- Corresponding Author:
- Mala D. Menon*
Department of Pharmaceutics, Bombay College of Pharmacy, Kalina, Santacruz (E), Mumbai-400 098
E-mail: maladmbcp@yahoo.com
| Date of Submission | 12 January 2012 |
| Date of Revision | 18 November 2012 |
| Date of Acceptance | 27 November 2012 |
| Indian J Pharm Sci., 2012, 74 (6): 521-526 |
Abstract
Cisplatin, a platinum compound, exerts its cytotoxic effects by coordinating to DNA where it inhibits both replication and transcription, and induces programmed cell death. It is used in the treatment of non-small cell lung cancer. In the present study, an attempt was made to achieve better treatment of lung cancer by direct lung delivery of cisplatin microparticulate systems, which helps to localize the drug in the lungs, and also provide sustained action. Cisplatin-loaded chitosan microspheres were prepared by emulsification and ionotropic gelation method, and characterized for drug content, particle size, densities, flow properties, moisture content, and surface topography by SEM and in vitro drug release was evaluated in simulated lung fluid at 37 0 at pH 7.4. The respirable or fine particle fraction (FPF) was determined by using twin stage impinger (TSI). Further stability evaluation of cisplatin-loaded DPI systems was carried out at 25 0 /60% RH and at 40°/75% RH.
Keywords
Cisplatin, dry powder inhalation, microspheres, pulmonary delivery
Lungs provide a large surface area and hence can be explored as a potential site for the systemic delivery of drugs. Also, for local effects, the mode of inhalational delivery system is noninvasive, which requires relatively small doses for efficacy, and avoids first pass metabolism [1-7].
Delivery platforms such as microspheres, liposomes, complexes with cyclodextrins, and conjugation with macromolecules have been explored for inhalational application [8]. Dry powder inhalation (DPI) technology is a particularly attractive approach over chlorofluorocarbons-based systems. DPI devices such as single-dose (e.g., Rotahaler®) to multiunit dose devices (in a blister pack) are available in the market. The DPI delivery systems are designed to ensure generation of a fine particulate system, which can overcome the impaction barrier that prevents the entry of any foreign matter inside the lungs and thereby deliver the therapeutic agents into the lungs.
Cisplatin is mainly used in treatment of non-small cell lung carcinoma, in combination with gemcitabine, paclitaxel, docetaxel, etoposide or vinorelbine [9]. Cisplatin shows its anticancer activity by coordinating to DNA where it inhibits both replication and transcription, and induces programmed cell death [10].
In the present study, chitosan-loaded cisplatin microspheres were prepared and evaluated for dry powder inhalation delivery of cisplatin for local action of the drug in lungs, and thereby achieve improved therapy in lung cancer.
Materials and Methods
Cisplatin was obtained as a gift sample from Themis Laboratories Pvt. Ltd., Mumbai, India. Chitosan was obtained from Central Institute of Fisheries, Mumbai, India, while a-lactose monohydrate was a generous gift from Burculo Domo, Netherlands. Citric acid (CA), liquid paraffin (LLP), dichloromethane (DCM), Span 80 were purchased from S.D. Fine-Chem., Mumbai, India. All the other solvents and chemicals were of analytical grade and were obtained from S.D. Fine-Chem., Mumbai, India.
