- *Corresponding Author:
- Yu Wang
Department of Cardiology, Chinese People’s Liberation Army General Hospital, Haidian, Beijing 100853, China
E-mail: wyu0519@163.com
| Date of Received | 23 December 2022 |
| Date of Revision | 06 February 2023 |
| Date of Acceptance | 03 October 2023 |
| Indian J Pharm Sci 2023;85(5):1321-1328 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Brown and white adipose tissues contribute differently to cardiovascular risk factors, yet the precise mechanisms remain elusive. Exosomes-tiny vesicles containing ribonucleic acid, proteins and lipids mediate inter-organ communication. We hypothesized that exosomes secreted by brown and white adipose tissues carry distinct microRNA profiles that differentially modulate myocardial ischemia/reperfusion injury. Male rats underwent 30 min left forearm ischemia, followed by reperfusion. Prior to reperfusion, exosomal secretions from brown and white adipose tissues were directly injected into the myocardium. Cardiac function was assessed through echocardiography, Evans blue and 2,3,5-triphenyltetrazolium chloride dual staining, as well as hematoxylin and eosin and Masson trichrome staining. The microRNA content in the adipose tissue-derived exosomes was analyzed using microarray and bioinformatics approaches, followed by quantitative polymerase chain reaction for real-time microRNA expression profiling. Protein levels modulated by microRNA-34c-3p agomir or antagomir were examined using proteomic analysis. Exosomal secretions from brown and white adipose tissues exerted distinct effects on myocardial ischemia/reperfusion injury. Compared to white adipose tissue, exosomes from brown adipose tissue significantly upregulated 55 microRNAs and downregulated 34 microRNAs. Genes involved in various biological processes-such as positive recirculatory aquaculture system signaling regulation, negative target of rapamycin signaling regulation, circadian rhythm mediation, autophagy and FOXO, mitogen-activated protein kinases, and Wnt signaling pathways are targeted by these differentially expressed microRNAs. Most notably, quantitative polymerase chain reaction analyses identified significant increases in microRNA-133a-3p and microRNA-378, and a decrease in microRNA-34c-3p. Further, microRNA-34c-3p agomir treatment led to increased expression of the cardiac gene G protein subunit alpha I3. Exosomes derived from brown and white adipose tissues differentially influence myocardial ischemia/ reperfusion injury, potentially due to distinct microRNA contents. Among them, miR-34c-3p stands out for its role in exacerbating myocardial ischemia/reperfusion injury through its target hub gene, G protein subunit alpha I3.
Keywords
Exosome, adipose, microRNA, ischemia reperfusion, angioplasty
Globally, Acute Myocardial Infarction (AMI) is a major source of mortality and morbidity[1]. While prompt revascularization therapies such as Percutaneous Coronary Intervention (PCI), Percutaneous Transluminal Coronary Angioplasty (PTCA), Coronary Artery Bypass Grafting (CABG), and early thrombolysis have proven effective for AMI treatment[2], these methods often induce reperfusion injuries. These complications manifest as myocardial stasis, reperfusion arrhythmia and microvascular dysfunction, leading to pathological consequences like myocardial necrosis, apoptosis, oxidative stress and inflammation. This considerably mitigates the therapeutic benefits of revascularization[3]. Studies indicate that ischemiareperfusion injuries contribute to nearly 50 % of the final myocardial infarction area[4]. Therefore, there is an urgent need to explore the pathogenesis of ischemia-reperfusion injuries and identify novel therapeutic targets.
The cardiovascular system is affected differently by the two types of adipose tissue, white and brown[5], each having its own distinct effects. Brown adipose tissue has been associated with a lower incidence of metabolic heart diseases and is present in conditions such as type 2 diabetes, dyslipidemia, ischemic diseases, and cerebrovascular diseases, among others[6]. Conversely, increased visceral fat (white adipose tissue) correlates with elevated cardiovascular risk[7-9]. As the largest endocrine organ, adipose tissue regulates various bodily functions through the secretion of numerous hormones and cytokines[10].
Emerging research suggests that brown adipose tissue improves cardiac function by activating Adenosine A2A Receptor (A2AR) receptors via Fibroblast Growth Factor 21 (FGF21) secretion and by specifically releasing dimers 12 and 13[11,12]. In contrast, saturated fatty acids released by white adipocytes, particularly palmitic acid, induce inflammation and dysfunction related to obesity[13,14]. However, the underlying mechanisms for these divergent effects remain unclear.
