrutinib was less than purity threshold. There was no interference of any other peak of DP, impurity, or matrix observed which ensure the selectivity of method. Assay study of stressed samples performed in order to perform the mass balance study. Assay results were more than 98.0 % in every stressed condition. Specificity chromatogram of ibrutinib and its ten DPs are given in fig. 4. Method specificity and peak purity data are given in Table 10. The max plot chromatogram of ibrutinib and all the DPs are given in supplementary fig. S8- S18. Neutral degradation study was carried out different time interval such as 0 min, 1 h, 4 h and 8 h. At each interval, before injection, sample was filtered through 0.22 μm filter. The drug substance was found to stable in neutral degradation condition. The results are given in Table 11 and fig. S19.
Figure 4: Specificity chromatogram of ibrutinib and ten degradation products
DP-1 peak with tR of 4.1 min, DP-2 peak with tR of 4.6 min, DP-3 peak with tR of 5.4 min, DP-4 peak with tR of 5.7 min, DP-5 peak with
tR of 6.0 min, DP-6 peak with tR of 6.5 min, ibrutinib peak with tR of 7.3 min, DP-7 peak with tR of 8.0 min, DP-8 peak with tR of 8.6 min,
DP-9 peak with tR of 9.6 min and DP-10 peak with tR of 10.4 min.
| Degradation products | Relative Retention time | Purity angle | Purity threshold | Resolution |
|---|---|---|---|---|
| DP-I | 0.56 | 0.547 | 3.775 | - |
| DP-II | 0.62 | 0.386 | 2.954 | 6.0 |
| DP-III | 0.74 | 1.660 | 17.120 | 12.4 |
| DP-IV | 0.78 | 4.236 | 26.431 | 3.7 |
| DP-V | 0.81 | 0.691 | 6.526 | 3.9 |
| DP-VI | 0.88 | 8.048 | 44.667 | 7.9 |
| Ibrutinib | 1.00 | 0.277 | 0.607 | 12.2 |
| DP-VII | 1.09 | 11.107 | 90.000 | 9.0 |
| DP-VIII | 1.17 | 0.624 | 7.218 | 7.5 |
| DP-IX | 1.31 | 0.485 | 2.445 | 13.4 |
| DP-X | 1.42 | 10.788 | 90.000 | 9.8 |
aMass balance =Percemtage Assay+percentage Sum of all impurities+percentage Sum of all degradants
Purity angle and purity calculated for each degradation product
formed
Table 10: Method Specificity And Peak Purity Data
Acid degradation study was carried out different time interval such as 0 min, 1 h, 4 h and 8 h. At each interval, stressed sample was neutralized with 1M NaOH and filtered through 0.22 μm filter before analysis. Only one degradation product (DP-I, Rt 4.1 min) of 18.0 % was formed during acid hydrolysis. The results are given in Table 11 and fig. S20. Alkaline degradation study was carried out different time interval such as 0 min, 1 h, 4 h and 8 h. At each interval, stressed sample was neutralized with 1M HCl and filtered through 0.22 μm filter before analysis. Five major DP’s (Rt 4.1, 4.6, 6.0, 8.6 and 9.6 min) of 28.10 % were formed during alkaline hydrolysis. The results are given in Table 11 and fig. S21.
| Stress Condition | Assay of ibrutinib (%) | Observation | aMass Balance (%) |
|---|---|---|---|
| Untreated Sample | 99.34 | - | |
| Neutral degradation | 99.24 | No major degradation observed | 99.35 |
| Acid degradation | 81.39 | Unknown degradant of 18.0 % was observed | 99.39 |
| Basic degradation | 71.14 | Five unknown degradation products of 28.10 % were formed. | 99.24 |
| Oxidative degradation | 82.65 | Five unknown degradation products of 17.00 % were formed. | 99.65 |
| Photolytic degradation | 99.52 | No major degradation observed | 99.61 |
| UV degradation | 99.55 | No major degradation observed | 99.55 |
| Thermal degradation | 99.48 | No major degradation observed | 99.59 |
aMass balance=Percentage Assay+Percentage Sum of all impurities+Percentage Sum of all degradants
Mass balance activity at different stress conditions were performed.
Table 11: Forced Degradation Study Of Ibrutinib
Oxidative degradation study was carried out different time interval such as 0 min, 1 h, 4 h and 8 h. At each interval, stressed sample was filtered through 0.22 μm filter before analysis. Five major DP’s (Rt 5.5, 5.7, 6.5, 8.0 and 10.4 min) of 17.00 % were formed during oxidative degradation. The results are shown in Table 11 and fig. S22. Ibrutinib drug substance was found to stable when exposed to photo stability chamber (1.2 million lux h) and UV light. There was no signification degradation observed. The results are given in Table 11 and fig. S23-24. Thermal degradation study was carried out by keeping 100 mg sample in duplicate in Petri dish and sealed. One petridish exposed to dry oven at a temperature of 105° for 24 h and other kept as control. After desired time period (24 h), stressed sample and control sample was diluted with diluent to produce a concentration of 100 μg ml-1 and filtered with filter paper before injection. Ibrutinib was found to be stable under thermal (105° for 24 h) stress conditions. The results are given in Table 11 and fig. S25.