Preparation of chitosan microspheres
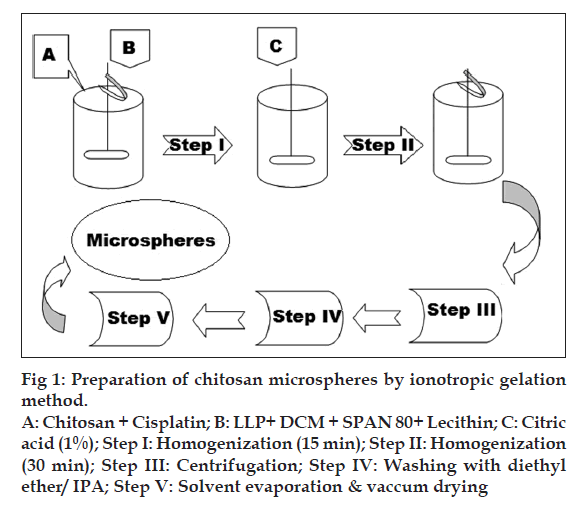
Microspheres were prepared by emulsificationionotropic gelation and heat crosslinking method as shown in fig. 1. Aqueous solutions of chitosan and cisplatin (in 0.5% w/v acetic acid) were emulsified in oil phase (100-200 ml) consisting of dichloromethane and light liquid paraffin using homogenizer (Silverson, India) for 15 min. Span 80 was used as an emulsifier and lecithin as a coemulsifier and deaggregating agent. Crosslinking solution (CA) was added to this emulsion, and homogenization was continued for another 30 min. Slow addition of this emulsion into previously heated and maintained (120°) light liquid paraffin (50 ml) on a rotamantle (Remi, India) was carried out, and stirring was continued for another one hour. This emulsion was then allowed to cool to room temperature with continuous stirring at same speed. Centrifugation at 10000 rpm for 10 min was carried out in order to separate the microspheres. To remove the oil, sediment was dispersed in diethyl ether, and this dispersion was again centrifuged for 3 min at the same speed (n=3). The sediment obtained was then dried in oven at 50-60º, passed through 100-mesh sieve and stored in a glass vial in a vacuum desiccator. Various parameters such as concentration of crosslinking agent citric acid, concentration of drug, and effect of molecular weight of chitosan were optimized with respect to drug content and particle size.
Figure 1: Preparation of chitosan microspheres by ionotropic gelation
method.
A: Chitosan + Cisplatin; B: LLP+ DCM + SPAN 80+ Lecithin; C: Citric
acid (1%); Step I: Homogenization (15 min); Step II: Homogenization
(30 min); Step III: Centrifugation; Step IV: Washing with diethyl
ether/ IPA; Step V: Solvent evaporation & vaccum drying
Development of microsphere-based dry powder inhalation formulations
DPI formulations of microspheres were prepared with and without carrier. Lactohale® 200 and 100 were mixed in equal quantities in a polybag, and this mixture was sieved to obtain particles in the range of 63-90 µm. The microspheres were mixed with lactose carrier (63-90 µm) in mass ratio of 1:6, using a cyclomixer (Remi, India) for 5 min; further, the mixture was passed through 100# and stored in a glass vial in a desiccator.
Characterization of chitosan microspheres
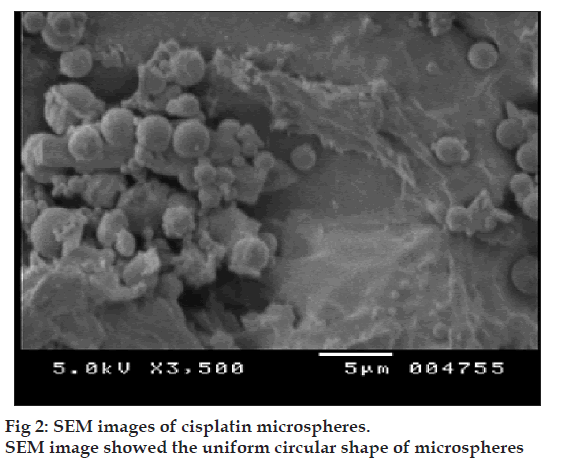
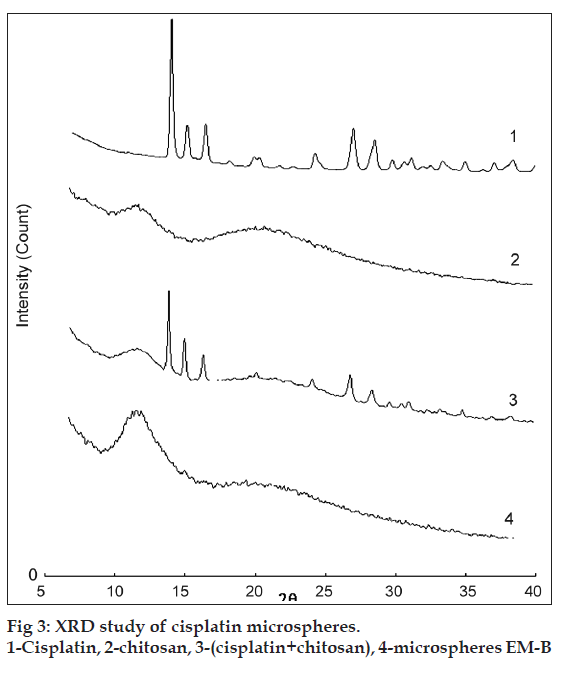
The microspheres were characterized for various physicochemical properties such as appearance, microscopic observation for aggregation, density, flow properties, moisture content (Karl Fischer method). The particle size distribution of the microspheres was assessed on a Malvern Mastersizer (Malvern Instruments, USA). The shape and surface morphology of chitosan microspheres, prepared by emulsification, were observed by scanning electron microscopy (CAMECA, SEM Probe-model SU 30, France). Solid state characterization of cisplatin microspheres was carried out by X-ray diffraction (Philips X? Pert MPD diffractometer, Philips, Holland).
