- *Corresponding Author:
- H. Mdiuni
Department of Biology, School of Sciences, 67149-67346, Razi University, Kermanshah, Iran
E-mail: mahdiuni@gmail.com
| Date of Submission | 22 July 2016 |
| Date of Revision | 12 March 2017 |
| Date of Acceptance | 14 September 2017 |
| Indian J Pharm Sci 2017;79(6): 900-906 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study ferric reducing antioxidant power assay, 2,2-diphenyl-1-picrylhydrazyl and oxygen radical absorbance capacity assays were employed to measure the antioxidant activity of flower and leaf extracts of Ferulago angulata. Ferric reducing antioxidant power assay of the extracts showed that the antioxidant activities of leaf extracts were higher than that of flower extracts. 2,2-diphenyl-1-picrylhydrazyl values showed similar trend to ferric reducing antioxidant power assay. A slow kinetic behavior was found within methanol extracts of both plant parts (EC50 of 0.071 mg/ml and 0.12 mg/ml for leaf methanol extract and flower methanol extract, respectively), estimated by kinetic mode of 2,2-diphenyl-1-picrylhydrazyl assay. The oxygen radical absorbance capacity assay indicated higher values for methanol extracts of plant parts compared to that of ethanol extracts. Except for oxygen radical absorbance capacity assay, a significant positive correlation was found between ferric reducing antioxidant power, 2,2-diphenyl-1-picrylhydrazyl and Folin-Ciocalteu assays, suggesting that phenolic compounds have an important role in reducing power and antiradical properties of the extracts.
Keywords
Antioxidant activity, DPPH, Ferulago angulata, FRAP, ORAC
Reactive oxygen species (ROS) are side products of numerous biochemical reactions; particularly in electron transfer chain reactions in mitochondria [1]. Excessive ROS in the body can lead to accumulative damages in vital macromolecules including proteins, lipids and DNA, resulting in oxidative stress. Oxidative stress, imbalance between oxidants and antioxidants in favor of the oxidants [2,3] has been considered as a cause of cardiovascular diseases [4,5] and cancer [6]. Epidemiological studies have shown an inverse relationship between consumption of antioxidants, especially phenolic compounds, and mortality from cardiovascular disease [7]. Owing to these statistical facts, the use of synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) has prevailed as food additives in the food industry to extend shelf life and to inhibit lipid rancidity [8,9]. However, need for natural antioxidants was augmented due to concerns about the safety of synthetic antioxidants [10-12]. Therefore, efforts to identify new antioxidants from natural sources have being increased. These natural antioxidants have potential to design nutraceuticals, which can assist to prevent oxidative damages in the body.
In an effort to introduce endemic natural antioxidants, antioxidant capacity of alcoholic and hydroalcoholic extracts of aerial parts of Ferulago angulata were investigated. The aerial parts of this plant have traditionally added to different food products such as oil ghee to prevent rancidity and to impart a pleasant odour. F. angulata, locally called Chavir in west of Iran, is a member of genus Ferulago belonging to the Apiaceae family [13]. This plant is grown in the spring at an altitude of 1900-3200 m above sea level.
To measure antioxidant activities, three assays were used, ferric-reducing antioxidant power (FRAP) assay, 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay and oxygen radical absorbance capacity (ORAC) assay. Additionally, the Folin-Ciocalteu (FC) method was used to determine the total phenolic content (TPC) of the extracts. On the basis of mechanism of reaction, FRAP, DPPH and FC assays are categorized as electrontransfer methods, while ORAC assay is classified as a hydrogen-transfer method[14]. In addition, to determine the contribution of phenolic compounds to the antioxidant capacities of the extracts, correlations were evaluated among the applied methods by correlation coefficients analysis.
