- *Corresponding Author:
- A. A. Heda
Department of Pharmaceutical Analysis and Quality Assurance, Royal College of Pharmacy and Health Sciences
E-mail: aaheda@rediffmail.com
| Date of Submission | 19 July 2010 |
| Date of Revision | 28 November 2011 |
| Date of Acceptance | 05 December 2011 |
| Indian J Pharm Sci, 2011, 73 (6): 696-699 |
Abstract
A new simple, selective, rapid, precise and accurate reverse phase HPLC method has been developed for simultaneous estimation of granisetron and dexamethasone. The method was developed using CPS Hypersil CN column (250×4.6 mm I.D.) with a mobile phase consisting of acetonitrile:buffer (100 mM Triethylamine adjusted to pH 3.0 with o-phosphoric acid) in ratio of 25:75 at a flow rate of 2 ml/min. Detection was carried out at 242 nm. The developed method was evaluated for various system suitability parameters and validated for linearity, accuracy, precision, LOD, LOQ as per ICH guidelines. It was also evaluated for bench top stability and freeze/thaw stability. The proposed method can be used for the estimation of these drugs in their combined dosage forms.
Keywords
dexamethasone, granisetron, RP-HPLC, validation
Granisetron (GRA) is a potent and selective 5-hydroxytryptamine (5-HT3) receptor antagonist with antiemetic activity indicated for prevention and treatment of nausea and vomiting associated with cytotoxic chemotherapy and radiotherapy, and postoperative nausea and vomiting [1]. Dexamethasone (DEX) is a synthetic glucocorticoid commonly used for allergic rhinitis, prevention of nausea and vomiting induced by cancer chemotherapy, cerebral edema and ophthalmic disorders [1].
Literature survey showed that co-administration of steroids increases the antiemetic efficacy of 5-HT3 receptor antagonist [2-4]. GRA combined with DEX was found to be the most effective regimen for prevention of emesis induced by moderately emetogenic chemotherapy [5-6]. Hence, a combined dosage form of GRA and DEX can be considered as a new and favorable avenue for research. As a prerequisite to the development of combined dosage form of GRA and DEX, a robust analytical method would be required, which would be applicable not only for assay purpose but also for drug release studies from different dosage forms. Literature survey revealed UV/Vis spectrophotometric and HPLC methods for the determination of DEX [7-9] and GRA [10-11] individually. No method has been reported in the literature for simultaneous estimation of GRA and DEX. Hence, the purpose of present work was to develop and validate a simple, rapid, accurate and precise RP-HPLC method for simultaneous estimation of GRA and DEX in a given mixture.
The chromatographic separation was performed on HPLC system (Dionex, Germering, Germany) with Chromeleon® acquisition software (Version 6.70) equipped with P680 HPLC pump, ASI-100 automated sample injector and UVD170U UV detector. The column used was CPS Hypersil CN (250×4.6 mm I.D.) column. Mobile phase filtered through 0.22 μm Nylon filter (Millipore) using filtration assembly with vacuum pump (Rocker pump 400, Today’s) and ultrasonicated using ultrasonic water bath (Model UCB 100, Spectralab) for degassing. GRA and DEX were obtained as gift samples from Wokhardt Ltd., Aurangabad and Cadila Ltd. Ahmedabad, respectively. Acetonitrile HPLC grade (Merck Ltd.), Triethylamine (Merck Ltd.) and o-phosphoric acid (Qualigens fine chemicals Ltd.) were purchased.
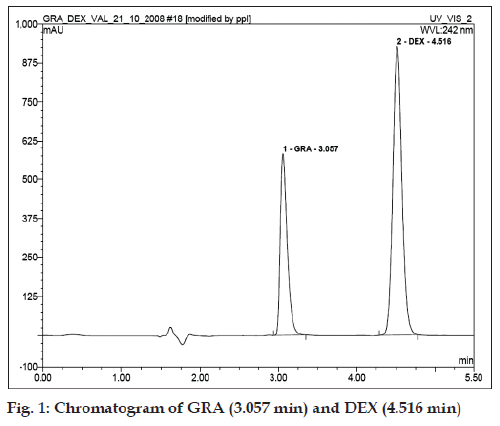
Different combinations of mobile phase were tried and a mobile phase consisting of acetonitrile:buffer (100 mM triethylamine adjusted to pH 3.0 with o-phosphoric acid) in ratio of 25:75 at flow rate of 2 ml/min was optimized which gave sharp peak with minimum tailing. Optimum wavelength for detection and quantification was 242 nm at which the best detector response was obtained for both drugs. The chromatographic conditions were optimized with run time of 5 min. Suitability of chromatographic system was monitored by calculating tailing/asymmetry factor and theoretical plates (Table 1).