Determination of drug content
The content of cisplatin in the microspheres was determined by UV spectrophotometric assay of the drug after extracting from the microspheres with dimethylformamide (DMF). Ten milligrams of microspheres was accurately weighed and dispersed in DMF for 24 h. It was kept overnight for complete extraction of drug and after filtration, the absorbance was measured at 309 nm against blank on a UV spectrophotometer (160A Shimadzu, Japan). The amount of cisplatin was extrapolated from the standard curve of cisplatin in DMF. Loading efficiency of these microspheres was calculated from the actual drug loading of the microspheres and the theoretical drug loading. All samples were analyzed in triplicate.
Determination of residual solvents by gas chromatography
GC (Varian CP-3800-GC chromatograph with FID Detector) was used for the determination of the level of residual organic solvents (IPA, acetone and DCM) in the microsphere samples. The analysis were performed by the static head-space method using Varian WS m software.
In vitro drug release
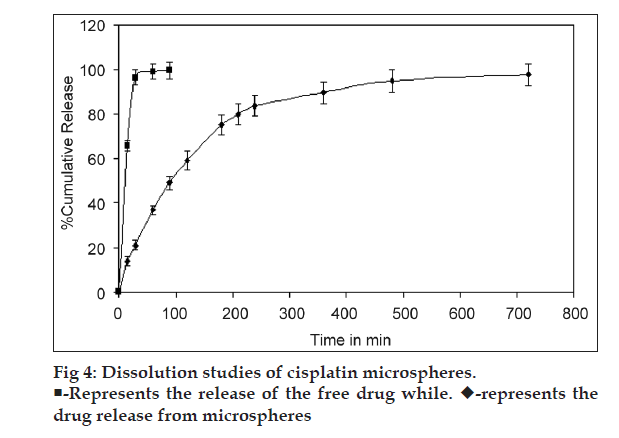
The dissolution test for cisplatin-loaded DPI system was performed by the flow through dissolution method (USP Apparatus 4) using Sotax Dissotest CE70 (Sotax, Basel, Switzerland) (n=3). The dissolution was carried out in simulated lung fluid [11] (SLF) pH 7.4; maintained at temperature 37±0.2º with the flow rate 0.2 ml/min. The collection of sample fractions was carried out at predetermined intervals; the drug content of aliquot fractions was determined by measuring the absorbance at 301 nm against blank. The amount of drug released was extrapolated from the standard curve for cisplatin in SLF (pH 7.4).
In vitro drug deposition studies by twin stage impinger
The respirable fine particle fraction (FPF) of the cisplatin microspheres was determined using a twin-stage impinger, apparatus A (TSI), official in British Pharmacopoeia. The Rotahaler®, a single unit dose dry powder inhaler device, was chosen to characterize the aerosol performance of the powder samples. All studies were carried out at constant flow rate of 60±5 l/min. The fractions collected in each stage were kept overnight in DMF for complete extraction of drug and after filtration, the drug deposited at different stages was analyzed for drug content by UV spectrophotometry.
Stability studies
Stability evaluation of selected batch of microspheres was carried out at 25±2°, 60% RH for 12 months and 40±2°, 75% RH for six months. Samples were evaluated for drug content, particle size, FPF, and in vitro release profiles.
In vitro cell cytotoxicity
In vitro cytotoxicity of the developed microspheres was evaluated in A549 and HOP 62 lung cancer cell lines. The IC50 value was calculated after plotting molar concentration versus growth inhibition (%).
Results and Discussion
Cisplatin is a chemotherapeutic agent, used in the treatment of various types of cancers. It is administered as intravenous infusion in saline. Microparticulate DPIs, such as microspheres, can be explored for patient friendly route of administration and sustained delivery of cisplatin by pulmonary drug delivery [12]. Most of the preparation methods for microspheres report glutraldehyde as crosslinking agent but due to toxicity of glutraldehyde, researchers are exploring alternate methods like spray drying and ionic gelation using agents like citric acid, sodium sulfate, and tripolyphosphate [13,14].