Secreted by cell membranes, exosomes are lipid bilayer vesicles with diameters ranging from 30 to 150 nm, playing a role in enabling inter-organ communication[15]. They encompass a diverse range of bioactive compounds, including nucleic acids, lipids and proteins[16-20]. New research has brought attention to the involvement of exosomes from adipose tissue in a range of pathological processes. For instance, exosomes secreted by brown adipose tissue have been shown to protect against myocardial ischemia-reperfusion injuries when stimulated by exercise[21]. On the other hand, white adipose tissue-derived exosomes can exacerbate cardiac conditions in diabetic mice[22].
This study pioneers the investigation of differential microRNA (miRNA) expression in exosomes secreted by brown and white adipose tissues. We discovered that miR-34C-3P and specific exogenous genes in white adipose tissue can worsen myocardial injury due to ischemia and reperfusion under normal conditions.
Materials and Methods
Animal models:
Male Sprague-Dawley rats of adult age (weighing between 250-280 g) were procured from Specific Pathogen Free (SPF) Biotechnology located in Beijing, China. The rodents were accommodated within a controlled environment where the temperature was consistently maintained at 22°±2°, following a 12 h light/dark pattern. Animals had unrestricted access to both food and water. The Animal Ethics Committee of the General Hospital of the People's Liberation Army granted approval for all animal care and surgical procedures. These actions adhered to the guidelines set by the National Institutes of Health for the ethical treatment and utilization of experimental animals. Rats were allocated at random to various experimental groups in order to conduct subsequent assessments including cardiac ultrasound, Evans blue/2,3,5- Triphenyltetrazolium Chloride (TTC) staining, and miRNA analysis.
Myocardial ischemia-reperfusion model:
Rats were anesthetized using 60 mg/kg sodium pentobarbital (i.p.) and placed in a supine position. A rodent ventilator was used for ventilation, and electrocardiogram leads were connected. The chest cavity was incised, and the left coronary artery was secured with a 6-0 suture thread. Criteria for successful ischemia induction included ST-segment elevation and pallor of the anterior myocardial wall. After 30 min of ischemia, reperfusion was confirmed by the return of normal myocardial color. Exosomes (100 μg in 50 μl Phosphate Buffer Saline (PBS)) were microinjected into the ischemic myocardium 5 min prior to reperfusion.
Exosome isolation:
Rats were humanely euthanized using an excessive amount of carbon dioxide, and this was followed by disinfection using 75 % alcohol. Brown and white adipose tissues were aseptically harvested, minced into approximately 1 mm3 pieces, and cultured in serum-free Dulbecco's Modified Eagle Medium (DMEM) for 24 h. Exosomes were extracted from the conditioned medium using Exoquick TC exosome isolation reagent (System Biosciences, Palo Alto, California, United States of America (USA)), following the manufacturer's protocol.
Evans blue/TTC staining:
After a lapse of 24 h following reperfusion, the coronary artery was reoccluded, and a solution containing 1 % Evans blue dye was introduced into the aorta. Hearts were then harvested, frozen, and sectioned. At 37°, 1 % TTC solution was incubated in sections for 15 min. Photographs were taken with a Canon camera and then subjected to analysis utilizing the ImageJ software.
Histological staining:
Cardiac tissues were immersed in 4 % paraformaldehyde for a minimum of 24 h to ensure fixation. Following this, the tissues were embedded in paraffin and subsequently sliced into sections that were 4 μm thick. Staining with Hematoxylin and Eosin (HE) as well as Masson's Trichrome was carried out, and the resultant images were obtained through a Nikon Eclipse Ts optical microscope based in Tokyo, Japan.
Echocardiography:
The measurement of Left Ventricular Ejection Fraction (LVEF) and Left Ventricular Fractional Shortening (LVFS) was conducted through cardiovascular ultrasound by skilled professionals using an unbiased technique, facilitated by Toronto Visual Ultrasound in Canada.
Quantitative Real-Time Polymerase Chain Reaction (PCR):
Total Ribonucleic Acid (RNA) was extracted using a specialized kit (RE-03111; FOR gene), followed by reverse transcription using a rapid Genomic Deoxyribonucleic Acid (GDNA) isolation RT Supermix kit (KR118, Beijing Tiangang). Relative expression of miRNA was quantified using Be- STARTM-QPCR-RT and BeSTARTM-qPCR-Mastermix kits (Decibels Relative to Isotropic (DBI)). Expression levels were calculated using the 2-ΔΔCT method.
miRNA Sequencing and Bioinformatics:
miRNA sequencing was performed using the miRNA4.0 chip. Target genes with differential expression were pinpointed and examined via Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, adhering to predefined standards for statistical relevance.
Western blotting:
Proteins were isolated with Radio-Immunoprecipitation Assay (RIPA) buffer and then separated using Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE). After being transferred onto a Polyvinylidene Fluoride (PVDF) membrane, non-specific interactions were inhibited with 5 % skim milk.