Materials and Methods
F. angulata Boiss. was collected from the Dalani, mountainous region in west of Iran, during April and the so collected material was authenticated in the Department of Botany, Razi University, Kermanshah, Iran. A voucher herbarium specimen (No: 580) was deposited in the herbarium of the Agriculture Department of Razi University (HARU). 2,2 ?-azobis (2-amidinopropane) dihydrochloride (AAPH) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich, GmbH, Munich, Germany. 2,4,6-tripyridyl-striazine (TPTZ), fluorescein (FL), 2,2-diphenyl-1- picrylhydrazyl (DPPH), iron(III) chloride hexahydrate (FeCl3.6H2O), sodium tungstate (Na2WO4.2H2O), sodium molybdate (Na2MoO4.2H2O), sodium carbonate (Na2CO3), sodium acetate (C2H3NaO2), sodium sulphate (Na2SO4·10H2O) and all of the solvents used in the present work were purchased from Merck, Darmstadt Germany. All solvents and reagents were of analytical grade.
Sample preparation and extraction procedure
After harvesting, aerial parts of F. angulata were dried at room temperature 20-25° and darkness for 7 d. The dried flowers and leaves were ground in a blender and 50 g of them were put in a Soxhlet apparatus separately and were mixed with petroleum ether (~3 times) until discoloration. Afterwards, the two almost resin-free suspensions were centrifuged at 2700 g for 10 min, the pellets obtained were re-mixed with 500 ml ethanol and were stirred for 4 d. After centrifugation at 2700 g for 15 min, the supernatants were evaporated under vacuum to dryness and the resultant powders (henceforth referred to as ethanol extract of the flower (EF) or the leaf (EL) stored at 4° until analysis. The yield of dried flower and leaf extracts was about 7% (w/w) of the starting dried flower and leaf. Subsequently, the EF and EL powders were re-dissolved in methanol (50%):chloroform (1:1 v/v) mixture separately, and the methanol phases henceforth referred to as methanol extract of the flower (MF) or the leaf (ML) were evaporated under vacuum to dryness.
TPC assay
The TPC in flower (EF and MF) and leaf (EL and ML) extracts was determined according to the FC method [15,16] with some modifications. Appropriate dilutions of the extracts (50 μl) were added to FC reagent (50 μl, 0.2 N) and double distilled water (800 μl). After 5 min, sodium carbonate (100 μl, 0.5 M) was added. The mixtures were incubated for 1 h at room temperature and the absorbance of the resulting light blue color solution was measured at 725 nm using a Cary-100 Bio spectrophotometer (Varian, Palo Alto, California, USA). Trolox was used as the standard antioxidant. The standard curve was linear between 0 and 200 μM Trolox. Results were presented as μmol of Trolox equivalent per gram of dry mass of plant extracts (μmolTE/gDM).
FRAP assay
The FRAP assay was performed according to Benzie and Strain [17,18] with some modifications. The stock solutions included 300 mM acetate buffer, pH 3.6, 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl3.6H2O solution. The fresh working solution (FRAP solution) was prepared by mixing 25 ml acetate buffer, 2.5 ml TPTZ solution, and 2.5 ml FeCl3.6H2O solution and then warmed up to 37° before use. Flower (EF and MF) and leaf (EL and ML) extracts (10 μl) were allowed to react with 990 μl of the FRAP solution for 30 min in the dark condition. Readings of the colored products were then taken at 595 nm using a Cary-100 Bio spectrophotometer. To express ferric ion reducing power of the extracts, Trolox was used as a standard antioxidant. The standard curve was linear between 5 and 20 μM Trolox. Results were expressed in μmolTE/gDM.
DPPH assay
The DPPH assay was done in kinetic and nonkinetic modes according to the method of Miliauskas et al. [19] and of Brand-Williams et al. [20] with some modifications. In non-kinetic mode, 50 μl of the plant extracts (EF, EL, MF and ML) were mixed with 950 μl of the DPPH solution (6×10–5 M DPPH in methanol) for 24 h in the dark. The absorbance of the bleached products was then recorded at 515 nm using a Cary-100 Bio spectrophotometer. To express antiradical power of the extracts, Trolox was used as a standard antioxidant. The standard curve was linear between 5 and 50 μM Trolox. Results were expressed in μmolTE/gDM.
