- *Corresponding Author:
- N. Mallikarjuna Rao
Department of Pharmaceutical Sciences, Jawaharlal Nehru Technological University, Kakinada, India
E-mail: mallimpharmmba@gmail.com
| Date of Submission | 28 May 2016 |
| Date of Revision | 13 August 2016 |
| Date of Acceptance | 18 August 2016 |
| Indian J Pharm Sci 2016; 78(4):492-497 |
This is an open access article distributed under terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows other the remix, tweak, and build up to the non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
A simple, accurate, selective and stability-indicating RP-HPLC method was developed for the simultaneous estimation of pseudoephedrine, ambroxol and desloratadine in bulk and their tablet formulations. Effective chromatographic separations were achieved on a Hypersil BDS C8, 250×4.6 mm, 5 μ column with reverse phase elution. The mobile phase composed of 0.01 M potassium dihydrogen phosphate and acetonitrile in the ratio of 50:50 v/v at a flow rate of 1.0 ml/min. The detection was carried out at 220 nm. The retention times were 2.2 min for pseudoephedrine, 3.4 min for ambroxol and 5.5 min for desloratadine. The linearity ranges for pseudoephedrine, ambroxol and desloratadine were 15 to 90 μg/ml, 30 to 180 μg/ml and 2.5 to 15 μg/ml, respectively with correlation coefficient 0.999. The developed method was validated statistically with respect to linearity, range, precision, accuracy, specificity, robustness, ruggedness, detection and quantification limits and also subjected to stress conditions like acidic and alkaline hydrolysis, oxidation, photolysis and thermal degradation. The method was accurate, precise, specific, rapid and found to be suitable for the analysis of commercial samples.
Keywords
Stability-indicating, pseudoephedrine, ambroxol, desloratadine, RP-HPLC
Pseudoephedrine hydrochloride (PSD) is (1S,2S)- 2-methylamino-1-phenylpropan-1-ol hydrochloride and official in European Pharmacopoeia and U.S. Pharmacopoeia. Ambroxol hydrochloride (AMB) is trans-4-[(2-amino-3,5-dibromobenzyl)amino] cyclohexanol hydrochloride and official in British Pharmacopoeia. Desloratadine (DLT) is 8-chloro- 6,11-dihydro-11-(4-piperdinylidene)-5H-benzo[5,6] cyclohepta[1,2-b] pyridine is non pharmacopeial drug. Figure 1 shows chemical structures of three analytes. The three drug combination in the ratio of 30:60:5 mg PSD, AMB and DLT, respectively in tablet dosage form is available under the brand name Nucope-AD. A few methods have been reported individually or in combination with other drugs including simultaneous estimation of AMB and loratadine by spectrophotometric method [1], high performance liquid chromatographic (HPLC) method [2,3], simultaneous estimation of AMB and DLT by spectrophotometric method [4-6], HPLC [7,8], HPTLC [9]. Since no methods were reported for the simultaneous estimation of PSD, AMB and DLT in bulk and pharmaceutical dosage forms, an attempt was made to develop a simple, accurate, precise and rugged method was developed for the estimation of these drugs simultaneously in bulk and tablet formulations.
Materials and Methods
HPLC grade acetonitrile, potassium dihydrogen phosphate AR Grade and potassium hydroxide AR grade were purchased from SD Fine-Chem, Mumbai, India were used in the study. Reference samples were obtained from M/s. Richer Pharmaceuticals, Hyderabad, India.
Chromatographic conditions
A HPLC system equipped with Waters e2695 separation module with HPLC instrument provided with photo diode array (PDA) detector, autosampler with Empower 2 software from Waters corporation, Milford, USA was employed in the study. The chromatographic separations were achieved on a Hypersil BDS C8, 250×4.6 mm, 5 μ column.
