- *Corresponding Author:
- N. B. Gadge
Department of Pharmacognosy and Phytochemistry, KLE University’s College of Pharmacy, JNMC Campus, Nehru Nagar, Belgaum-590 010, Karnataka, India
E-mail: navneetgadge@indiatimes.com
| Date of Submission | 27 October 2010 |
| Date of Revision | 3 May 2011 |
| Date of Acceptance | 10 May 2011 |
| Indian J Pharm Sci, 2011, 73 (3): 306-311 |
Abstract
The present study was aimed to investigate the diuretic effects of aqueous and crude ethanol extracts of Bombax ceiba L. fruits (family, Bombacaceae) using acute model in rats. A single individual dose of aqueous and ethanol extract of B. ceiba fruit (200 mg/kg and 400 mg/kg, p.o., each), frusemide and hydrochlorothiazide, (25 mg/kg, p.o., each) as reference diuretic drugs, were administered orally to dehydrated rats. Control group rats were fed with normal saline (25 ml/kg, p.o.). All rats were caged in metabolic cages in pairs and their urine output was monitored at 5 and 24 h intervals. Both extracts significantly increased the urine output in higher doses. Although, the onset of this diuretic action was gradual (within 5 h), it lasted throughout the studied period (up to 24 h). Further, the intensity of diuresis induced by aqueous extract (400 mg/kg) in 5 h was almost similar to that of frusemide and hydrochlothiazide. Aqueous extract of B. ceiba fruit also caused marked increase in urinary Na+ and K+ levels. However, the routine urinalysis showed non-significant alterations in pH and specific gravity by either dose of crude extracts of B. ceiba fruits. These effects demonstrate possible diuretic actions of B. ceiba fruit extracts and support its folklore use in various urinary ailments. Further studies need to be done to characterize the active phytoconstituents from fruits.
Keywords
Diuretics, frusemide, bombax ceiba, hydrochlorothiazide, urine
Diuretics are the drugs that increase the rate of urine flow and adjust the volume and composition of body fluids in a variety of clinical situations such as acute and chronic renal failure, cirrhosis of liver and hypercalciuria. Drug-induced diuresis is beneficial in many life-threatening disease conditions such as congestive heart failure (CHF), nephritis, hypertension and pregnancy toxemia [1]. A number of diuretics like mannitol, thiazides, frusemide, and spironolactone are used in practice [2]. However, most diuretic drugs have been associated with numerous adverse effects such as electrolyte imbalance, metabolic alterations, development of new-onset diabetes, activation of the renin-angiotensin and neuroendocrine systems, and impairment of sexual function [3]. Eshaghian et al. (2006) reported that higher loop diuretic dosages identify patients with CHF at particularly high risk for mortality [4].
In this scenario, the need for novel diuretics such as plant-based substances, which are considered to be relatively safe and possessing lower potential for adverse effects, is advocated. Many indigenous drugs have been cited in Sanskrit text in Ayurvedic systems of medicine, claiming to possess diuretic actions. In this regard, many plants have been studied systematically to establish the scientific validity to their diuretic potentials; however, yet, the need for truly satisfactory, safe and efficacious diuretic drug persists.
bombax ceiba L. Syn. Bombax malabaricum Schott. (Family: Bombacaceae), is a tall deciduous tree, found throughout the hotter forest regions of India and Ceylon. Young fruits of B. ceiba are used traditionally as an ethnomedicine for various ailments including calculous affections, chronic inflammation and ulceration of the bladder and kidneys [5]. Stem, root, flower, fruit and leaves of B ceiba have been reported to contain many important phytoconstituents including alkaloids, glycosides, phytosterols and triterpenoids (lupeol and beta-sitosterol), proteins, phenolic compounds (naphthalene derivatives, mangiferin, shamimin, kaempferol and quercetin) and tannins [6-10]. In recent decades, many potential uses of different extracts of B ceiba such as moderate oxytocic activity and cardiac stimulant properties [11], hypotensive and hypoglycaemic activity [12], analgesic and antioxidant activity [13,14] have been reported. In another study, the use of B. ceiba as a traditional anti-inflammatory agent [15] has been reported.