In kinetic mode, 50 μl of different concentrations (50, 100, 200, 300 μg/ml in methanol) of the MF and ML were mixed with 950 μl of DPPH solution and the decrease in absorbance was recorded at 515 nm at time intervals of 30 s until 3 h and thereafter at time intervals of 2 h until the reaction reached a steady state. For each MF and ML concentration tested, the reaction kinetics were plotted by use of the Eqn., %DPPH=100×[DPPH]rem/[DPPH]t=0, where, [DPPH]rem is remaining DPPH concentration at different times and [DPPH]t=0 is amount of DPPH at initial time. From these graphs, the percentage of DPPH remaining at the steady state for each concentration of MF or ML was determined and these values were plotted versus ML or MF concentrations to calculate EC50 (the amount of antioxidant necessary to decline the initial DPPH concentration by 50%), using the exponential model: y=a×exp(–x)/t1)+y0, where, a is the slope and y0 is the intercept.
ORAC-FL assay
The ORAC assay was done according to the procedure described by Ou et al. [21,22] with some modifications. The reaction was carried out in 75 mM phosphate buffer (pH 7.4), and the final reaction mixture was 500 μl. Samples (EF, EL, MF and ML, 200 μl) and FL (200 μl; 78 nM, final concentration) solutions were put in the florescence cuvette. The mixture was preincubated for 10 min at 37°. AAPH solution (100 μl; 221 μM, final concentration) was added and the cuvette was immediately put in the fluorescence spectrophotometer (Cary Eclipse, Varian, Palo Alto, California, USA) spectrofluorimeter with jacketed cell holder in which temperature was controlled by an external thermostated water circulation system. Fluorescence was recorded at 1 min intervals until it reached less than 5% of initial intensity (excitation wavelength 485 nm, emission wavelength 535 nm). For blank (FL+AAPH), phosphate buffer was used instead of the antioxidant solution, and for standard solutions, different concentrations of Trolox were used as an antioxidant. Antioxidant curves (fluorescence versus time) were normalized to the curve of the blank corresponded to the same assay by multiplying original data by the factor fluorescenceblank, t=0/fluorescencesample, t=0. From the normalized curves, the area under the fluorescence decay curve (AUC) was calculated as: AUC=1+f1/f0+f2/f0+f3/f0+...+fn/f0, where, f0 is the initial fluorescence read at 0 min and f1 is the fluorescence reading at time i. The net AUC corresponding to a sample was calculated by subtracting the AUC corresponding to the blank. Regression equations between net AUC and antioxidant concentration were calculated for all the samples. ORAC-FL values were calculated as: ORACvalue=CTrolox×[(AUCsample–AUCblank)/(AUCTrolox– AUCblank)], where, CTrolox is molarity of Trolox. Final results were expressed in μmol of Trolox equivalent per gram of dry mass of the extrac ts (μmolTE/gDM).
Results and Discussion
The TPC values of the ethanol and methanol extracts of flower (EF, MF) and leaf (EL, ML) ranged from 0.4 to 1 μmolTE/gDM (Table 1). The highest phenolic content obtained using ethanolic extraction followed by methanolic extraction. Thus, protocols using methanol (50%):chloroform mixture would be the choice method to concentrate phenolic substances of high polarity. Antioxidant properties of phenolic compounds are directly depended on their structure. Having hydroxyl groups attached to aromatic rings, phenolic compounds are potentially able to quench free radicals by a resonance-stabilized mechanism [16,23]. As it will be shown in the following sections, there are good relationships between phenolic content values of the extracts and antioxidant activities measured by FRAP and DPPH methods.
| Type of extract | TPC value μmolTE/gDM±SD |
FRAP value μmolTE/gDM±SD |
DPPH value μmolTE/gDM±SD |
|---|---|---|---|
| EL | 0.7±0.04 | 185.4±16.8 | 321.92±8.8 |
| EF | 0.365±0.006 | 98.7±1.8 | 264±15.04 |
| ML | 0.95±0.03 | 360±3.1 | 393.28±1.92 |
| MF | 0.565±0.015 | 142.3±7.8 | 300.64±4.64 |
Table 1: Antioxidant Activity of Different Extracts from F. Angulata
Ferric ion reducing capacities of the F. angulata extracts have listed in Table 1. The trend for the reducing activities was similar to the TPC measured by FC method. Again, the ML with FRAP value of 360 μmolTE/gDM had the highest reducing activity followed by EL, MF and EF. Compared with vegetables such as spinach, broccoli, purple onion, white cabbage, tomato and carrot [24], the ferric reducing potential of the F. angulata extracts is markedly greater. However, it must be noted that the solvents used to extract of the antioxidant compounds in the cited study were different from the solvents used in our investigation.
