- *Corresponding Author:
- B. P. Srinivasan
Department of Quality Assurance, Delhi Institute of Pharmaceutical Sciences and Research (DIPSAR), Goverment of N. C. T. of Delhi, M. B. Road, Pushp Vihar, Sector-3, New Delhi-110 017 India
E-mail: bpsrinivasan@yahoo.com
| Date of Submission | 18 January 2010 |
| Date of Revision | 2 July 2010 |
| Date of Acceptance | 2 October 2010 |
| Indian J. Pharm. Sci., 2010, 72 (5): 667-671 |
Abstract
A simple, sensitive, precise and robust reverse-phase high-performance liquid chromatographic method for analysis of ivabradine hydrochloride in pharmaceutical formulations was developed and validated as per ICH guidelines. The separation was performed on SS Wakosil C18AR, 250Χ4.6 mm, 5 μm column with methanol:25 mM phosphate buffer (60:40 v/v), adjusted to pH 6.5 with orthophosphoric acid, added drop wise, as mobile phase. A well defined chromatographic peak of Ivabradine hydrochloride was exhibited with a retention time of 6.55±0.05 min and tailing factor of 1.14 at the flow rate of 0.8 ml/min and at ambient temperature, when monitored at 285 nm. The linear regression analysis data for calibration plots showed good linear relationship with R=0.9998 in the concentration range of 30-210 μg/ml. The method was validated for precision, recovery and robustness. Intra and Inter-day precision (% relative standard deviation) were always less than 2%. The method showed the mean % recovery of 99.00 and 98.55 % for Ivabrad and Inapure tablets, respectively. The proposed method has been successfully applied to the commercial tablets without any interference of excipients.
Keywords
Ivabradine, RP-HPLC, accuracy, precision, robustness, specificity.
Ivabradine hydrochloride, 3- [3-({ [(7S)-3,4- dimethoxybicyclo [4.2.0]octa-1,3,5-trien-7-yl] methyl}methylamino)propyl]-1,3,4,5-tetrahydro- 7,8-dimethoxy-2H-3-benzazepin-2-one hydrochloride is a new bradycardiac agent. Its activity is a result of a direct effect on the sinus node, reducing the slope of the spontaneous diastolic depolarization. It is a selective sinus node If inhibitor used in the treatment of angina pectoris in patients unable to take beta blockers [1-5]. Literature survey revealed that, analytical methods like LC with fluorescence detector and LC with tandem mass spectrometric detector for determination of ivabradine in biological samples were reported [6-9] . However, till date no assay procedure has been reported for the determination of this drug in bulk and pharmaceutical formulations. Hence there is a need to develop a simple assay procedure for the determination of this drug in tablet formulations. Owing to the wide spread use of HPLC in routine analysis, it is important that well validated HPLC method has to be developed for estimating ivabradine hydrochloride. The aim of this study is development of a simple, precise, rapid and accurate reverse phase HPLC method for estimation of ivabradine hydrochloride in pharmaceutical formulations as per ICH guidelines which is easily adaptable as a routine in quality testing laboratories in industry and academic institutes [10].
The pure sample of ivabradine hydrochloride used in the development of this analytical method was gifted by Ind-Swift Labs, Jammu, India. Methanol and water were of HPLC grade (Merck India Ltd., Mumbai, india). All other reagents were of AR grade and were purchased from Merck Chemicals, India. Commercially available Ivabrad and Inapure tablets, claimed to contain 7.5 mg and 5 mg ivabradine, respectively were procured from local Pharmacy.
An isocratic HPLC with two Waters 515 HPLC pump, Waters Inline Degasser AF, 717 plus Auto sampler, 2998 PDA Detector and RP C18 Column (SS Wakosil C18AR, 250×4.6 mm, packed with 5 μm particle size) was used. The HPLC system was equipped with Empower software. For HPLC, the mobile phase used was a mixture of methanol and phosphate buffer (pH 6.5) in the ratio of 60:40 v/v; it was filtered before use through a 0.22 μm membrane filter in a Millipore filtration assembly and pumped from the respective solvent reservoirs to the column at a flow rate of 0.8 ml/min. The run time was set at 10 min and column was maintained at ambient temperature. The volume of injection is 10 μl. Prior to the injection of drug solutions, the column was equilibrated for at least 30 min with the mobile phase flowing through the system. The eluent was monitored at 285 nm and data acquired was stored and analyzed with the software.