Notably, dried young fruits of B. ceiba are given in calculus affections and chronic inflammation and ulceration of the bladder and kidneys including strangury and other forms of dysuria [5]; however, the rationale behind its use is not yet scientifically established. In the present study, an effort has been made to ascertain the scientific validity to diuretic effects of B. ceiba using experimental model in rats.
Young fruits of B. ceiba were collected during April 2008, from Navi Mumbai, Maharashtra, India and authenticated at the Botanical Survey of India, Govt. of India, Ministry of Environment and Forests, Pune, India. A voucher specimen of the plant (NBGHIP-1) was deposited in the BSI herbarium. Further, fruits were dried in shade and ground to get coarse powder (approx. 40 mesh size). Hydrochlorothiazide (HTZ) and frusemide were procured as gift sample from Indoco Remedies Ltd., Navi Mumbai, Maharashtra, India. Urocolor 10 test strips (Standard Diagnostics Inc., South Korea) were used for routine urinalysis.
The ethanol extract (EtE, 10% w/v) was prepared using 95% v/v ethanol by Soxhlet method at temperature of 60 to 70 degree C (yield 14.23% w/w) and aqueous extract (AqE, 10% w/v) was prepared using chloroform water by maceration method for 7 days at room temperature (yield 12.04% w/w). Extracts were concentrated under vacuum. A suspension of AqE and EtE in 0.5% w/v sodium carboxymethyl cellulose was prepared for oral administration.
Healthy male Wister rats weighing between 150 to 200 g were procured from Shri Venkateshwara Enterprises, Bangalore, Karnataka, India. The animals were acclimatized to standard laboratory conditions (temperature: 22±30) and maintained on 12:12 h light: dark cycle. They were provided with regular rat chow (Lipton India Ltd., Mumbai, India) and drinking water ad libitum. The animal care and experimental protocol was in accordance with the committee for the purpose of control and supervision of experiments on animals (CPCSEA), New Delhi, India. An approval from institutional animal ethical committee (IAEC) was obtained wide resolution number JNMC/IAEC/ Res-2/7/2008 dated 19th December 2008.
An acute oral toxicity study was carried out as per the guidelines number 423 set by organization for economic co-operation and development (OECD) [16]. Based on the cut-off value of the median lethal dose (LD50), the therapeutically effective dose was derived.
The method of Lipschitz et al. with some modifications was used to study the diuretic activity in male Wistar rats [17]. Rats, fasted and deprived of water for 18 h prior to the experiment, were divided in seven groups containing eight rats in each. Group 1, serving as control, received normal saline (25 ml/ kg). Group 2 and 3 received frusemide (25 mg/kg) and HTZ (25 mg/kg) in saline as reference diuretic drugs, respectively [1,17]. Group 4 and 5 received the AqE (200 and 400 mg/kg) in saline, respectively and group 6 and 7 received the EtE (200 and 400 mg/kg) in saline, respectively. All groups received a single individual dose by oral route using gastric intubation method.
Immediately after dosing, a pair of two rats per cage was kept in metabolic cages and urine was collected at 5 h and 24 h intervals. Routine urinalysis including quantitative determination of pH and specific gravity along with presence of occult blood, bilirubin, urobilinogen, ketone bodies, proteins, nitrite, glucose, and leucocytes in urine was carried out using Urocolor test strips for urine samples of control and extract treated rats. Urine volume (ml/100 g) and Na+, K+ and Cl- concentrations (mEq/l/100 g) in the urine were determined [1,18] and various indices for diuretic action were calculated [17,19,20].
Diuretic index= urine volume of test group/urine volume of control group; Saluretic index= urinary excretion of electrolyte of test group/urinary excretion of electrolyte of control group; Natriuretic index= urinary excretion of Na+/urinary excretion of K+; Ion quotient= urinary excretion of Cl-/sum of urinary excretion of Na+ and K+
Results were expressed as mean±SEM. Significance of difference between control and treated group was determined by unpaired Student′s t-test using statistical package (Graphpad Prism, Version 4.00.255). Level of significance chosen was P<0.05.