The DPPH assay is technically a simple and reproducible method for evaluating radical scavenging capacities of plant extracts, fruits, olive oil and wine [20]. DPPH radical scavenging activities of the EL, EF, ML and MF extracts were in the range of 260 to 390 μmolTE/gDMs (Table 1), when they were calculated non-kinetically. Interestingly, similar trends were observed for the FRAP, TPC and DPPH values (ML>EL>MF>EF).
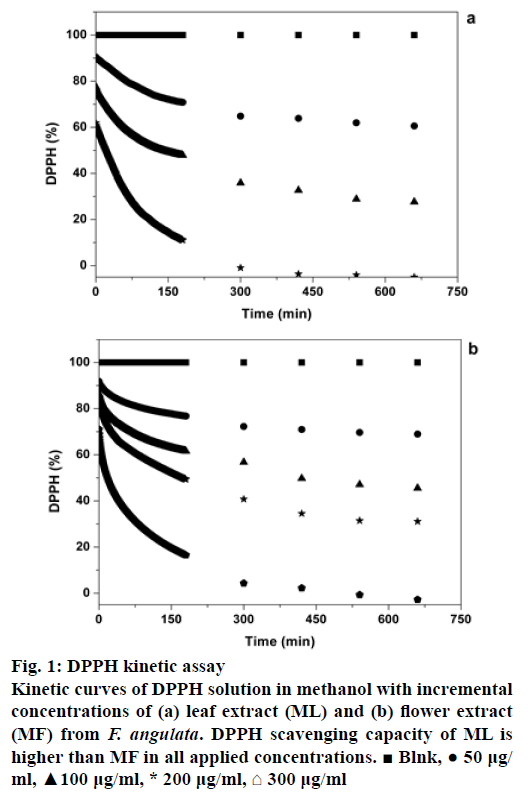
In order to evaluate antiradical behaviours of ML and MF extracts kinetically, the time evolutions of remaining DPPH for each concentration of the extracts were plotted (Figure 1). Depending on the rate of reaching to the steady state, there are three types of kinetic reactions: a rapid kinetics (<1 min), b intermediate kinetics (5-30 min) and c slow kinetics (>1 h) [20]. From the graphs illustrated in Figure 1, it is obvious that antioxidant compounds available in the ML and MF extracts have slow kinetic behaviours. Furthermore, the free radical scavenging activities of ML and MF, representing by EC50, were 0.071 mg/ml and 0.12 mg/ml, respectively. These values are in the range of EC50 of known antioxidants such as curcumin, BHT, thymol and carvacrol, having EC50 values of 0.0078, 0.02, 0.16 and 0.25 mg/ml, respectively [25].
Figure 1: DPPH kinetic assay
Kinetic curves of DPPH solution in methanol with incremental concentrations of (a) leaf extract (ML) and (b) flower extract (MF) from F. angulata. DPPH scavenging capacity of ML is higher than MF in all applied concentrations. ? Blnk, ? 50 μg/ml, ?100 μg/ml, * 200 μg/ml, ? 300 μg/ml
Among the methods employed in this study, only the ORAC assay is based on hydrogen atom transfer (HAT) mechanism. Besides, in contrast to DPPH assay, the ORAC assay employs a biologically relevant radical [14,23,24], and it is the only method quantifying both inhibition time and degree of inhibition for an antioxidant [21].