The membranes were subsequently exposed to primary antibodies specific for Tumor Susceptibility Gene 101 (TSG101) and Cluster of Differentiation (CD)-81, followed by the appropriate secondary antibodies. Band patterns were captured with a gel imaging system (AI 600, GE Healthcare, USA) and quantification was done through ImageJ software.
In vivo miRNA modulation:
miRNA-agomirs or antagomirs (10 nmol) were microinjected into the myocardium at five different sites. After 48 h, hearts were harvested for subsequent ischemia-reperfusion or protein expression analysis.
Statistical analysis:
The data is shown as the mean±standard deviation. For comparing two groups, the Student's t-test was utilized; for multiple groups, one-way Analysis of Variance (ANOVA) followed by a Newman-Keuls test was applied. A p<0.05 was deemed to be statistically meaningful.
Results and Discussion
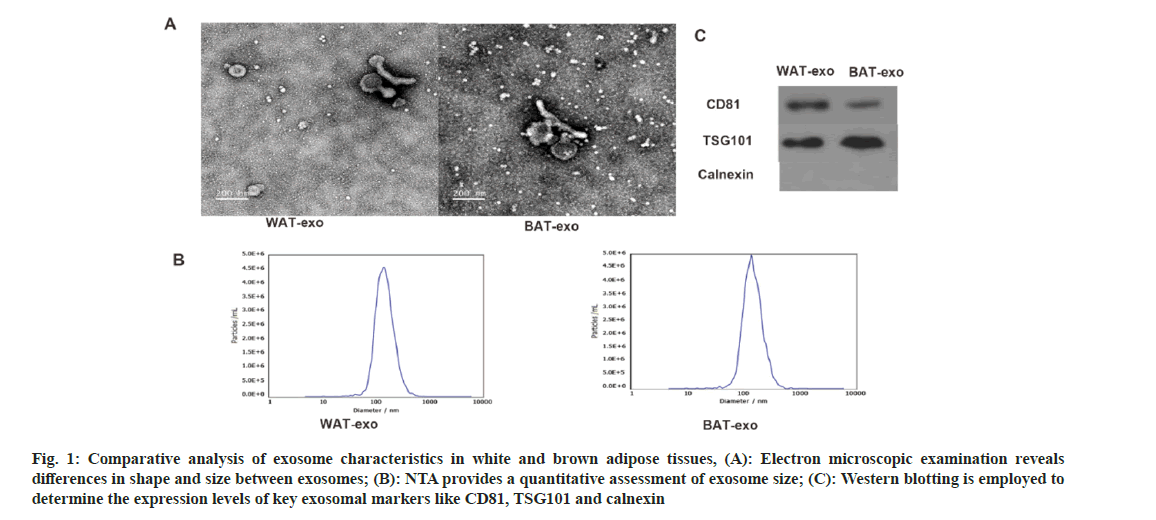
Electron microscopy revealed that the isolated vesicles display a cup-shaped or circular morphology, which is consistent with the typical appearance of extracellular vesicles (fig. 1A). Nanoparticle Tracking Analysis (NTA) confirmed that the vesicles from both groups had a uniform size distribution with an average diameter of approximately 100 nm (fig. 1B), aligning with known exosome dimensions. The Western blot tests confirmed the presence of well-known exosomal markers CD81 and TSG101 in both vesicle samples. Notably, CD81 was more abundantly expressed in white adipose tissue-derived vesicles, whereas TSG101 was higher in brown adipose tissue-derived vesicles (fig. 1C). These findings confirm the successful isolation of extracellular vesicles from adipose tissue.
