- *Corresponding Author:
- E. Robins
Direction Technique, Valois SAS, Route des Falaises, 27100 Le Vaudreuil, France
E-mail: emmanuelle.robins@valois.com
| 26 October, 2007 | |
| Indian J Pharm Sci, 2007, 69 (5): 724-726 |
Abstract
A hydrofluoroalkane (HFA) based salbutamol formulation was developed so that 120 µg of salbutamol sulphate per shot would exit the valve over 200 doses. This formulation was designed to be physically stable upon visual observation and it would give reproducible aerosol performances [1-3]. The stability of the resulting metered dose inhaler was also evaluated under accelerated storage conditions (40°/75%RH) up to 6 mo on a small scale and up to 3 mo on a larger scale batch. The aim was also to match the innovative Ventolin® HFA product in terms of in vitro performances.
Materials and Methods
Excipients selected for evaluation were polyethylene glycol 300 (at levels of 0.15 to 5% w/w, supplied by Fluka, France) and ethanol (at levels of 0.2 to 2% w/w, supplied by Prolabo, France). The pressurised metered dose inhalers (pMDIs) were prepared on a laboratory scale by a two step filling process: introduction of the drug in each canister, crimping of the metering valve onto a standard anodised aluminium canister (supplied by Presspart, Great- Britain) and filling of propellant through the valve. Other pMDIs were prepared as a one step filling process by introducing the HFA salbutamol suspension formulation through a metering valve previously crimped onto a canister.
Formulations were evaluated with regard to drug stability upon storage, delivered dose uniformity (DDU) over 10 actuations at 28.3 l/min. Combinations of different formulations together with different valve configurations were tested with 0.5 mm outlet orifice diameter actuators.