Preparation of mobile phase
Weighed and dissolved 1.36 g of potassium dihydrogen phosphate in 1000 ml of water (adjusted pH to 6.5±0.05 with dilute potassium hydroxide) and acetonitrile in the ratio of 50:50 v/v was filtered through 0.22 μ membrane filter and degassed. Mobile phase was used as diluent. The mobile phase was filtered and sonicated before use. The flow rate of the mobile phase was maintained at 1.0 ml/min. The column temperature was maintained at 30° and the detection of the drug was carried out at 220 nm.
Preparation of standard solution
Weighed accurately 30.0 mg of PSD, 60.0 mg of AMB and 5.0 mg of DLT on a Sartorius semi-micro balance model-CPA225D and transferred in to a 50 ml volumetric flask. The solution was sonicated and the resulting solution was diluted with the mobile phase to get a working standard solution containing 600 μg/ml PSD, 1200 μg/ml AMB and 100 μg/ml DLT. Working solutions were prepared by diluting the working standard solution in the concentration ranges 15-90 μg/ml, 30-180 μg/ml, and 2.5-15.0 μg/ml for PSD, AMB and DLT, respectively. Each concentration was injected thrice under described chromatographic conditions. The calibration curve constructed peak area against concentration.
Sample preparation
Weighed accurately previously weighed and crushed 20 tablet powder equivalent to one tablet weight containing 30 mg of PSD, 60 mg of AMB and 5 mg of DLT. This powder was transferred to a 50 ml volumetric flask, and volume is made up to the mark in mobile phase, sonicated to dissolve and filtered through Whatman filter paper. It was further diluted 10 ml of the stock solution to 100 ml with mobile phase.
Forced degradation and stability-indicating tests [10]
Weighed accurately 30.0 mg of PSD, 60.0 mg of AMB and 5.0 mg of DLT, and transferred to 50 ml volumetric flask and dissolved in mobile phase with sonication and the resulting solution was diluted with the mobile phase to get a working standard solution containing 600 μg/ml PSD, 1200 μg/ml AMB and 100 μg/ml DLT.
To determine acid degradation, 10 ml of 1 N HCl was added to 10 ml of stock solution, kept at 80° for about 6 h in a water bath, cooled volume was made up to 100 ml with the mobile phase and filtered through 0.22 μ membrane filter.
To determine alkali-induced degradation, 10 ml of 1 N NaOH was added to 10 ml of stock solution, heated at 80° at 6 h in a water bath, allowed to cool, volume made up to 100 ml with mobile phase and filtered through a 0.22 μ membrane filter.
To determine oxidative degradation 5 ml of 3% H2O2 was added to 10 ml of stock solution, heated at 80° for 3 h in a water bath, allowed to cool, volume made up to 100 ml with mobile phase and filtered through a 0.22 μ membrane filter.
Thermal degradation was determined by keeping 10 ml of stock solution at 80° for 10 days, allowed to cool, volume made up to 100 ml with mobile phase and filtered through a 0.22 μ membrane filter.
Results and Discussion
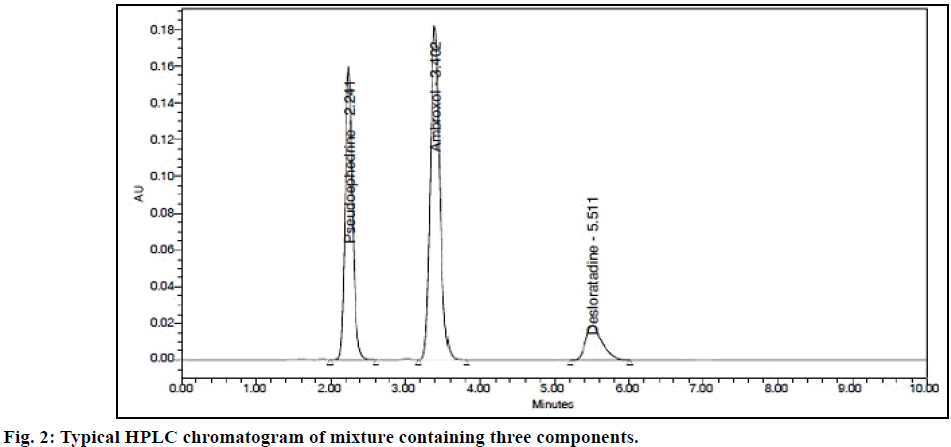
Method validation was performed following ICH Q2 guideline specifications [11]. System suitability is an integral part of the method validation and performed to evaluate the parameters like tailing factor, theoretical plates, resolution and %RSD for replicate injections. The results were within the limits and were presented in Table 1 while the Figure 2 displayed the system suitability chromatogram.