Fig. 1: Comparative analysis of exosome characteristics in white and brown adipose tissues, (A): Electron microscopic examination reveals differences in shape and size between exosomes; (B): NTA provides a quantitative assessment of exosome size; (C): Western blotting is employed to determine the expression levels of key exosomal markers like CD81, TSG101 and calnexin
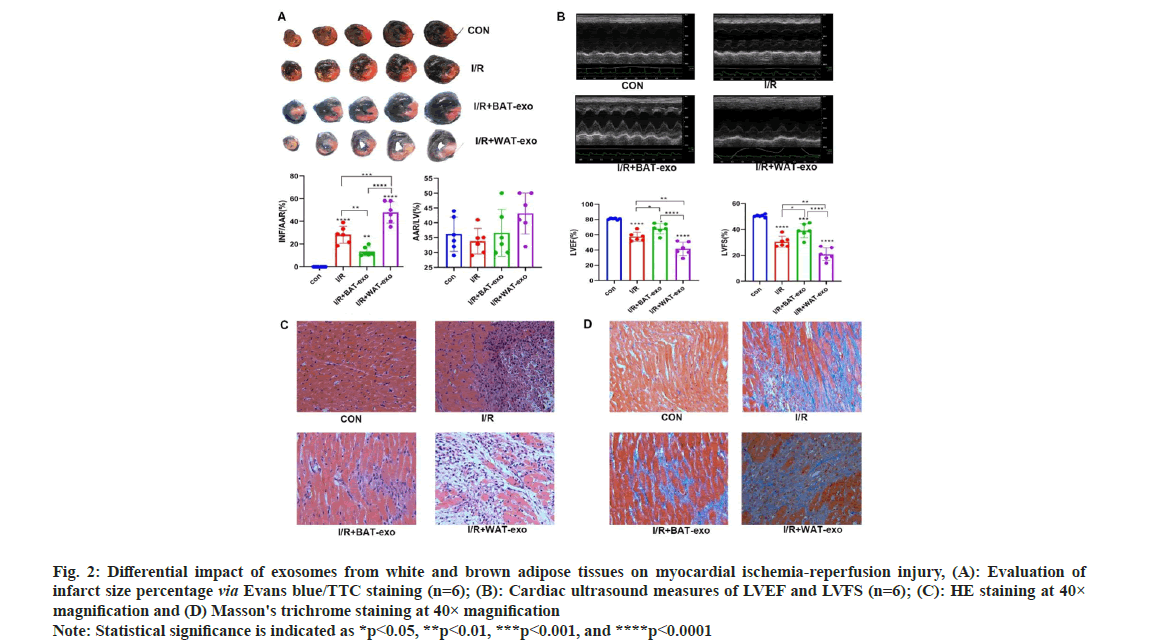
The Evans/TC staining indicated successful establishment of a rat model for ischemiareperfusion myocardial injury (fig. 2). The area of infarction in the Ischemia/Reperfusion (I/R)+BATexosome group was notably reduced compared to the I/R+PBS group (p<0.01). Conversely, the infarct area in the I/R+WAT-exosome group was notably larger than in the I/R+PBS group (p<0.01). Further, the I/R+BAT-exosome group demonstrated a significant improvement in left ventricular function (p<0.05), whereas the I/R+WAT-exosome group showed a significant decline (p<0.01). These findings suggest that brown adipose tissuederived extracellular vesicles exert protective effects against myocardial ischemia-reperfusion injury, whereas those from white adipose tissue exacerbate it.