Orally administered to rats with starting dose of 2000 mg/kg, AqE and EtE of B. ceiba increased urination and defecation. No mortality was observed with starting dose. However, two rats among each group died with the confirmatory dose of both extracts. Further, treatment with the lower dose (300 mg/kg, p.o.) of AqE and EtE was found to be safer and the mortality rate was reduced to zero. Hence, therapeutic doses were calculated from 1/10th and 1/5th of the LD50 cut-off dose (2000 mg/kg) for both extracts.
Table 1 showed the effect of oral administration of AqE and EtE in rats. The results clearly indicate that no abrupt change in pH and specific gravity was seen in any of the extract treated groups. Also, the urinary excretion of bilirubin, urobilinogen, leucocytes, ketone bodies and nitrite were found to be below detectable limits among all experimental groups (data not given). Higher doses (400 mg/kg) of AqE and EtE showed traces of proteins in urine, while, other groups were devoid of preoteinuria and glucosuria.
| Group | Dose (mg/kg) | pH | Specific gravity | Glucose | Protein |
|---|---|---|---|---|---|
| Control | --- | 6.92 ± 0.15 | 1.022 ± 0.002 | Absent | Absent |
| AqE | 200 | 7.25 ± 0.21 | 1.027 ± 0.002 | Absent | Absent |
| 400 | 7.08 ± 0.20 | 1.023 ± 0.003 | Absent | Trace | |
| EtE | 200 | 7.17 ± 0.25 | 1.018 ± 0.004 | Absent | Absent |
| 400 | 7.00 ± 0.26 | 1.015 ± 0.003 | Absent | Trace |
Table 1: Effect of bombax ceiba fruit extracts on miscellaneous urinary parameters in control and experimental rats
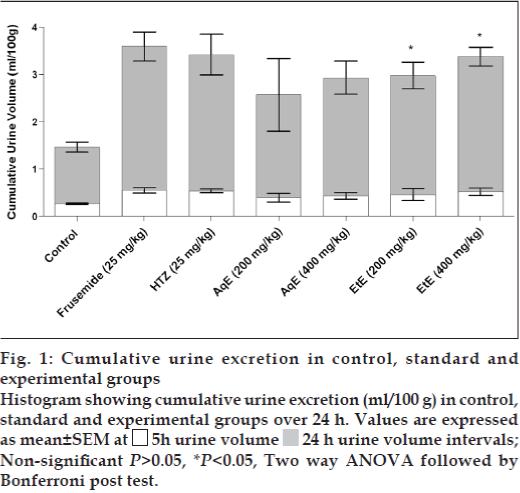
Among the reference diuretic drugs, frusemide and HTZ-induced excretion values of water of more than 100%, when compared to the untreated control group (Table 2). Both crude extracts in either dose significantly increased the urine output. A gradual onset of diuretic action was observed within first 5 h comparable to all three reference diuretic drugs. This effect lasted up to 24 h showing significantly (P<0.05) increased urine excretion volume (fig. 1). The intensity of diuresis induced by EtE (400 mg/ kg) in 5 h was almost similar to that of frusemide and HTZ (Table 2). In comparison with AqE, the EtE showed higher diuretic index.
| Groups | Dose (mg/kg, p.o.) | 5 h | 24 h | ||
|---|---|---|---|---|---|
| Urine excretion(ml/100 g) | Diureticindex | Urine excretion(ml/100 g) | Diureticindex | ||
| Control | - | 0.26 ± 0.02 | 1.00 | 1.20 ± 0.11 | 1.00 |
| Frusemide | 25 | 0.55 ± 0.06* | 2.12 | 3.04 ± 0.31* | 2.53 |
| HTZ | 25 | 0.54 ± 0.04* | 2.08 | 2.88 ± 0.43* | 2.40 |
| AqE | 200 | 0.39 ± 0.09 | 1.51 | 2.18 ± 0.57 | 1.82 |
| 400 | 0.43 ± 0.07* | 1.66 | 2.50 ± 0.35* | 2.08 | |
| EtE | 200 | 0.46 ± 0.13 | 1.77 | 2.52 ± 0.28* | 2.10 |
| 400 | 0.52 ± 0.08* | 2.01 | 2.86 ± 0.20* | 2.38 | |
Table 2: Effect of bombax ceiba fruit extracts on urine excretion volume
Figure 1: Cumulative urine excretion in control, standard and experimental groups Histogram showing cumulative urine excretion (ml/100 g) in control, standard and experimental groups over 24 h. Values are expressed as mean±SEM at  5h urine volume
5h urine volume  24 h urine volume intervals; Non-significant P>0.05, *P<0.05, Two way ANOVA followed by Bonferroni post test.