| Parameter | Results | ||
|---|---|---|---|
| Pseudoephedrine | Ambroxol | Desloratadine | |
| % RSD of peak area | 0.17 | 0.32 | 049 |
| % RSD of retention time | 0.19 | 0.19 | 0.13 |
| Tailing factor (T) | 1.15 | 1.16 | 1.38 |
| Theoreticalplate(N) | 3239 | 3278 | 3116 |
| Resolution (R) | - | 5.35 | 6.48 |
Table 1: System Suitability Results
In the blank chromatogram, there were no peaks observed at the retention times of PSD, AMB and DLT, and also the degradation studies showed that there was no interference with degradants, purity angle was less than the purity threshold for the sample solution indicating that the method is specific. To determine the accuracy of the proposed method, recovery experiments were conducted; known concentration of pure drug was spiked at three different levels, 50, 100 and 150% and was calculated. Accuracy was calculated as the percentage of recovery and the results were tabulated in Table 2.
| Parameter | Amountadded(µg) | Amount recovered(µg) | % recovery | Mean % recovery |
|---|---|---|---|---|
| Pseudoephedrine | ||||
| 50% level | 30.0 | 29.79 | 99.31 | 99.31 |
| 100%level | 60.0 | 59.89 | 99.81 | 99.81 |
| 150%level | 90.0 | 89.54 | 99.49 | 99.49 |
| Ambroxol | ||||
| 50% level | 60.0 | 59.5 | 99.17 | 99.17 |
| 100%level | 120.0 | 120.42 | 100.35 | 100.35 |
| 150%level | 180.0 | 180.18 | 100.1 | 100.1 |
| Desloratadine | ||||
| 50% level | 5.0 | 4.94 | 98.71 | 98.71 |
| 100% level | 10.0 | 10.005 | 100.05 | 100.05 |
| 150% level | 15.0 | 15.04 | 100.27 | 100.27 |
Table 2: Accuracy Data
The precision was evaluated at three levels, repeatability, reproducibility and intermediate precision each level of precision was investigated by six replicate injections of concentrations 120, 100 and 10 μg/ml PSD, AMB and DLT, respectively. The result of precision was expressed as percentage relative standard deviation (% RSD) and was tabulated in Table 3.
| Parameter | Pseudoephedrine | Ambroxol | Desloratadine |
|---|---|---|---|
| Repeatability | |||
| RSD of Retention time | 0.19 | 0.19 | 0.13 |
| RSD of Peak Area | 0.17 | 0.32 | 0.49 |
| Reproducibility | |||
| RSD of Retention time | 0.13 | 0.11 | 0.11 |
| RSD of Peak Area | 0.59 | 0.56 | 0.49 |
| Intermediate Precision | |||
| RSD of Retention time | 0.20 | 0.13 | 0.09 |
| RSD of Peak Area | 0.17 | 0.29 | 0.49 |
Table 3: Precision Studies
The linearity of the measurement was evaluated by analyzing different concentrations (25% to 150%) of the standard solutions of PSD, AMB and DLT. Calibration curve was constructed by plotting concentration against mean peak area and the regression equation was computed. The summary of the parameters is shown in Table 4. Estimation of limit of detection (LOD) and limit of quantification (LOQ) considered the acceptable signal-to-noise ratios 3:1 and 10:1, respectively. LOD and LOQ determined were 0.3235 μg/ml and 0.98 μg/ ml for PSD, 1.2344 and 3.741 μg/ml for AMB and 0.1575 μg/ml and 0.4772 μg/ml for DLT, respectively.