Fig. 2: Differential impact of exosomes from white and brown adipose tissues on myocardial ischemia-reperfusion injury, (A): Evaluation of infarct size percentage via Evans blue/TTC staining (n=6); (B): Cardiac ultrasound measures of LVEF and LVFS (n=6); (C): HE staining at 40× magnification and (D) Masson's trichrome staining at 40× magnification Note: Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001
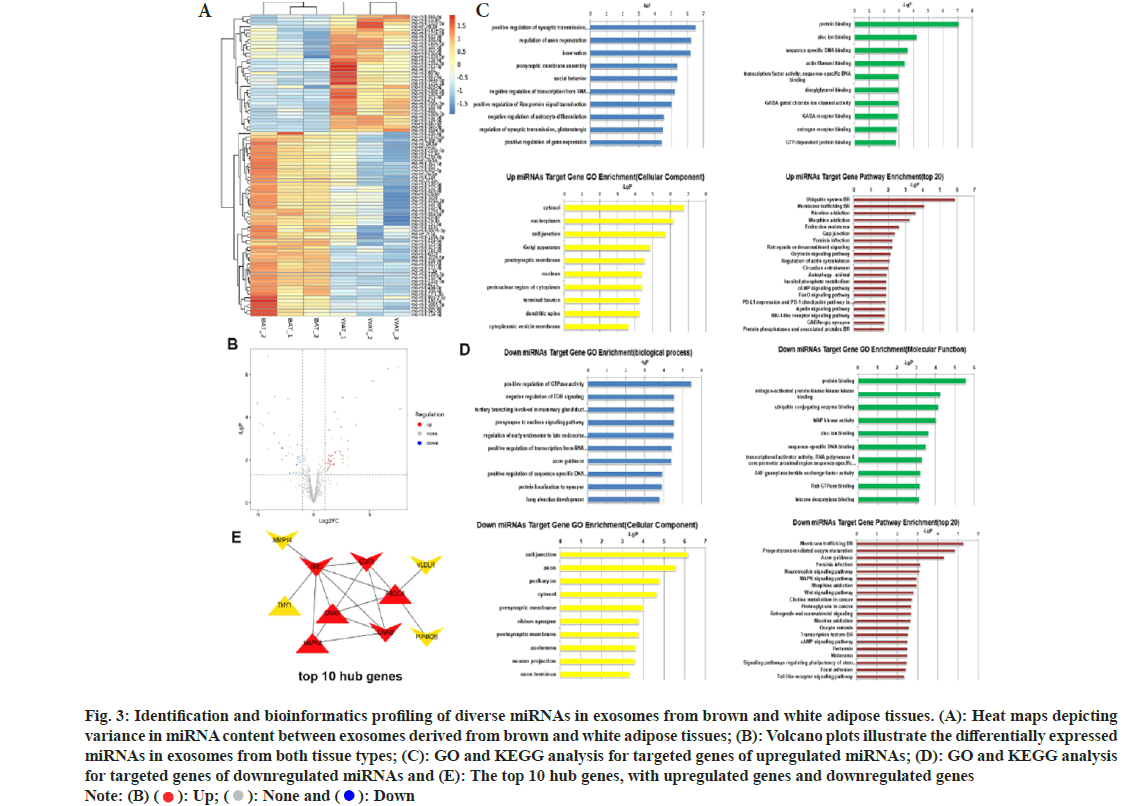
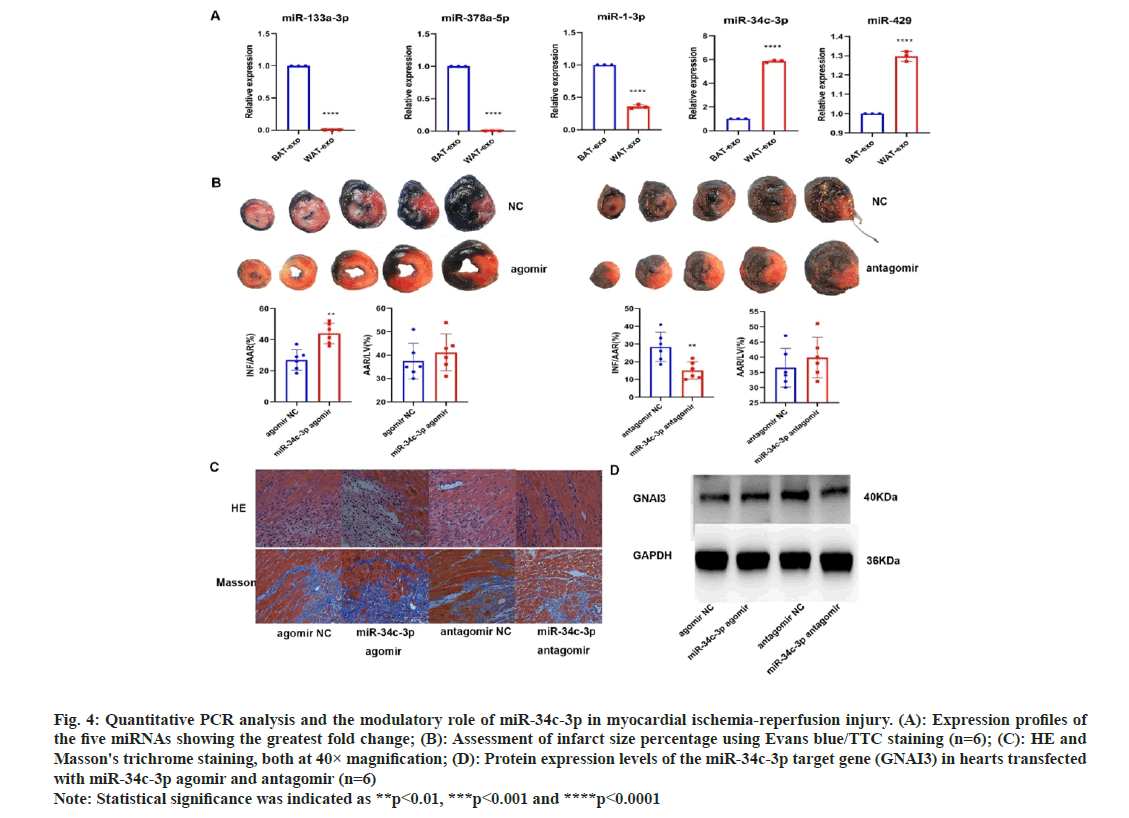
To explore the underlying mechanisms, miRNA sequencing was performed on both brown and white adipose tissue-derived vesicles. A total of 55 miRNAs were significantly unregulated, and 34 were downregulated in brown adipose tissue relative to white adipose tissue (fig. 3A and fig. 3B). Candidate target genes corresponding to these differentially expressed miRNAs were discovered via a cross-analysis of Miranda and TargetScan databases. The GO and KEGG pathway analysis for these target genes were also performed (fig. 3C-fig. 3E). Additionally, Hub gene targets were identified using Hubba software for proteinprotein interaction analysis. Among differentially expressed miRNAs, mir-133a-3p and mir-378a-5p were notably higher in brown adipose tissue, while mir-34c-3p and mir-429 were lower (fig. 4A), as confirmed by qPCR.