| Parameters | GRA | DEX |
|---|---|---|
| Retention time (min) | 3.057 | 4.516 |
| Tailing/asymmetry factor | 1.29 | 1.10 |
| Theoretical plates | 6188 | 7216 |
| No. of data points | 5 | 5 |
| Linearity (μg/ml) | 25-400 | 25-400 |
| Correlation coefficient (r2) | 0.9999 | 0.9999 |
| Slope (m) | 0.14 | 0.313 |
| Intercept (c) | 0.729 | -0.161 |
| Recovery (%) | 101.28 | 100.02 |
| LOD (μg/ml) | 1.69 | 0.0966 |
| LOQ (μg/ml) | 5.125 | 0.2928 |
| Precision (RSD) | ||
| Intraday (n=3) | 1.5 | 0.4756 |
| Interday (n=3) | 0.1292 | 0.5292 |
| Repeatability (n=6) | 0.5115 | 0.2755 |
| Bench top stability (RSD; n=3) | 0.832 | 1.1 |
| Freeze/thaw stability (RSD; n=3) | 3.92 | 8.75 |
Table 1: Validation and system suitability Parameters for rp-hplc method
Standard stock solutions of 1 mg/ml of GRA and DEX were prepared separately using solvent mixture of ethanol:PEG 400:water (20:20:60). Aliquots 0.25, 0.5, 1, 2, and 4 ml of each standard solution of 1 mg/ml were transferred in a series of 10 ml calibrated volumetric flasks and volume was made up to mark with solvent mixture to give binary mixtures of various concentrations i.e. 25, 50, 100, 200 and 400 μg/ml of GRA and DEX each respectively. An aliquot (20 μl) was injected onto the column and chromatogram was recorded. A representative chromatogram is given in (fig. 1). Area under the curve was calculated and calibration curves were obtained by plotting them versus respective concentration. The equations of the regression lines obtained were, for GRA, y=0.140x+0.729 and For DEX, y=0.313x–0.161. Where y is area under curve and x is concentration.
The method was validated by studying various ICH parameters viz. linearity, accuracy, precision, limit of detection (LOD) and limit of quantification (LOQ). Also, bench top stability, freeze/thaw stability and system suitability parameters of the method were evaluated [12-13]. The linearity of the method was determined at five concentration levels ranging from 25, 50, 100, 200 and 400 μg/ml of GRA and DEX each respectively. The accuracy of the method was determined by recovery study at three levels 80, 100 and 120%. Each solution was injected in triplicate and the percentage recovery was calculated. Recovery was within range of 100 ± 2% which indicates accuracy of the method. The intra-day and inter-day precision study carried by estimating responses at three different concentrations of GRA and DEX (50, 100, 150 μg/ml each), three times on a same day and on three different days. Repeatability of method was estimated by 6 replicate injections of each standard stock solution. The results were reported in terms of percentage relative standard deviation (RSD) in Table 1. The value of %RSD of not more than 1.5% indicated that the developed method was precise. LOD and LOQ were calculated for GRA and DEX as per ICH guidelines.
Bench top stability of GRA and DEX was determined by analysis of 100 μg/ml each of binary standard solution which was kept at room temperature for 72 h. The freeze/thaw stability was studied by analysis of 100 μg/ml of binary standard solution exposed to three freeze/thaw cycles; each cycle consist of removing samples from freezer, thawing them to room temperature, keeping at room temperature for 4 h and re-freezing at -20º.
In present work, the chromatographic conditions were optimized to achieve best resolution and peak shapes for GRA and DEX. The developed RP-HPLC method was found to be accurate and precise with acceptable values of LOD and LOQ (Table 1). The drug solutions were found to be stable only during bench top stability studies. The newly reported RP-HPLC method was found to be simple, fast, accurate, precise and sensitive and thus, can be used for routine analysis of GRA and DEX in their pharmaceutical dosage forms.
Acknowledgments
Authors are grateful to Wockhardt Ltd., Aurangabad and Cadila Ltd., Ahmedabad for providing gift samples of GRA and DEX, respectively.
References
- Sweetman SC, Martindale: The Complete Drug Reference. 35th ed. London: Pharmaceutical Press; 2007, p. 1334,1526-602
- Dexamethasone, Granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. The Italian group for antiemetic research. N Engl J Med 1995;332:1-5.
- Dexamethasone alone or in combination with Ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. The Italian group for antiemetic research. N Eng J Med 2000;342:1554-9.
- Wiser W, Berger A. Practical management of chemotherapy-induced nausea and vomiting. Oncology 2000;19:637-45.
- Latreille J, Pater J, Johnston D. Use of dexamethasone and granisetron in the control of delayed emesis for patients who receive highly emetogenic chemotherapy. J Clin Oncology 1998;16:1174-8.
- Sanger GH, Dott CS. Pharmaceutical Compositions Containing Granisetron And Dexamethasone. United State Patent US 5929059; 1999.
- Gallego JM, Arroyo JP. Simultaneous determination of dexamethasone and trimethoprim by liquid chromatography. J Pharm Biomed Anal 2002;30:1255-61.
- Grippa E, Santini L, Castellano G. Simultaneous determination of hydrocortisone, dexamethasone, indomethacin, phenylbutazone and oxyphenbutazone in equine serum by high-performance liquid chromatography. J Chromatogr B 2000;738:17-25.
- Zivanovic L, Zecevic M, Markovic S, Petrovic S, Ivanovic I. Validation of liquid chromatographic method for analysis of lidocaine hydrochloride, dexamethasone acetate, calcium dobesilate, butylhydroxyanisol and degradation product hydroquinone in suppositories and ointment. J Chromatogr A 2005;1088:182-6.
- Pinguet F, Bressolle F, Martel P, Salabert D, Astre C. High-performance liquid chromatographic determination of granisetron in human plasma. J Chromatogr B 1996;675:99-105.
- Moffat AC, Osselton MD, Widdop B. Clarke’s Analysis of Drugs and poisons. Vol 2, 3rd ed. Great Britain: Pharmaceutical Press; 2004, p.887, 1263.
- ICH, Q2(R1), Harmonised Tripartite Guideline. Validation of analytical procedures: Text and methodology; Nov. 2005.
- Trissel LA. Trissels stability of compounded formulations. 3rd ed. Washington, DC: American Pharmacists Association; 2005. p. 199-200.