| Parameter | Pseudoephedrine | Ambroxol | Desloratadine |
|---|---|---|---|
| Linearity range(µg/ml) | 15 to 90 | 30 to 180 | 2.5 to 15 |
| Correlation co-efficient | 0.999 | 0.999 | 0.999 |
| Slope | 19380 | 13786 | 28705 |
| Y-intercept | -12178 | -16085 | -3795 |
Table 4: Regression Equation Parameters
The robustness of the method was evaluated by subjecting the 100% test concentration to small but deliberate changes in the chromatographic conditions like, flow rate, mobile phase composition and column temperature and the results were shown in Table 5. The ruggedness of the proposed method was determined by analysing the same sample using different columns, different analysts, and on different instruments.
| Parameter | Variation | Chromatographic Conditions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retention time | Area | Theoretical Plates | Tailing Factor | ||||||||||
| PSD | AMB | DLT | PSD | AMB | DLT | PSD | AMB | DLT | PSD | AMB | DLT | ||
| Flow change | 0.9 ml/min | 2.553 | 3.868 | 6.274 | 1327568 | 1891871 | 324566 | 3474 | 3601 | 3230 | 1.18 | 1.17 | 1.39 |
| 1 ml/min | 2.241 | 3.402 | 5.511 | 1140569 | 1621587 | 281024 | 3239 | 3278 | 3116 | 1.15 | 1.16 | 1.38 | |
| 1.1 ml/min | 2.005 | 3.038 | 4.908 | 1035509 | 1474678 | 254981 | 3982 | 2991 | 2897 | 1.14 | 1.15 | 1.38 | |
| Temperature | 25° | 2.257 | 3.512 | 6.450 | 1158053 | 1647565 | 284220 | 2212 | 3102 | 2527 | 1.17 | 1.16 | 1.41 |
| 30° | 2.241 | 3.402 | 5.511 | 1140569 | 1621587 | 281024 | 3239 | 3278 | 3116 | 1.15 | 1.16 | 1.38 | |
| 35° | 2.029 | 3.299 | 4.792 | 1161505 | 1654521 | 283817 | 2239 | 3525 | 4172 | 1.16 | 1.15 | 1.29 | |
| Wavelength | 218 nm | 2.227 | 3.496 | 5.55 | 1163102 | 1659076 | 283013 | 2223 | 3554 | 4154 | 1.16 | 1.15 | 1.28 |
| 220 nm | 2.241 | 3.402 | 5.511 | 1140569 | 1621587 | 281024 | 3239 | 3278 | 3116 | 1.15 | 1.16 | 1.38 | |
| 222 nm | 2.223 | 3.495 | 5.448 | 1161021 | 1654354 | 284565 | 2246 | 3564 | 4176 | 1.15 | 1.15 | 1.28 | |
Table 5: Robustness Study
The stability studies of the standard solution were conducted at intervals of 24 h and 48 h at room temperature. There were no significant changes observed in system suitability parameters like theoretical plates, tailing factor, retention time and resolution. Hence the standard solution was found to be stable up to 48h on the bench top. The stability study of the mobile phase was conducted at intervals of 24 h and 48 h at room temperature. There were no significant changes observed in system suitability parameters like peak area, theoretical plates, tailing factor, retention time and resolution. Hence the mobile phase is stable up to 48 h at room temperature. The proposed method was applied successfully for the analysis of PSD, AMB and DLT in tablet dosage forms, satisfactory results were obtained and the results were summarized in Table 6.