A standard stock solution of ivabradine hydrochloride (1 mg/ml) was prepared in HPLC grade water. To study the linearity range of the drug, serial dilutions were made from standard stock solution in the range of 30-210 μg/ml with HPLC grade water. Each of these drug solutions (10 μl) was injected, into the HPLC system to obtain the chromatogram. From these, the area under the peak of the drug was noted and the data of peak area plotted against the corresponding concentrations were treated by linear least–square regression analysis.
For intraday assay precision, the sample injection and measurement of peak areas were carried out 3 times with in a day by using three different concentrations of 90, 120 and 150 μg/ml and were expressed in terms of % RSD. For interday assay precision, the sample injection and measurement of peak areas were carried out 3 times for 3 days by using the same concentrations as that of intra day assay precision, and were expressed in terms of % RSD. By introducing slight changes in organic phase of mobile phase composition (±2 %), pH (±0.2), flow rate (±0.1 ml/min) and the wavelength (±1 nm), the effects of the results were examined.
Recovery studies was confirmed by standard addition method and were carried out by applying the method to pre analyzed samples to which known amount of standard drug corresponding to 12, 24 and 36 μg/ ml was spiked. At each level of the amount, three determinations were performed. This was done to check for the recovery of the drug at different levels in the formulations.
Lactose, talc, starch, magnesium stearate and microcrystalline cellulose were added as excipients to three different concentrations of 90, 120 and 150 μg/ ml of standard ivabradine hydrochloride solutions and analyzed by the proposed method. Paired t-Test was applied to compare the results.
After equilibration of column with mobile phase, the chromatograms were recorded and peak response i.e., peak area was measured for a concentration of 120 μg/ml. Other parameters like tailing factor, capacity factor, number of theoretical plates were calculated and compared with standard values.
Twenty tablets containing Ivabradine hydrochloride as the active ingredient were weighed and their average weight was calculated. Tablets were then crushed and powdered in a mortar and pestle. A portion of the powder equivalent to 25 mg of the drug (on the label claim basis) was transferred to a 25 ml volumetric flask. The drug was dissolved in by adding 10 ml of HPLC grade water to the flask with constant stirring for 15-20 min on a magnetic stirrer. The volume was made up to the mark with HPLC grade water to prepare stock solution of 1 mg/ml. The resulting solution was centrifuged at 3000 rpm for 5 min and then filtered through 0.45 μ nylon syringe filter and the filtrate was further diluted to give the concentration of 120 μg/ml and then analyzed by the proposed method. The analysis was carried out for 6 times and the mean peak area of six such determinations was calculated. The same procedure was used to estimate the content of the drug in both the brands Ivabrad 7.5 mg and Inapure 5 mg, respectively.
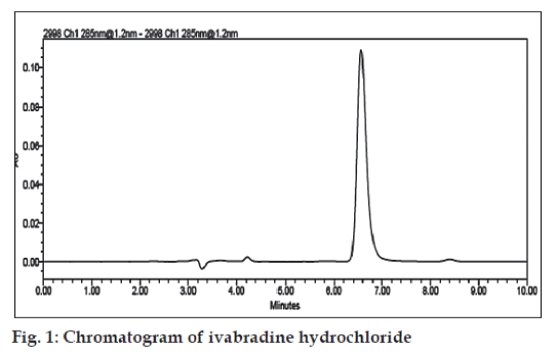
To achieve sharp peak under isocratic conditions, mixtures of methanol and phosphate buffer in different combinations were tried as mobile phase on a C18 stationary phase. A binary mixture of methanol and phosphate buffer (pH 6.5) in 60:40 v/v proportions was proved to be the most suitable of all combinations since chromatographic peak was better defined and free from tailing with this system. Under the above-mentioned chromatographic conditions, the retention time of 6.55±0.05 min (fig.1) was obtained for ivabradine hydrochloride.