24 h urine volume intervals; Non-significant P>0.05, *P<0.05, Two way ANOVA followed by Bonferroni post test.
An increase in the urinary excretion of electrolytes (Na+, K+ and Cl-) between 20 to 90%, exhibited by the reference diuretic drugs over 24 h period, was found significant (P<0.01) in comparison with the control group (Table 3). Both extracts of B. ceiba fruit showed significant increase in urinary Na+ and Cl- levels and a reduction in the osmolarity of urine. Except with EtE (400 mg/kg) showing a significant (P<0.05) increase in urinary excretion of K+, the kaliuretic changes with other extracts were non-significant. An increase in Na+ excretion shown by EtE over a period of 24 h was significant (P<0.05) compared to control group. Although AqE administration resulted in moderate diuresis, the rise in urinary electrolyte excretion was less than 50%; whereas, EtE treatment showed nearly 30 to 70% increase in urinary electrolyte excretion, when compared to control group (Table 3). Moreover, the higher dose (400 mg/kg) of EtE showed significant saluretic activity.
| Groups | Dose(mg/kg, p.o.) | Concentration of ions(mEq/l/100 g)† | Saluretic index | Natriuretic index | Ion quotient | ||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl- | Na+ | K+ | Cl- | ||||
| Control | - | 2.88 ± 0.23 | 1.08 ± 0.13 | 3.72 ± 0.13 | 1.00 | 1.00 | 1.00 | 2.67 | 0.94 |
| Frusemide | 25 | 5.43 ± 0.27* | 1.47 ± 0.09* | 5.52 ± 0.24* | 1.89 | 1.36 | 1.48 | 3.69 | 0.80 |
| HTZ | 25 | 5.00 ± 0.19* | 1.29 ± 0.05 | 5.02 ± 0.16* | 1.73 | 1.20 | 1.35 | 3.86 | 0.80 |
| AqE | 200 | 3.18 ± 0.23 | 1.18 ± 0.04* | 3.71 ± 0.22 | 1.10 | 1.10 | 1.00 | 2.69 | 0.85 |
| 400 | 3.40 ± 0.21 | 1.28 ± 0.04* | 3.97 ± 0.08* | 1.18 | 1.19 | 1.07 | 2.65 | 0.85 | |
| EtE | 200 | 3.95 ± 0.23* | 1.10 ± 0.09 | 4.13 ± 0.10* | 1.37 | 1.01 | 1.11 | 3.63 | 0.82 |
| 400 | 4.98 ± 0.33* | 1.21 ± 0.07 | 4.93 ± 0.28* | 1.73 | 1.12 | 1.32 | 4.13 | 0.80 | |
n (number of pairs in each group) = 4; Values are mean±SEM; *P<0.05 vs Control (Saline 25 mg/kg, p.o.); Student’s t-test (unpaired); †Urine collected for 24 h after treatment.
Table 3: Effect of bombax ceiba fruit extracts on urinary excretion of electrolytes
Diuresis occurs by mainly two phenomena including, net increase in urine volume (water excretion) and elevated excretion of electrolytes (solutes) in the urine [21]. These processes result from suppression of renal tubular reabsorption of water and electrolytes into the blood stream. The thiazide diuretics inhibit Na+/Cl- symporter (co-transporter system) in the distal convoluted tubule, by competing for the Cl- binding site and thereby increasing the excretion of Na+ and Cl-, while the loop diuretic reference drug, frusemide, increases the urine output and urinary excretion of Na+ by inhibiting Na+/K+/Cl- symporter in the thick ascending limb of Loop of Henle [21].