| Drug | Labeled amount (mg/tab) | Amount found (mg/tab) | % of Assay |
|---|---|---|---|
| Pseudoephedrine | 30 | 29.76 | 99.21 |
| Ambroxol | 60 | 59.522 | 99.20 |
| Desloratadine | 5 | 4.969 | 99.37 |
Table 6: Assay Results
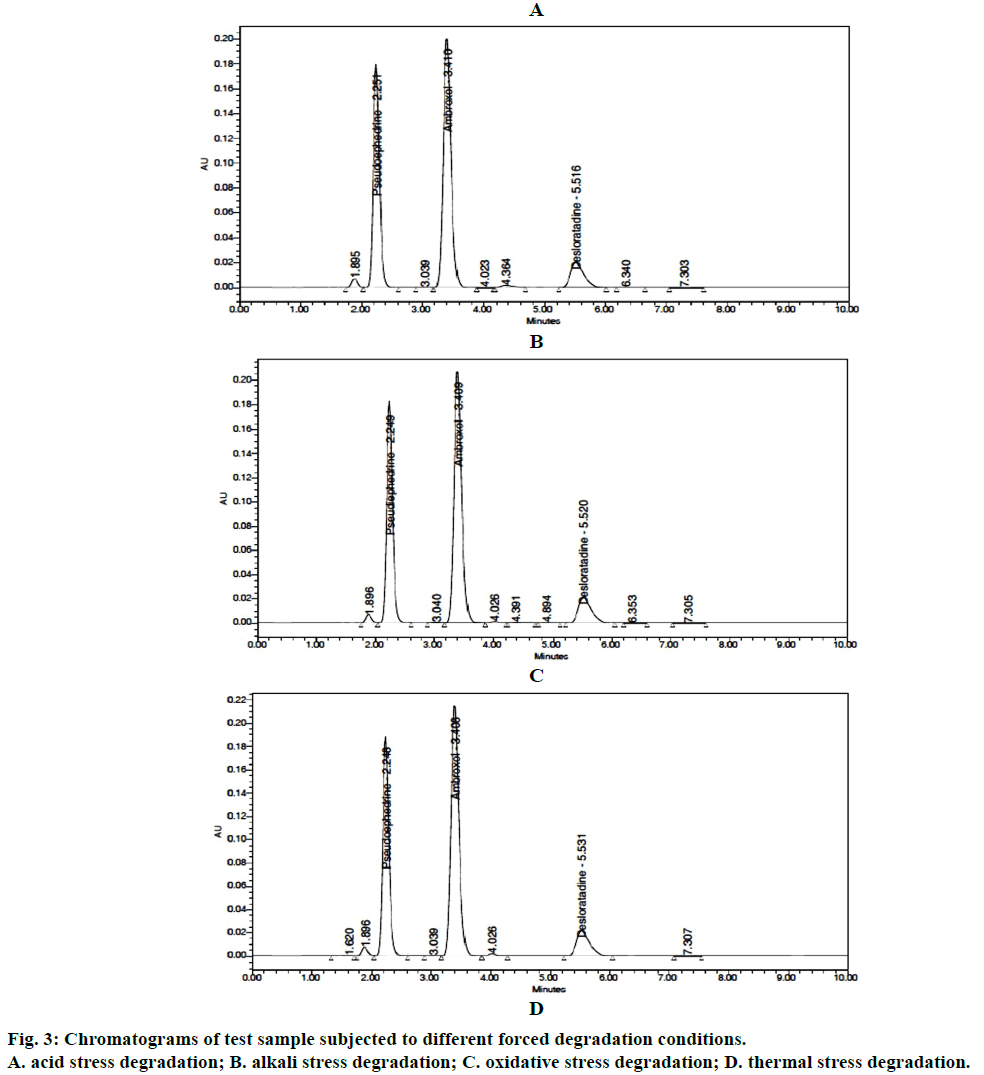
Since no interference of blank and degradants, the HPLC results showed that the three active ingredients PSD, AMB and DLT. Purity angle was less than the purity threshold and hence the proposed method was the specific and revealed its stability-indicating power. The results were summarized in Table 7. Figure 3 displays chromatograms of samples subjected to different stress degradations.