In the present investigation, chitosan microspheres were prepared by emulsification and crosslinking method. Citric acid was used as a crosslinking agent. Table 1 represents optimization of various variables such as amount of crosslinking agent, concentration of drug, and molecular weight of chitosan, which showed significant effect on particle size, drug content, and dissolution.
| Ingredients | EM-A | EM-B* | EM-C | EM-D | EM-E | EM-F* | EM-G* |
|---|---|---|---|---|---|---|---|
| Drug:Polymer | 1:2 | 1:5 | 1:8 | 1:5 | 1:5 | 1:5 | 1:5 |
| Chitosan (mg) | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| Acetic acid 0.5%) (ml) | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Cisplatin (mg) | 250 | 100 | 62.5 | 100 | 100 | 100 | 100 |
| DCM (ml) | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
| LLP (ml) | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Span 80 (%) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Lecithin (%) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| CA (1%) (ml) | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Particle size d50 (µm) | 7.8±1.6 | 5.2±1.19 | 5.1±1.4 | 4.6±0.64 | 4.9±1.22 | 11.8±0.8 | 7.3±1.24 |
| Drug content (%) | 80.7±1.2 | 79.2±2.9 | 62.9±2.1 | 66.9±3.5 | 70.4±4.1 | 88.9±2.5 | 92.2±2.1 |
DCM=Dichloromethane, LLP=Liquid petroleum, CA=Citric acid. *EM-B was the optimized batch of chitosan-based cisplatin microsphere. EM-B=Chitosan M.W 70,000, EM-F=Chitosan M.W 120,000, EM-G=M.W 160,000
Table 1: Optimization of the Chitosan-Based Cisplatin Microspheres
The size of the microspheres was increased from 4.6 µm to 5.2 µm when volume of citric acid (1%) was increased from 5 ml to 15 ml (Table 1; EM-E, EM-D and EM-B). In presence of excess ionotropic gelating agent, fusion of smaller microspheres occurs, leading to rise in chitosan microsphere size [15]. The multifunctional citric acid acts here to facilitate inter-microparticle binding of the chitosan.
The sizes of the drug-loaded chitosan?CA microspheres also increased with an increase in the molecular weight of chitosan. Low molecular weight chitosan?CA microspheres (M.W 70,000; EM-B) were smaller (5.2 µm) than medium (7.5 µm) (M.W 120,000; EM-F) and high (11.8 µm) molecular weight (M.W 160,000; EM-G) chitosan?CA microspheres. Increasing the amount of drug with respect to polymer decreases the drug content in microspheres. This may be due to smaller size of microspheres and long heating time (1 h) during preparation of microspheres [16].
Prepared microspheres were observed under microscope. All the batches of microspheres were found to be spherical in shape without any aggregation, and no precipitation of drug was observed. The physicochemical properties of optimized chitosan?CA microspheres are shown in Table 2. The shape and surface morphology of chitosan microspheres, prepared by emulsification, were observed by scanning electron microscopy (fig. 2). A few disintegrated particles were found after cross-linking. This may be due to disintegration of some of the microspheres during heating with aqueous CA.
| Property | Observations |
| Physical appearance | Light brown, odorless powder |
| Densities (g/ml) | |
| Bulk density | |
| Aerated | 0.368 |
| Packed | 0.515 |
| True density | 0.946 |
| Particle size distribution | |
| Mean particle size (µm) | 5.2±1.19 |
| Daero (µm) | 2.71 |
| Flow Properties- | |
| Carr’s index | 28.48 |
| Angle of repose (°) | 35.3±0.4 |
| Moisture content (%) | 4.10 |
| Yield (%) | 71.5 |
| Drug content (%) | 79.2±2.9 |
Table 2: Physiochemical Properties of Cisplatin Microspheres
The characteristic XRD spectra of pure drug (cisplatin), chitosan microspheres and physical mixture of drug, and chitosan are presented in (fig. 3). Characteristic crystalline peaks of cisplatin of pure drug indicates the presence of crystalline cisplatin. Peaks of cisplatin crystals are also present in physical mixture (reduced in intensity). However, XRD pattern of the cisplatin microspheres did not show any peaks. These results indicated that cisplatin is present in the amorphous form after its encapsulation in the chitosan?CA matrix.