The linear regression data for the calibration curves (n=4) showed a good linear relationship over concentration range 30-210 μg/ml with respect to the peak area as depicted in Table 1. The intraday precision and interday precision were expressed in terms of % RSD and found below 0.86 and 0.53, respectively. The results of intra and interday variation of Ivabradine hydrochloride at three different concentrations levels of 90, 120 and 150 μg/ml are depicted in Table 2.
| Parameter | Value |
|---|---|
| Linearity parameters | |
| Linearity range (n=4) | 30-210 (μg/ml) |
| Regression coefficient (R2) | 0.9998 |
| Correlation coefficient (R) | 0.9998 |
| Slope | 13588 |
| Intercept | 24483 |
| System suitability Parameters | |
| Mean AUC | 1586275 |
| Retention time | 6.56 |
| Tailing factor | 1.14 |
| Capacity factor | 1.16 |
| No. of Theoretical plates | 2599.6 |
| Precision | |
| Repeatability (% RSD) | 0.48 |
| Intermediate precision (% RSD) | 0.44 |
| % Assay±SD | |
| Ivabrad (7.5 mg) | 99.38%±0.68 |
| Inapure (5 mg) | 99.29±0.47 |
AUC - Area under curve; RSD - Relative standard deviation; SD - Standard deviation.
Table 1: Summary of validation parameters
| Precision | Conc. range (μg/ml) | Conc. found (μg/ml) | SD (n=3) | % RSD (n=3) | ±CI |
|---|---|---|---|---|---|
| Intraday | 90 | 88.89 | 0.76 | 0.86 | 0.86 |
| Variation | 120 | 118.97 | 0.33 | 0.28 | 0.37 |
| (Repeatability) | 150 | 150.94 | 0.46 | 0.30 | 0.52 |
| Interday | 90 | 89.14 | 0.47 | 0.53 | 0.53 |
| Variation | 120 | 119.24 | 0.49 | 0.41 | 0.55 |
| (Intermediate | 150 | 150.49 | 0.58 | 0.38 | 0.65 |
| Precision) |
Conc.- Concentration; SD - Standard deviation; RSD - Relative standard deviation; CI - Confidence interval.
Table 2: Intraday variation & interday variation
The method was found to be robust for slight changes in the organic phase of mobile phase composition (±2 %), pH (±0.2), flow rate (±0.1 ml/min) and the wavelength (±1 nm) during the development of the method. The proposed method when used for extraction and subsequent estimation of ivabradine hydrochloride from pharmaceutical dosage forms after spiking with 12, 24 and 36 μg/ml of additional drug afforded mean % recovery of 99.00 and 98.55 for Ivabrad and Inapure tablets respectively as depicted in Table 3.
| Brand Name | Amount spiked (μg/ml) | Total conc. (μg/ml) | Conc. found (μg/ml) | % recovery±SD (n=3) | ±CI | %REE |
|---|---|---|---|---|---|---|
| IVABRAD | 12 | 132 | 131.08 | 99.30±0.44 | 0.50 | -0.70 |
| 24 | 144 | 142.60 | 99.03±0.29 | 0.33 | -0.98 | |
| 36 | 156 | 153.96 | 98.69±0.31 | 0.35 | -1.33 | |
| INAPURE | 12 | 132 | 130.71 | 99.02±0.18 | 0.20 | -0.99 |
| 24 | 144 | 141.52 | 98.27±0.43 | 0.49 | -1.76 | |
| 36 | 156 | 153.47 | 98.38±0.07 | 0.08 | -1.65 |
Conc. - Concentration; SD - Standard deviation; CI - Confidence interval; REE - Relative error estimate.