| Stress condition | % Assay of active ingredient | Purity angle | Purity threshold | ||
|---|---|---|---|---|---|
| PSD | AMB | DLT | |||
| Acid degradation (1N HCl for 6 h) | 88.1 | 84.7 | 89.4 | 0.829 | 1.071 |
| Alkali degradation (1M NaoH for 6 h) | 89.3 | 89.0 | 87.1 | 0.757 | 0.989 |
| Thermal degradation (80° for 10 days) | 90.9 | 85.2 | 88.8 | 0.981 | 1.082 |
| Photolytic (UV 200 Watt hours) | 90.0 | 89.1 | 88.8 | 0.763 | 0.921 |
Table 7: Degradation Studies
A simple, specific and reliable reverse phase HPLCDAD method was developed for the estimation of PSD, AMB and DLT in their pharmaceutical formulation. The three compounds were subjected to forced degradation applying several stress conditions. The proposed method successfully separated all the three compounds with degradants, the active contents were estimated. The proposed method is specific and stability-indicating. Hence the developed method can be adapted to regular quality control analysis.
Acknowledgements
The authors thank M/s Richer Pharmaceuticals, Hyderabad for providing standards and lab facilities. The authors also thank Department of Pharmaceutical Analysis, Jawaharlal Nehru Technological University, Kakinada, Department of Pharmaceutical Analysis and Quality Assurance, Andhra University, Vishakhapatnam, India for their encouragement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ilangovan P, Chebrolu SNK, Asha P. Simultaneous estimation of ambroxolhydrochloride and loratadine in tablet dosage form by using UV spectrophotometric method. Int J Pharma Bio Sci 2011;2:338-44.
- Krishnaveni N, Meyyanathan SN, Rajinikanth BR, Suresh R, Jeyaprakash MR, Arunadevi SB, et al. A RP-HPLC method for simultaneous estimation of ambroxol hydrochloride and loratadine in pharmaceutical formulation. Res J Pharm Tech 2008;1:366-9.
- Sateesh PL, Pavithra V, Bishupada B, Nagarjun Reddy G. Method development and validation of ambroxol hydrochloride and loratadine by RP-HPLC in tablet dosage form. Int J Pharm Sci 2013;3:370-4.
- Sharma E, Nehal JS. Development and validation of first order derivative spectrophotometric method for simultaneous estimation of ambroxol hydrochloride and desloratadine hydrochloride in combined tablet dosage form. Int J Pharm Res Bio Sci 2012;1:155-66.
- Sharma EA, Shah NJ. Development and validation of dual wavelength UV spectrophotometric method for simultaneous estimation of ambroxol and hydrochloride and desloratadine hydrochloride in their combined tablet dosage form. Int J Pharm Sci Res 2012;3:2584-9.
- Sharma EA, Shah NJ. Development and validation of Q-absorbance ratio method for simultaneous estimation of ambroxol and desloratadine in combined tablet dosage form. Int J Pharm ChemSci 2012;1:773-8.
- Moses PF, Prathap S, Raja A, Banji D. Analytical method development and validation for simultaneous estimation of ambroxol and desloratadine in its pharmaceutical dosage forms by RP-HPLC. World J Pharm PharmSci 2013;2:6246-62.
- Babu G, Shirin K, Rajapandi R. RP-HPLC Method for the simultaneous estimation of ambroxol hydrochloride and desloratadine in pure and dosage form. Der Pharmacia Lett 2013;5:391-6.
- Sharma EA, Shah NJ. Development and validation of HPTLC method for simultaneous estimation of ambroxol hydrochloride and desloratadine hydrochloride in their combined tablet dosage form. Int Res J Pharm 2012;3:305-8.
- MallikarjunaRao N, GowriSankar D. Development and validation of stability-indicating HPLC-DAD method for simultaneous determination of emtricitabine, elvetegravir, cobicistat and tenofovir in their tablet dosage forms. Indian J Pharm Ed Res 2016;50:205-11.
- ICH, Q2(R1), Harmonised Tripartite Guidelines, Validation of Analytical Procedures, Text and Methodology, London; 2009.