The dissolution studies of chitosan microspheres of cisplatin were performed in SLF (pH 7.4) using flow through cell. In the present investigation, results indicate that the release behavior of cisplatin is related to interaction of CA with chitosan. Addition of CA during the preparation of the microspheres resulted in prolongation of release. High crosslinking density of chitosan?CA matrix might have resulted in less swelling ability; therefore, the release of the drug is decreased [17]. The release behavior of cisplatin from chitosan?CA microspheres has shown a biphasic release pattern with an initial burst effect, followed by a subsequent slower release. Initial burst release from cisplatin microspheres (37% in 1 h) may be due to drug adhered on surface of microspheres. After initial burst, drug was released in sustained manner up to 12 h from the microspheres compared to free drug (released immediately in SLF) as shown in fig. 4. The dissolution data were plotted as the percentage of cisplatin release against the square root of time. Linearity was observed with the plot because the correlation coefficient (R2) was 0.911. This indicates that the release of cisplatin from chitosan? CA microspheres followed Fick?s law of diffusion. Similar finding by Nishioka et al. has been reported for diclofenac sodium from chitosan microspheres [17].
Residual organic solvents can change the cystallinity of the bulk drug substance, leading to changes in dissolution properties and problems with formulation of the finished product. In the present investigation, residual solvent observed in microspheres is isopropyl alcohol 0.5 ppm and dichloromethane 24.8 ppm, which are far below the ICH limits for isopropyl alcohol (5000 ppm) and dichloromethane (600 ppm). During the process of preparation, these microspheres were heated to 120° for 1 h and further dried in oven (50-60°), which may have resulted in very low and acceptable levels of these solvents.
In the present study, in vitro inhalation performance of microspheres was evaluated using twin stage glass impinger. Chitosan microspheres, when formulated as DPI formulation without using any carrier particles, resulted in low FPF value. This observation describes the importance of the addition of lactose carrier in formulating the DPI. In this study, the lactose was sieved, and only 63-90 µm fraction was used, effect of fines was ignored, which has been reported to increase the in vitro performance of DPI formulations [18-20]. The FPF of cisplatin was significantly improved when lactose was used as carrier. The cisplatin microspheres showed higher FPF value compared to uncoated cisplatin both with and without lactose. The data revealed that the microspheres:lactose mass ratio of 1:6 significantly improved the DPI performance of both plain drug and microsphere-based formulation.
Stability studies of the developed DPI formulation filled in hard gelatin capsules (size 3) and placed in HDPE container was carried out kept at 40°/75% RH and 25°/60% RH. No changes in color, odor, or physical appearance were evident upon storage. The particle sizes of samples stored at 25°/60% RH and at 40°/75% RH are reported in Table 3 and have not changed significantly. The FPF value was slightly decreased as shown in Table 3. This could be attributed to an increase in moisture content and subsequent increase in particle size or agglomeration during storage (Table 3). No significant change in drug content of samples kept at 40°/75% RH and 25°/60% RH was evident, indicating the stability of formulation for a period of six months.
| Physical | 25º/60% RH | 40º/75% RH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| appearance | Off white to very light | Off white to very light | |||||||
| (%) | |||||||||
| brown, odorless powder | brown, odorless powder | ||||||||
| Initial | 6 Month 12 month | Initial | 3 Month 6 month | ||||||
| Drug | 63.9±1.5 60.4±2.1 | 59.7±2.6 | 63.9±1.5 59.8±3.4 59.1±2.3 | ||||||
| content | |||||||||
| (w/w) | |||||||||
| Moisture | 4.10 | 4.49 | 4.83 | 4.10 | 4.85 | 4.89 | |||
| content | |||||||||
| (w/w) | |||||||||
| FPF | 33.4±2.4 28.7±2.7 | 27.2±3.3 | 33.4±2.4 27.2±3.1 23.2±3.8 | ||||||
FPF=Fine particle fraction
Table 3: Stability Study of Cisplatin Microspheres
Cisplatin has shown cytotoxic effect on A549 human lung cancer cells but was unable to attain IC50 values for HOP-62 human lung cancer cells (Table 4). Similar observations were reported by Liang et al., and have confirmed that human lung adenocarcinoma cells are resistant to the cytotoxic effects of cisplatin [21]. Formulation process especially does not change any cytotoxic effect of cisplatin in chitosan matrix and hence retained its cell-killing effect. Microspheres showed higher IC50 value compared to free drug due to slower release from the chitosan matrix.