Fig. 3: Identification and bioinformatics profiling of diverse miRNAs in exosomes from brown and white adipose tissues. (A): Heat maps depicting variance in miRNA content between exosomes derived from brown and white adipose tissues; (B): Volcano plots illustrate the differentially expressed miRNAs in exosomes from both tissue types; (C): GO and KEGG analysis for targeted genes of upregulated miRNAs; (D): GO and KEGG analysis for targeted genes of downregulated miRNAs and (E): The top 10 hub genes, with upregulated genes and downregulated genes Note: (B) ( ): Up; (
): Up; ( ): None and (
): None and ( ): Down
): Down
Fig. 4: Quantitative PCR analysis and the modulatory role of miR-34c-3p in myocardial ischemia-reperfusion injury. (A): Expression profiles of the five miRNAs showing the greatest fold change; (B): Assessment of infarct size percentage using Evans blue/TTC staining (n=6); (C): HE and Masson's trichrome staining, both at 40× magnification; (D): Protein expression levels of the miR-34c-3p target gene (GNAI3) in hearts transfected with miR-34c-3p agomir and antagomir (n=6) Note: Statistical significance was indicated as **p<0.01, ***p<0.001 and ****p<0.0001
The miRNA with the most significant changes between brown and white adipose tissue-derived vesicles, miR-34C-3P, was further investigated for its role in myocardial ischemia-reperfusion injury. Agomirs and antagomirs for miR-34C-3P were introduced into the ventricles of rats subjected to ischemic myocardial reperfusion. Evans/TC and histological staining (fig. 4B and fig. 4C) demonstrated that miR-34C-3P agomir increased the infarct size, tissue edema, inflammatory cell infiltration, and fibrosis, whereas its antagomir had the opposite effects. TargetScan prediction revealed G Protein Subunit Alpha I (GNAI) as a potential target gene of miR-34C-3P, and Western blotting confirmed changes in the expression of GNAI protein (fig. 4D). These findings suggest that miR-34C-3P aggravates myocardial ischemiareperfusion injury through the modulation of GNAI expression.
Over the past three decades, epidemiological studies have established that visceral adipose tissue (commonly known as white fat) is a significant marker for cardiovascular and metabolic diseases, as well as overall mortality risk[23-25]. These findings have been consistently validated through imaging techniques like Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). Yet, the mechanisms underlying the detrimental impact of visceral fat on cardiovascular health remain elusive[26-28].
Several studies have posted different theories. Some suggest that visceral adipose tissue accelerates heart aging by affecting fibroblast aging through skeletal protein production. Others point to the role of exocrine secretions from visceral fat in atherosclerosis by modulating macrophage activity[29-35]. Our study adds a new dimension to this ongoing discussion by focusing on the effects of exocrine secretions from visceral adipose tissue during normal ischemic reperfusion in myocardial injuries.
Interestingly, our findings indicate that the composition of miRNAs differs substantially between brown and white adipose tissues, affecting the myocardial ischemia-reperfusion injury in distinct ways. For instance, miR-133a-3p and miR- 378 were found to be highly upregulated in brown adipose tissue and had protective roles during ischemic reperfusion, corroborating previous reports. Conversely, high levels of miR-34C-3P in white adipose tissue appeared to exacerbate ischemic reperfusion injuries[36-38].
Bioinformatics analysis further revealed several signaling pathways and key genes associated with ischemic reperfusion myocardial injury. Our results point to the potential regulatory roles of these miRNAs and pathways in myocardial damage, thereby underscoring the possible therapeutic implications of brown and white adipose tissues in metabolic and cardiovascular diseases[39].
Our study also highlights the importance of miR- 34C-3P in ischemic myocardial reperfusion injury. This miRNA was not only markedly altered between brown and white adipose tissues but also appeared to regulate the key gene G Protein Subunit Alpha I3 (GNAI3), contributing to the exacerbation of ischemic reperfusion injuries. Hence, miR- 34C-3P could serve as a new therapeutic target for managing ischemic reperfusion injuries in myocardial tissues[40-42].
In summary, our research provides a novel understanding of the role of exocrine secretions from visceral adipose tissue in myocardial ischemia-reperfusion injury, and it sheds light on possible therapeutic avenues for metabolic and cardiovascular diseases. Further studies are needed to elucidate the precise mechanisms by which these miRNAs and associated pathways exert their effects.
In this study, we have elucidated the distinct roles played by exosomes originating from brown and white adipose tissues in the context of myocardial ischemia-reperfusion injury. We also identified unique miRNA profiles associated with these exosomes. Specifically, our findings suggest that the exosome/miR-34c-3p/GNAI3 axis derived from white adipose tissue may offer a novel therapeutic target for treating myocardial ischemia-reperfusion injury.