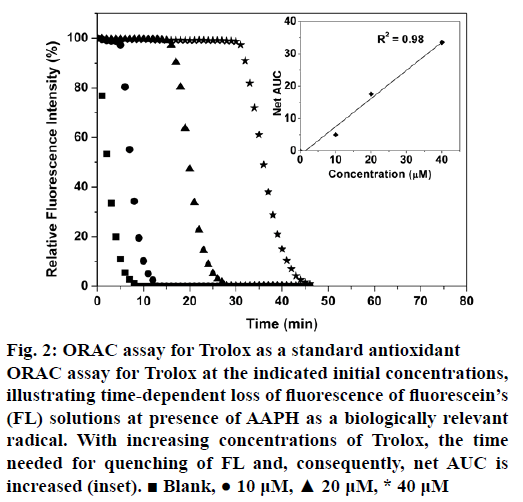
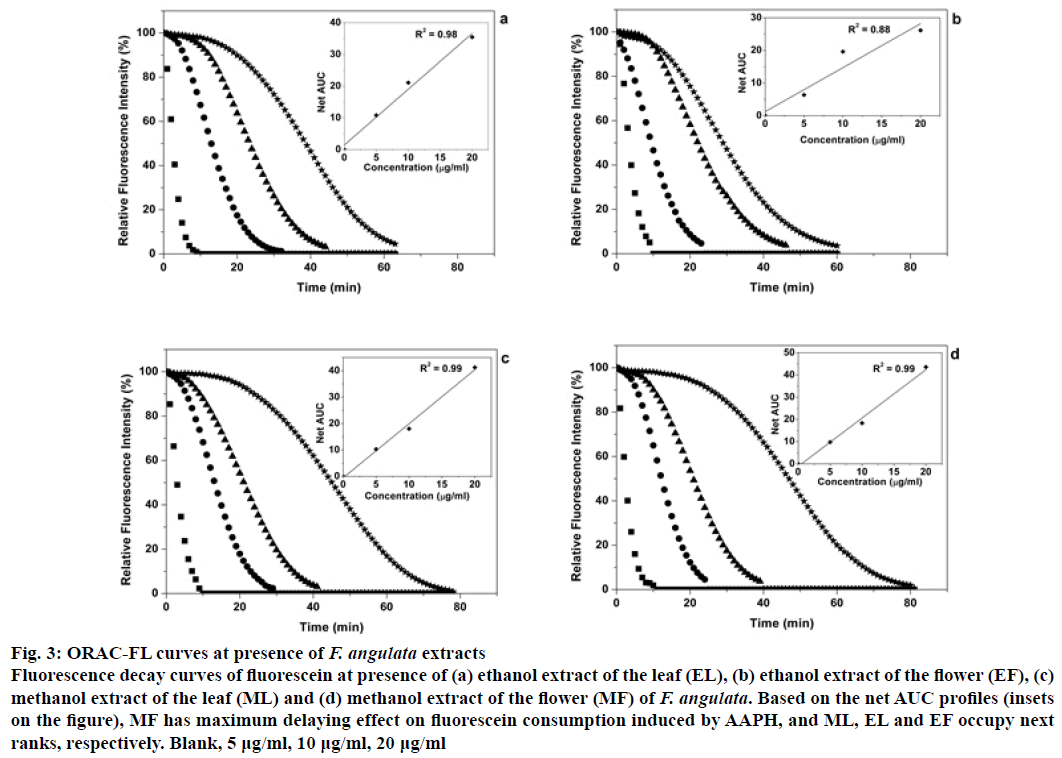
Figure 2 illustrates FL decay curves for Trolox as a standard antioxidant over a concentration range of 0-40 μM. There is a positive correlation between the concentration of Trolox and required time for the decay in fluorescence intensity of FL. The net area under the curve (net AUC) was plotted as a function of Trolox concentrations. Similarly, fluorescence decay curves and corresponding net AUCs were plotted for EL, EF, ML and MF extracts over concentration ranges of 0-20 μg/ml (Figure 3a-d). From the ORAC values of the extracts (Table 2), calculated by the formula given in the section 2.7, MF showed the highest value (6326 μmolTE/gDM), while EF had the least (4042 μmolTE/gDM). Compared to several known extracts, the flower and leaf extracts of F. angulata show higher antioxidant capacities. For example, extracts from black tea leaves and blueberry have the ORAC values of 1629 and 2792 μmolTE/gDM, respectively [26], whereas those values for the extracts of F. angulata range from 4042 to 6326 with the same units (Table 2). However, it was noticed that the methods and the solvents used by Atala et al. were different from ours [26]. Notably, F. angulata extracts’ ORAC values were much lower than that of grape skin with the ORAC value of 15675 μmolTE/gDM [21]. This high potential antioxidant activity is also observed for citrus families using different extraction methods [27] and different parts of the orangery [28,29].