| Treatment | A 549 human | HOP 62 human |
|---|---|---|
| lung cancer | lung cancer | |
| cells (µM) | cells (µM) | |
| Adriamycin (ADR) | 28.77 | 6.83 |
| Cisplatin (Cis) | 40.82 | 1643.6 |
| Cisplatin microspheres (Cis-SD) | 67.13 | 2314.7 |
IC50 values represent the cytotoxicity potential of the developed cisplatin microsphere
Table 4: Ic50 Value of Different Treatments
In the present investigation, cisplatin-loaded chitosan microspheres were successfully developed, optimized, and evaluated for its suitability for pulmonary sustained drug delivery. Further in vivo safety and deposition studies in suitable animal models will give better insight for clinical applications of the inhalable cisplatin microspheres.
Acknowledgments
The authors are thankful to BRNS-DAE for providing financial assistance for the present investigation (Sanction no. 2004/35/5/BRNS).
References
- Courrier HM, Butz N, Vandamme TF. Pulmonary drug delivery systems: Recent developments and prospects. Crit Rev Ther Drug Carrier Syst 2002;19:425-98.
- Groneberg DA, Witt C, Wagner U, Chung KF, Fischer A. Fundamentals of pulmonary drug delivery. Respir Med 2003;97:382-7.
- Zeng XM, Martin GP, Marriott C. The controlled delivery of drugs to the lung. Int J Pharm 1995;124:149-64.
- Witek TJ. The Fate of Inhaled Drugs: The pharmacokinetics and pharmacodynamics of drugs administered by aerosol. Respir Care 2000;45:826-30.
- Clark AR. Pulmonary Delivery Technology: Recent Advances and Potential for the New Millennium. In: Hickey AJ, editor. Pharmaceutical Inhalation Aerosol Technology. 2nd ed. New York: Marcel Dekker Inc.; 2004. p. 563-84.
- Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci 1986;75:433-8.
- Shenfield GM, Evans ME, Paterson JW. Absorption of drugs by the lung. Br J Clin Pharm 1976;3:1218-23.
- Adjei A, Gupta P. Dry-powder inhalation aerosols. In: Adjei AL, Gupta PK, editors, Inhalation Delivery of Therapeutic Peptides and Proteins. New York: Marcel Dekker; 1997. p. 625-65.
- Clegg A, Scott D, Hewitson P. Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbinein non small cell lung cancer: A systematic review. Thorax 2002;57:20-8.
- Pieter P, Stephen J. Cisplatin and related drugs. In: Bertino JR editor, Encyclopedia of cancer. New York: Academic Press; 1996. p. 392-410.
- Davies NM, Feddah MR, A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm 2003;255:175-87
- Martinac A, Filipovic-Grcic J, Voinovich D, Perissutti B, Franceschinis E. Development and bioadhesive properties of chitosan-ethylcellulose microspheres for nasal delivery. Int J Pharm 2005;291:69-77.
- Mi F, Wong T, Shyu S, Chang S. Chitosan microspheres: Modification of polymeric anti-physical properties of spray dried microspheres to control the release of antibiotic drug. J Appl Polym Sci 1999;71:747-59.
- Berthold K, Cremer J, Kreuter J. Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for antiinflammatory drugs. Control Release 1996;39:17-25.
- Anal A, Willem F, Carmen R. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int J Pharm 2006;312:166-73.
- Lim L, Wan L, Thai P. Chitosan microspheres prepared by emulsification and ionotropic gelation. Drug Dev Ind Pharm 1997;23:981-5.
- Nishioka Y, Kyotani S, Okamura M, Miyazaki M, Okazaki K, Ohnishi S et al. Release characteristics of cisplatin chitosan microspheres and effect of containing chitin. Chem Pharm Bull 1990;38:2871-3.
- Staniforth J. Improvement in dry powder inhaler performance: Surface passivation effects. Proc Drug Deliv Lungs (London) 1996;7:86-9.
- Lucas P, Anderson K, Potter U, Staniforth J. Enhancement of small particle size dry powder aerosol formulations using an ultra low density additive. Pharm Res 1999;16:1643-7.
- Louey M, Razia S, Stewart P. Influence of physicochemical carrier properties on the in vitro aerosol deposition from interactive mixtures. Int J Pharm 2003;252:87-98.
- Liang CH, Liu LF, Shiu LY, Huang YS, Chang LC, Kuo KW. Action of solamargine on TNFs and cisplatin resistant human lung cancer calls. Biochem Biophys Res Commun 2004;322:751-8.