Authors’ contributions:
The study was designed, data were collected and analysed, and the manuscript was drafted by Hangshun Li and Qinglei Zhu. Financial support was provided by Yu Wang. All authors have read and approved the final version of the manuscript for publication.
Acknowledgements:
Yu Wang and Qinglei Zhu have contributed same to this work.
Funding:
This research was generously supported by the National Natural Science Foundation of China (No.81670218).
Ethical approval:
All animal care and surgical procedures in this study were conducted in accordance with ethical guidelines and received approval from the Animal Ethics Committee of the Chinese People’s Liberation Army General Hospital, Beijing, China.
Conflict of interests:
The authors declared no conflict of interests.
References
- Chen HY, Xiao ZZ, Ling X, Xu RN, Zhu P, Zheng SY. ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med 2021;27(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Bugger H, Pfeil K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis 2020;1866(7):165768.
[Crossref] [Google Scholar] [PubMed]
- Xiao H, Zhang M, Wu H, Wu J, Hu X, Pei X, et al. CIRKIL exacerbates cardiac ischemia/reperfusion injury by interacting with Ku70. Circ Res 2022;130(5):e3-17.
[Crossref] [Google Scholar] [PubMed]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357(11):1121-35.
- Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res 2021;128(7):951-68.
[Crossref] [Google Scholar] [PubMed]
- Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med 2021;27(1):58-65.
- Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins and cardiovascular disease. Arteriosclerosis 1990;10(4):497-511.
[Crossref] [Google Scholar] [PubMed]
- Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, et al. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diabetes 2003;52(1):172-9.
[Crossref] [Google Scholar] [PubMed]
- Sironi AM, Petz R, de Marchi D, Buzzigoli E, Ciociaro D, Positano V, et al. Impact of increased visceral and cardiac fat on cardiometabolic risk and disease. Diabetic Med 2012;29(5):622-7.
[Crossref] [Google Scholar] [PubMed]
- Froy O, Garaulet M. The circadian clock in white and brown adipose tissue: Mechanistic, endocrine and clinical aspects. Endocr Rev 2018;39(3):261-73.
[Crossref] [Google Scholar] [PubMed]
- Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab 2018;28(3):476-89.
[Crossref] [Google Scholar] [PubMed]
- Pinckard KM, Shettigar VK, Wright KR, Abay E, Baer LA, Vidal P, et al. A novel endocrine role for the BAT-released lipokine 12, 13-diHOME to mediate cardiac function. Circulation 2021;143(2):145-59.
[Crossref] [Google Scholar] [PubMed]
- Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis and impaired angiogenesis. J Clin Invest 2017;127(1):74-82.
[Crossref] [Google Scholar] [PubMed]
- Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017;127(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose and methods for exosome isolation and analysis. Cells 2019;8(7):727.
[Crossref] [Google Scholar] [PubMed]
- Pegtel DM, Gould SJ. Exosomes. Annual Rev Biochem 2019;88:487-514.
- Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q, Bai M, et al. Adipocyte-derived exosomal MTTP suppresses ferroptosis and promotes chemoresistance in colorectal cancer. Adv Sci 2022;9(28):2203357.
[Crossref] [Google Scholar] [PubMed]
- Lin JR, Ding LL, Xu L, Huang J, Zhang ZB, Chen XH, et al. Brown adipocyte ADRB3 mediates cardioprotection via suppressing exosomal iNOS. Circ Res 2022;131(2):133-47.
[Crossref] [Google Scholar] [PubMed]
- Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev 2013;23(1):3-11.
[Crossref] [Google Scholar] [PubMed]
- Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542(7642):450-5.
- Zhao H, Chen X, Hu G, Li C, Guo L, Zhang L, et al. Small extracellular vesicles from brown adipose tissue mediate exercise cardioprotection. Circ Res 2022;130(10):1490-506.
[Crossref] [Google Scholar] [PubMed]
- Gan LU, Xie D, Liu J, Bond Lau W, Christopher TA, Lopez B, et al. Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation 2020;141(12):968-83.
[Crossref] [Google Scholar] [PubMed]
- Su M, Li W, Yuan Y, Liu S, Liang C, Liu HE, et al. Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner. Transl Res 2022;248:51-67.
[Crossref] [Google Scholar] [PubMed]
- Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS. Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol 2016;68(14):1509-21.
[Crossref] [Google Scholar] [PubMed]
- Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. International Atherosclerosis Society; International Chair on cardiometabolic risk working group on visceral obesity. Visceral and ectopic fat, atherosclerosis and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol 2019;7(9):715-25.