Table 3: Accuracy (standard addition method)
The absence of interfering peaks in the chromatogram suggests that the tablet excipients do not interfere with the estimation of the drug by the proposed HPLC method. Paired t-Test was applied to compare the results as depicted in Table 4. The system suitability parameters when compared to the standard values are within the limit. A single chromatogram was observed in the chromatogram of the drug samples from tablets. There was no interference from the excipients commonly present in the tablets. The drug content was found to be 99.38%±0.68 with a % RSD of 0.57 and 99.29±0.47 with a % RSD of 0.39 for Ivabrad (7.5 mg) and Inapure (5 mg) tablets, respectively.
| Conc. range (μg/ml) | Conc. found (μg/ml) | SD (n=3) | t-value |
|---|---|---|---|
| 90 | 88.89 | 0.76 | 0.77 |
| 90* | 89.04 | 0.74 | |
| 120 | 118.97 | 0.33 | 0.72 |
| 120* | 118.99 | 0.20 | |
| 150 | 150.92 | 0.46 | 0.83 |
| 150* | 151.00 | 0.61 |
90, 120 and 150 are unspiked std. drug while 90*, 120* and 150* are spiked with excipients; Conc. Is concentration; SD is standard deviation; The Critical t-value for two tail distribution student paired t-test having degree of freedom – 2 and level of confidence of 95 % is 4.30.
Table 4: Specificity
The developed RP-HPLC method provides a convenient and efficient method for the estimation of Ivabradine hydrochloride in dosage form. There was no interference from the excipients used in the tablet formulation and hence the method is suitable for analysis of tablets. The results of validation showed that the proposed method is simple, linear, precise, accurate and selective and is employed in routine assay of ivabradine hydrochloride in tablets.
Acknowledgements
The authors are thankful to Ind-Swift Labs, Jammu, India for providing gift sample of pure ivabradine hydrochloride.
References
- Parfitt K. Martindale: The Complete Drug Reference. 35th ed. London: Pharmaceutical Press; 2007. p. 1185.
- O’Neil MJ. The Merck Index: an Encyclopedia of Chemicals, Drugs and Biologicals. 14th ed. Whitehouse Station: Merck Research Laboratories, Division of Merck and Co., Inc.; 2006. p. 907.
- Ivabradine hydrochloride: Scientific discussion. URL:http://www.emea. europa.eu/humandocs/PDFs/EPAR/corlentor/32123005en6.pdf, [accessed on 2009 feb 03].
- Summary of Product Characteristics. URL:http://drugs- about.com/drugs/ procoralan/procoralan.pdf. [accessed on 2009 feb 27].
- Summary of Product Characteristics. URL:http://www.emea.europa. eu/humandocs/PDFs/EPAR/procoralan/H-597-PI-en.pdf. [accessed on 2009 feb 27].
- François-Bouchard M, Simonin G, Bossant, Boursier-Neyret C. Simultaneous determination of ivabradine and its metabolites in
- human plasma by liquid chromatography--tandem mass spectrometry. J Chromatogr B Biomed SciAppl 2000;745:261-9.
- Klippert P, Jeanniot JP, Polvé S, Lefèvre C, Merdjan H. Determination of ivabradine and its N-demethylated metabolite in human plasma and urine, and in rat and dog plasma by a validated high-performance liquid chromatographic method with fluorescence detection. J Chromatogr B Biomed SciAppl 1998;719:125-33.
- Prasad UK, Gray D, Purcell H. Review of the If Selective Channel Inhibitor Ivabradine in the Treatment of Chronic Stable Angina. AdvTher 2008;26:127-37.
- Duffull SB, Chabaud S, Nony P, Laveille C, Girard P, Aarons L. A
- Pharmacokinetic simulation model for ivabradine in healthy volunteer.Eur J Pharm Sci 2000;10:285-94.
- ICH, Q2(R1): Validation of Analytical Procedures: Text and Methodology, International Conference on Harmonization, Geneva, November 2005. http://www.ich.org/LOB/media/MEDIA417.pdf. [accessed on 2009 feb 27].