Figure 2: ORAC assay for Trolox as a standard antioxidant
ORAC assay for Trolox at the indicated initial concentrations, illustrating time-dependent loss of fluorescence of fluorescein’s (FL) solutions at presence of AAPH as a biologically relevant radical. With increasing concentrations of Trolox, the time needed for quenching of FL and, consequently, net AUC is increased (inset). ? Blank, ? 10 μM, ? 20 μM, * 40 μM
Figure 3: ORAC-FL curves at presence of F. angulata extracts
Fluorescence decay curves of fluorescein at presence of (a) ethanol extract of the leaf (EL), (b) ethanol extract of the flower (EF), (c) methanol extract of the leaf (ML) and (d) methanol extract of the flower (MF) of F. angulata. Based on the net AUC profiles (insets on the figure), MF has maximum delaying effect on fluorescein consumption induced by AAPH, and ML, EL and EF occupy next ranks, respectively. Blank, 5 μg/ml, 10 μg/ml, 20 μg/ml
| Type of extract | Extraction solvents (Solvent ratio) |
ORAC Value μmolTE/gDM |
|---|---|---|
| ML | Methanol (50%):chloroform (1:1) | 6176a |
| MF | Methanol (50%):chloroform (1:1) |
6326a |
| EL | Ethanol (100%) | 5140a |
| EF | Ethanol (100%) | 4042a |
| Black tea leaves | Acetone:water (4:1) | 1629[26] |
| Blueberry extracts | Acetone:water (4:1) | 2792[26] |
| Grape skin extracts | Acetone:water (4:1) | 15675[26] |
| Matricariarecutita(flower) | Water | 588[24] |
| Lavandulahybridagrosso (flower) | Water | 1181[24] |
| Actinidiachinensis(flower) | Water | 877[24] |
| Cistusladaniferus(leaf) | Water | 1410[24] |
Table 2: Comparison of ORAC Values between different extracts of F. Angulata and the well-known extracts
Correlations among the methods were calculated by regression analysis on results of various methods used in this study (Table 3). A significant positive correlation was found between FRAP and DPPH assays (R=0.97). Also, results of DPPH and FRAP assays were correlated significantly to the TPC concentration, determined by FC method, with coefficients of 0.97 and 0.88, respectively. These high correlation coefficients indicate that there are good relationships between phenolic compound concentration in F. angulata extracts and their antiradical capacity as well as ferric reducing power. In other words, higher phenolic content contributes to a higher antioxidant potential, a proposition that is reported previously [24,27,28]. Antioxidant potential of phenolic compounds is appertained to number of hydroxyl groups and other substitutions on their aromatic rings [30].
| Assay methods | FRAP | DPPH | TPC | ORAC |
|---|---|---|---|---|
| FRAP | 1 | |||
| DPPH | 0.97 | 1 | ||
| TPC | 0.88 | 0.97 | 1 | |
| ORAC | 0.17 | 0.4 | 0.6 | 1 |
Table 3: Correlation Coefficient
The lowest correlations were found between ORAC and FRAP (R=0.17), ORAC and DPPH (R=0.4) and ORAC and TPC (R=0.6) assays. In contrast to the TPC, DPPH and FRAP assays, the ORAC assay was based on HAT mechanism [23]. Moreover, the ORAC assay is the only method that can be used to evaluate the kinetic behaviors of antioxidants by calculation of area under the decay curves of FL [31]. These differences might explain the low correlation coefficients observed between the ORAC assay and the other methods.
In this investigation, a promising source of natural antioxidant compounds in a rather less studied plant F. angulata have been identified. The antioxidant activity of the aerial parts of F. angulata was estimated using several well-known methods, such as TPC, FRAP, DPPH and ORAC assays. The first three methods showed high degree of correlation among them for antioxidant activity. These findings suggest that high antiradical potential and reducing power of the alcoholic and hydroalcoholic extracts of the aerial parts of F. angulata correspond to a high phenolic content in these plant parts. Additional studies are needed to characterize the active compounds within these plant extracts that may be useful to introduce new nutraceutical agents.