In the present study, 25 mg/kg dose of frusemide and HTZ, each, showed significant diuresis in rats over a period of 24 h. In comparison, similar increase in urine excretion was found with the AqE and EtE of B. ceiba, when administered orally in dehydrated rats (Table 2). While, the different results obtained on electrolytic excretion by both extracts of B. ceiba suggest a difference in their comparative diuretic profile. AqE and EtE show a gradual rise in excretion of electrolytes (Na+, K+ and Cl-) in a dose-dependent manner. Particularly the AqE showed unsubstantial rise in urinary K+ levels. Further, there was no alkalization of urine. Collectively, these observations suggest that the AqE act as potassiumsparing diuretics in agreement with its natriuretic index [22-24].
Although the thiazide diuretics, like HTZ, increase only the urinary K+ level and alter the urinary Na+/K+ ratio [22,23], the administration of EtE in this study caused increase in urinary K+ levels along with urinary Na+ and Cl- levels without significant alteration in the Na+/K+ ratio. Such, activity of EtE reflect that is unlikely to predict the modus operandi of EtE as thiazide or loop diuretics. Moreover, the evidences suggest that especially the higher dose (400 mg/kg) of EtE act by kaliuretic action in conjunction with its saluretic activity. Further, the intensity of aquaresis and accompanied marked increase in urinary Na+ and K+ levels by the EtE of B. ceiba fruits were similar to that of furosemide and HTZ. Also, changes in urine pH were non-significant. These features strongly suggest that the EtE act more as a loop diuretic than as thiazide diuretic, mainly due to its higher natriuretic, kaliuretic and saluretic actions. Loop diuretics mainly inhibit the Na+/K+/Cl- symporter in the thick ascending Loop of Henle; thereby cause to increase natriuresis and kaliuresis [22-24]. Nevertheless, moderate acidification of urine is also seen with these diuretics [22,23,25]. Besides another important index, viz. the ion quotient in between 0.8 to 1.0, as exhibited by the experimental groups, suggests their unequivocal association with carbonic anhydrase inhibition [17].
The onset of the diuretic activity of the AqE and EtE was gradual till first 5 h after dose administration, which is in conformity with clinically, used synthetic loop diuretics [22,23]. Interestingly, in spite of the heavy loss of urinary Na+ and K+, there was a significant reduction in the osmolarity of urine in AqE and EtE treated rats. Considering the fact that inhibition of ADH causes polyurea with low osmolarity [25,26], it is possible that the AqE and EtE induced diuresis in the present study, may also be due to the impaired basal secretion of ADH and/or diminished sensitivity of uriniferous tubules to the action of ADH.
High ceiling loop diuretics are clinically used in patients with salt and water overload due to host of conditions. The observed mode of action of AqE and EtE in the present study, indicate that traditional practitioners may find B. ceiba fruits being useful as a non toxic natural therapeutic agent in the treatment of conditions such as pulmonary oedema, cardiac oedema and hypertension [22,23]. Besides the only limitation of increased risk of hypokalaemia, as with other therapeutically used loop diuretics, the diuretic actions of crude extracts B. ceiba fruits conclude the plant as an appealing alternative to presently available diuretic drugs. Though the onset of the diuretic action of the AqE and EtE was fairly gradual, it had a substantially prolonged duration of action. Thus, it is probable that it would curtail the frequency of administration of a diuretic drug.
The findings from present study support the folklore use of bombax ceiba fruits for their diuretic actions. The plant extracts, at the dose studied, do not seem to have renal toxicity in rats. Although, the phytochemistry of active components remain unidentified, based on the pattern of excretion of water and electrolytes, it appears that that there are at least two types of active principals present in these extracts, one having a frusemide-like activity and the other a HTZ-like activity. However, further systematic phytochemical studies are necessary to elucidate the probable structure activity relationship of biomolecules.
Acknowledgements
The corresponding author is especially thankful to Indian Council of Medical Research, New Delhi, India, for providing the financial assistance in the form of SRF.
References
- Agunu A, Abdurahamn EM, Andrew GO, Muhammed Z. Diuretic activity of stem bark extracts of Steganotaenia araliaceae Hoechst [Apiaceae]. J Ethnopharmacol 2005;96:471-5.