A novel, linear, accurate, specific, reliable and specific stability-indicating UPLC method was developed and fully validated for the determination of ibrutinib in the presence of its DPs. It is first reported highly sensitive UPLC method capable of separating ibrutinib and its ten DPs up to nano gram level. The drug under investigation was found to be more sensitive to acidic, basic and peroxide degradation conditions as it degraded by 18.0 28.10 % and 17.0 % respectively. Ibrutinib is found to be stable when exposed to neutral hydrolysis, photolytic and thermal degradation conditions. Validation of the RP-UPLC method as per the ICH guidelines demonstrates that the method is highly sensitive, linear, rapid, robust and stability-indicating. Therefore, work may be useful for estimation of ibrutinib in bulk and pharmaceutical dosage form and also very useful in further identification and characterization of different DPs when exposed to stress condition.
Conflict Of Insterest
The authors declare that there are no conflicts of interest.
Acknowledgements
I wish to thanks to department of pharmaceutical science, Amity University, Noida for encouragement and providing all the necessary research facilities to carry out RP-UPLC related work. I am also very much thankful to the MSN laboratory, Hyderabad for providing pure ibrutinib drug as gift sample.
References
- Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences 2010;107(29):13075-80.
- Neuman LL, Ward R, Arnold D, Combs DL, Gruver D, Hill W, et al. First-in-human phase 1a study of the safety, pharmacokinetics, and pharmacodynamics of the noncovalent bruton tyrosine kinase (BTK) inhibitor SNS-062 in healthy subjects. J Am Soc Hemat 2016;128:2032.
- Ponader S, Balasubramanian S, Pham LV, Chen J, Tamayo AT, Wang M, et al. Activity of Bruton's tyrosine kinase (Btk) inhibitor PCI-32765 in mantle cell lymphoma (MCL) identifies Btk as a novel therapeutic target. J Am Soc Hemat 2011;118:3688.
- Wang L, Martin P, Blum KA, Kahl BS, Maeda LS, Advani R, et al. The Bruton's tyrosine kinase inhibitor PCI-32765 is highly active as single-agent therapy in previously-treated mantle cell lymphoma (MCL): preliminary results of a phase II trial. J Am Soc Hemat 2016;3:324-30.
- Sheridan C. Companies in rapid pursuit of Btk immunokinase. Nat Biotechnol 2012;30:199-200.
- de Vries R, Smit JW, Hellemans P, Jiao J, Murphy J, Skee D, Snoeys J, Sukbuntherng J, Vliegen M, de Zwart L, Mannaert E. Stable isotope?labelled intravenous microdose for absolute bioavailability and effect of grapefruit juice on ibrutinib in healthy adults. Br J Clin Pharmacol 2016;81(2):235-45.
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood, J Am Soc Hemat 2012;119(5):1182-9.
- Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor-and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood J Am Soc Hemat 2012;119(11):2590-4.
- Pavlasova G, Borsky M, Seda V, Cerna K, Osickova J, Doubek M, et al. Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood J Am Soc Hemat 2016;128(12):1609-13.
- Seda V, Mraz M. B?cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Hematol 2015;94(3):193-205.
- Uppala V, Divya N, Charishma E, Harshavardan K, Shyamala M. Validated stability-indicating RP-HPLC method for determination of Ibrutinib. Am J Pharm Sci 2016;3(4):324-330.
- Wei LM, Xu ZX, Peng-fei LV, Yong-le Xue XW, Zhang M. A simple HPLC method for the determination of Ibrutinib in rabbit plasma and its application to a pharmacokinetic study. Lat Am J of Pharm 2016;35(1):130-4.
- de Vries R, Huang M, Bode N, Jejurkar P, Jong JD, Sukbuntherng J, et al. Bioanalysis of ibrutinib and its active metabolite in human plasma: selectivity issue, impact assessment and resolution. Bioanalysis 2015;7(20):2713-24.
- Chintala R, Golkonda R, Kapavarapu S. Validation of stability indicating RP-HPLC method for the assay of ibrutinib in pharmaceutical dosage form. Ana Chem 2016;16(1):7-19.
- Prasad SS, Mohan GK, Babu AN. A quality by design approach for development of simple and robust reversed phase stability indicating hplc method for estimation of ibrutinib and its impurities. Anal Methods 2019;12(3):1434-45.
- Veeraraghavan S, Viswanadha S, Thappali S, Govindarajulu B, Vakkalanka S, Rangasamy M. Simultaneous quantification of lenalidomide, ibrutinib and its active metabolite PCI-45227 in rat plasma by LC–MS/MS: Application to a pharmacokinetic study. J Pharm Biomed Anal 2015;107:151-8.
- Konduru N, Gundla R, Katari NK, Paidikondala K, Reddy AS, Jagadabi V. Development and Validation of a Stability-indicating Method for Ibrutinib: Identification and Separation of Degradation Products, Known and Genotoxic Impurities Using RP-HPLC/PDA and QDa Mass Detectors. Anal Chem Lett 2020;10(1):113-36.
- Gopireddy RR, Maruthapillai A, Arockia JS, Mahapatra S. Determination of potential genotoxic impurity hydrazine hydrate in ibrutinib by RP-liquid chromatography. Mater Today 2020.
- Mehta L, Naved T, Grover P, Bhardwaj M, Mukherjee D. LC and LC-MS/MS Studies for Identification and Characterization of New Degradation Products of Ibrutinib and Elucidation of their Degradation Pathway. J Pharm Biomed Anal 2020:113768.
- ICH Q2 (R1), Validation of Analytical Procedures. Text and Methodology. International conference on harmonisation 2005.
- ICH Q1A (R2), Stability testing of new drug substances and products. International conference on harmonisation 2003.
- ICH Q1B, Photo stability testing of new drug substances and products. International conference on harmonisation 1996.