Acknowledgements
Authors wish to thank Dr. Seyed Mohammad Masoumi, Assistant Professor of Botany, Razi University, Kermanshah, Iran for authenticating the plant Ferulago angulata Boiss. collected from the Dalani mountainous region and Dr. Hossein Fallahi of Razi University, Kermanshah, Iran for reviewing and making useful comments during manuscript preparation.
Financial assistance
Authors gratefully acknowledge the financial support received from Razi University, Kermanshah, Iran to carry out this study.
Conflicts of interest
There are no conflicts of interest.
References
- Kirkinezos IG, Moraes CT. Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol 2001;12:449-57.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;8:291-5.
- Ghasemzadeh A, Jaafar HZ, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010;15:4324-33.
- Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol 1997;26:1-13.
- Lampe JW. Health effects of vegetables and fruits: assessing mechanisms of action in human experimental studies. Am J Clin Nutr 1999;70:475-90.
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 1996;96:1027-39.
- Serdula MK, Byers T, Mokdad AH, Simoes E, Mendlein JM, Coates RJ. The association between fruit and vegetable intake and chronic disease risk factors. Epidemiology 1996;7:161-5.
- Adegoke GO, Kumar MV, Krishna AGG, Varadaraj MC, Sambaiah K, Lokesh BR. Antioxidants and lipid oxidation in foods: A critical appraisal. J Food Sci Tech Mys 1998;35:283-98.
- Wu N, Fu K, Fu YJ, Zu YG, Chang FR, Chen YH, et al. Antioxidant activities of extracts and main components of Pigeon pea [Cajanus cajan (L.) Millsp.] Leaves. Molecules 2009;14:1032-43.
- Iverson F. In vivo studies on butylated hydroxyanisole. Food Chem Toxicol 1999;37:993-7.
- Williams GM, Iatropoulos MJ, Whysner J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol 1999;37:1027-38.
- Sonkawade SD, Naik GR. In vitro evaluation of antioxidant properties of sugarcane extracts rich in dietarynucleotides. Int J Adv Biol Res 2015;5:243-50.
- Javidnia K, Miri R, Edraki N, Khoshneviszadeh M, Javidnia A. Constituents of the volatile oil of Ferulago angulata (Schlecht.) Boiss. from Iran. J Essent Oil Res 2006;18:548-50.
- Phipps SM, Sharaf MHM, Butterweck V. Assessing antioxidant activity in botanicals and other dietary supplements. Pharmacopeial Forum 2007;33:810-14.
- Folin O, Ciocalteu V. On tyrosine and tryptophan determinations in proteins. J Biol Chem 1927;73:627-49.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 1999;299:152-78.
- Benzie IF, Strain JJ. The Ferric reducing ability of plasma (FRAP) as a measure of ?antioxidant power?: the FRAP assay. Anal Biochem 1996;239:70-6.
- Wojdylo A, Oszmian´ski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 2007;105:940-9.
- Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 2004;85:231-7.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 1995;28:25-30.
- Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygenradical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem 2001;49:4619-26.
- da Silva JK, Cazarin CBB, Colomeu TC, Batista AG, Meletti LMM, Paschoal JAR, et al. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: In vitro and in vivo study. Food Res Int 2013;53:882-90.
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem 2005;53:1841-56.
- Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem 2009;57:1768-74.
- Vardar-Unlu G, Candan F, Sokmen A, Daferera D, Polissiou M, Sokmen M, et al. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J Agric Food Chem 2003;51:63-7.
- Atala E, Vásquez L, Speisky H, Lissi E, López-Alarcón C. Ascorbic acid contribution to ORAC values in berry extracts: an evaluation by the ORAC-pyrogallol red methodology. Food Chem 2009;113:331-5.
- Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 2006;94:19-25.
- Peng Wong S, Peng Leong L, William Koh JH. Antioxidant activities of aqueous extracts of selected plants. Food Chem 2006;99:775-83.
- Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citru sspecies peels and tissues. Pak J Pharm Sci 2009;22:277-81.
- Silva MM, Santos MR, CarocoG, Rocha R, Justino G, Mira L. Structure-antioxidant activity relationships of flavonoids: a re-examination. Free Radic Res 2002;36:1219-27.
- Cao G, AlessioHM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidant. Free Radic Bio Med 1993;14:303-11.