Results and Discussion
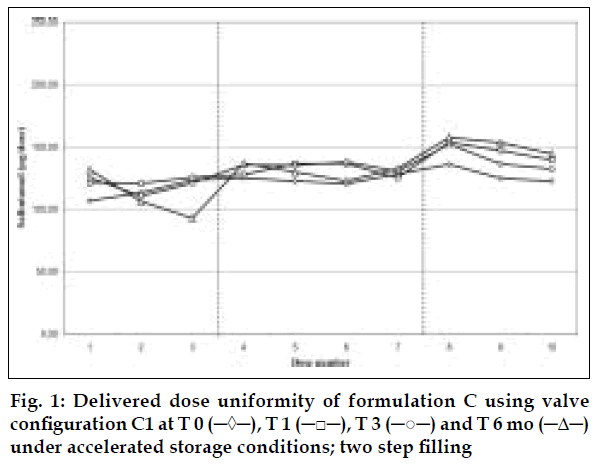
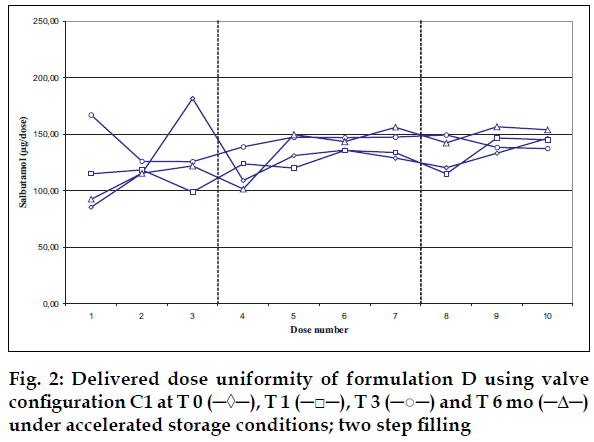
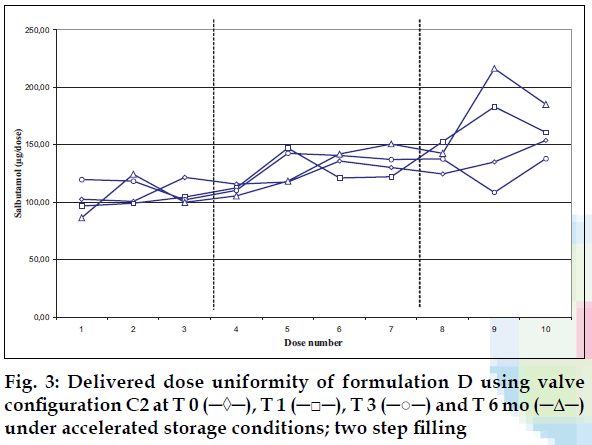
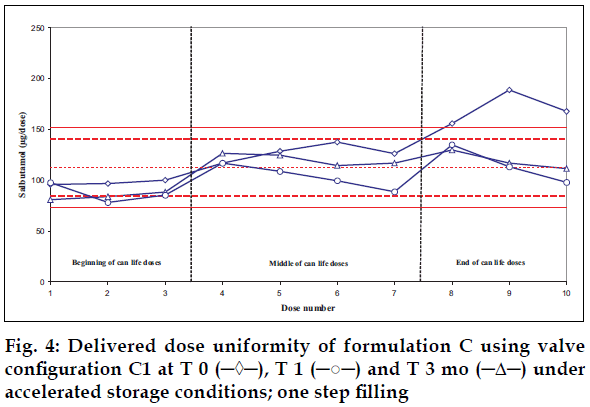
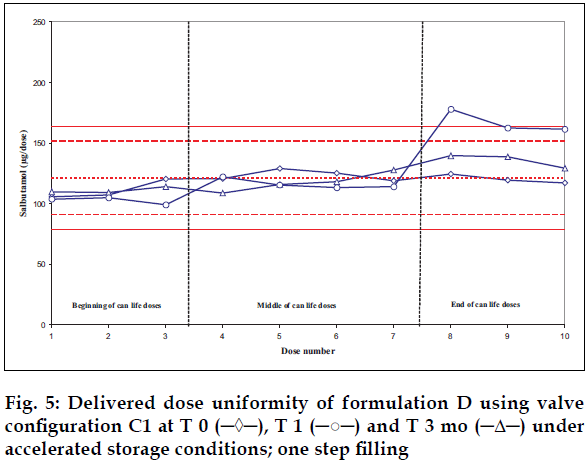
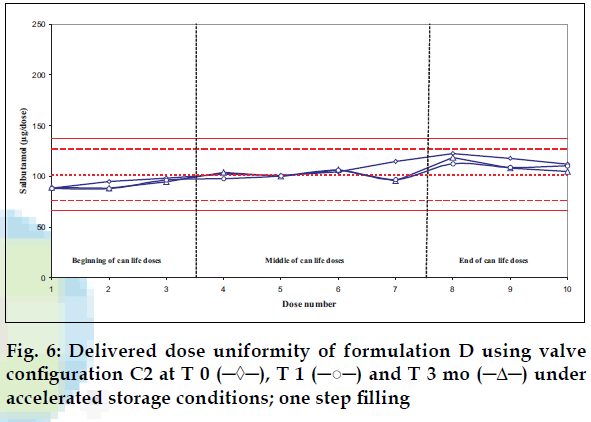
Delivered dose uniformity results obtained on a laboratory scale (two step filling process, n= 3) can be found in figs. 1 to 3. Delivered dose uniformity results obtained on a larger scale (one step filling process, n= 10) can be found in figs. 4 to 6.
A mixture of HFA propellants closely matching the density of the micronised salbutamol has been used to improve the suspension stability. The formulation is readily re-dispersible, and upon re-dispersion, does not flocculate quickly and so prevents irregular dosing of the drug. However, addition of excipients alters the inter particulates forces as well as interaction between the formulation and the valve components. Changes in DDU performances are then seen.
Valois offers a fully developed salbutamol HFA formulation. It has good DDU stability for at least 6 mo under 40°/75% RH storage conditions, together with the selected appropriate valve, canister and actuator.
Acknowledgements
The authors would like to thank Presspart for supplying the canisters.
References
- Purewal T. Formulation of metered dose inhalers: Metered dose inhalers technology. Interpharm Press: Buffalo Grove; 1998. p .1-8.
- Atkins P, Baker PN, Mathiesen D. The design and development of inhalation drug delivery systems: Pharmaceutical Inhalation Aerosol Technology. Marcel Dekker: New York; 1992. p. 168-71.
- Vervaet C, Byron PR. Drug-surfactant-propellant interactions in HFAformulations. Int J Pharm 1999;186:13-30.