- Singh RG, Singh RP, Usha KP. Experimental evaluation of diuretic action of herbal drug (Tribulus terrestris Linn.) on albino rats. J Res Edu Indian Med 1991;3:19-21.
- Gupta S, Neyses L. Diuretic usage in heart failure: A continuing conundrum in 2005. Eur Heart J 2005;26:644-9.
- Eshaghian S, Horwich T, Fonarow G. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006;97:1759-64.
- Kapoor LD. Handbook of ayurvedic medicinal plants. Boca Raton: CRC Press; 1990. p. 80-1.
- Mehra PN, Karnick CR. Pharmacognostic studies on Bombax ceiba Linn. Indian J Pharm 1968;30:284.
- Mukherjee J, Roy B. Chemical examination of Salmalia malabarica Schott Endl. (syn. Bombax malabaricum DC.). J Indian ChemSoc 1971;48:769.
- Seshadri V, Batta AK, Rangaswamy S. Phenolic components of Bombax malabaricum (Root-Bark). CurrSci 1971;40:630-1.
- Harish G, Gupta RK. Chemical constituents of Salmalia malabarica Schott etEndl. flowers. J Pharm Sci 1972;61:807.
- Seshadri V, Batta AK, Rangaswamy S. Phenolic components of Bombax malabaricum. Indian J Chem 1973;11:825.
- Misra MB, Mishra SS, Misra RK. Pharmacology of Bombax malabaricum(DC). Indian J Pharm 1968;30:165.
- Saleem R, Ahmad M, Hussain SA, Qazi AM, Ahmad SI, Qazi MH, et al. Hypotensive, hypoglycaemic and toxicological studies on theflavonol C-glycoside shamimin from Bombax ceiba. Planta Med 1999;65:331-4.
- Dar A, Faizi S, Naqvi S, Roome T, Zikr-ur-Rehman S, Ali M. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol Pharm Bull 2005;28:596-600.
- Vieira TO, Said A, Aboutabl E, Azzam M, Creczynski-Pasa TB. Antioxidant activity of methanolic extract of Bombaxceiba. Redox Rep 2009;14:41-6.
- Namsa ND, Tag H, Mandal M, Kalita P, Das AK. An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J Ethnopharmacol 2009;125:234-45.
- Anonymous. OECD series on testing and assessment, Number 24. Guidance document on acute oral toxicity testing. ENV/JM/ MONO(2001)4. Paris: Environment directorate, OECD; 2001.
- Lipschitz WL, Haddian Z, Kerpscar A. Bioassay of diuretics. J Pharmacol Exp Ther 1943;79:97-110.
- Swain SR, Sinha BN, Murthy PN. Anti inflammatory, diuretic and antimicrobial activities of Rungia pectinata Linn. And Rungiarepens Nees. Indian J Pharm Sci 2008;70:679-83.
- Martin-Herrera D, Abdala S, Benjume D, Gutierrez-Luis J. Diuretic activity of some Withania aristata Ait. fractions. J Ethno pharmacol 2008;117:496-9.
- Mukherjee PK. Evaluation of diuretic agents, in: Quality control of herbal drugs. New Delhi: Business Horizons; 2002.
- Jackson EK. Drugs affecting renal and cardiovascular function, In: Goodman and Gilman’s the Pharmacological Basis of Therapeutics. Hardman JC, Gilman AG, Limbird LE, editors. 9th ed. New York: Pergamon Press; 1996. p. 685-713.
- Rang HP, Dale MM, Ritter JM. Pharmacology. London: Churchill Livingstone; 1995. p. 367-84.
- British National Formulary. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2000. p. 63-9.
- Kreydiyyeh SI, Julnar V. Diuretic effect and mechanism of action of parsley. J Ethnopharmacol 2002;79:353-7.
- Osorio FV, Teitelbaum I. Mechanisms of defective hydroosmotic response in chronic renal failure. J Nephrol 1997;10:232-7.
- Mayne PD. Clinical chemistry. London: Edward Arnold; 1994. p. 2-24.