- Sawaki D, Czibik G, Pini M, Ternacle J, Suffee N, Mercedes R, et al. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation 2018;138(8):809-22.
[Crossref] [Google Scholar] [PubMed]
- Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc 2018;7(5):e007442.
[Crossref] [Google Scholar] [PubMed]
- Zhao X, Si L, Bian J, Pan C, Guo W, Qin P, et al. Adipose tissue macrophage-derived exosomes induce ferroptosis via glutathione synthesis inhibition by targeting SLC7A11 in obesity-induced cardiac injury. Free Radical Biol Med 2022;182:232-45.
[Crossref] [Google Scholar] [PubMed]
- Lee HA, Cho JH, Afinanisa Q, An GH, Han JG, Kang HJ, et al. Ganoderma lucidum extract reduces insulin resistance by enhancing AMPK activation in high-fat diet-induced obese mice. Nutrients 2020;12(11):3338.
[Crossref] [Google Scholar] [PubMed]
- Li M, Ding W, Tariq MA, Chang W, Zhang X, Xu W, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics 2018;8(21):5855-69.
[Crossref] [Google Scholar] [PubMed]
- Zhou R, Jia Y, Wang Y, Li Z, Qi J, Yang Y. Elevating miR-378 strengthens the isoflurane-mediated effects on myocardial ischemia-reperfusion injury in mice via suppression of MAPK1. Am J Transl Res 2021;13(4):2350.
[Google Scholar] [PubMed]
- Zhu Q, Hu F. Antagonism of miR-429 ameliorates anoxia/reoxygenation injury in cardiomyocytes by enhancing MO25/LKB1/AMPK mediated autophagy. Life Sci 2019;235:116842.
[Crossref] [Google Scholar] [PubMed]
- Jeremic N, Chaturvedi P, Tyagi SC. Browning of white fat: Novel insight into factors, mechanisms and therapeutics. J Cell Physiol 2017;232(1):61-8.
[Crossref] [Google Scholar] [PubMed]
- Rabinovich-Nikitin I, Rasouli M, Reitz CJ, Posen I, Margulets V, Dhingra R, et al. Mitochondrial autophagy and cell survival is regulated by the circadian Clock gene in cardiac myocytes during ischemic stress. Autophagy 2021;17(11):3794-812.
[Crossref] [Google Scholar] [PubMed]
- Xie M, Cho GW, Kong Y, Li DL, Altamirano F, Luo X, et al. Activation of autophagic flux blunts cardiac ischemia/reperfusion injury. Circ Res 2021;129(3):435-50.
[Crossref] [Google Scholar] [PubMed]
- Guo Y, Li Z, Shi C, Li J, Yao M, Chen X. Trichostatin A attenuates oxidative stress-mediated myocardial injury through the FoxO3a signaling pathway. Int J Mol Med 2017;40(4):999-1008.
[Crossref] [Google Scholar] [PubMed]
- Suchal K, Malik S, Gamad N, Malhotra RK, Goyal SN, Chaudhary U, et al. Kaempferol attenuates myocardial ischemic injury via inhibition of MAPK signaling pathway in experimental model of myocardial ischemia-reperfusion injury. Oxid Med Cell Longev 2016;2016:7580731.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Lu X. miR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/β-catenin signaling pathway via targeting MYBL2. J Cell Physiol 2019;234(12):22034-43.
[Crossref] [Google Scholar] [PubMed]
- Köhler D, Devanathan V, Bernardo de Oliveira Franz C, Eldh T, Novakovic A, Roth JM, et al. Gαi2-and Gαi3-deficient mice display opposite severity of myocardial ischemia reperfusion injury. PloS One 2014;9(5):e98325.
[Crossref] [Google Scholar] [PubMed]
- Alonso-Herranz L, Sahun-Espanol A, Paredes A, Gonzalo P, Gkontra P, Nunez V, et al. Macrophages promote endothelial-to-mesenchymal transition via MT1-MMP/TGFβ1 after myocardial infarction. Elife 2020;9:e57920.
[Crossref] [Google Scholar] [PubMed]
- Heliste J, Jokilammi A, Vaparanta K, Paatero I, Elenius K. Combined genetic and chemical screens indicate protective potential for EGFR inhibition to cardiomyocytes under hypoxia. Sci Rep 2021;11(1):16661.
[Crossref] [Google Scholar] [PubMed]
- Giricz Z, Makkos A, Schreckenberg R, Poling J, Lorchner H, Kiss K, et al. Swiprosin-1/EFhD-2 expression in cardiac remodeling and post-infarct repair: Effect of ischemic conditioning. Int J Mol Sci 2020:21(9):3359.
[Crossref] [Google Scholar] [PubMed]