- *Corresponding Author:
- K. Y. Hsu
College of Pharmacy, Taipei Medical University, 250 Wu-Hsing Street, Taipei 11031, Taiwan, R.O.C.
E-mail: kyhsu@tmu.edu.tw
| Date of Submission | 07 June 2014 |
| Date of Revision | 02 February 2015 |
| Date of Acceptance | 16 September 2015 |
| Indian J Pharm Sci 2015;77(5):573-578 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The aim of this study was to investigate differences in organic anion transporting polypeptide 1A2 activity among the Taiwanese population via an analysis of 3 pharmacokinetic studies completed in a total of 103 healthy male Taiwanese subjects. The pharmacokinetics of fexofenadine was measured as an indicator of organic anion transporting polypeptide 1A2 activity. Using the Kolmogorov-Smirnov test and quantile plots, the frequency distributions of area under the concentration-time curve and concentration were shown to be tri-modal and to represent 3 pharmacokinetic phenotypes. In a comparison with published data, the mean area under the concentration-time curve of fexofenadine in the Taiwanese subjects was similar to that in American, German, and Indian subjects, but significantly different from that in some Asian populations, including Korean and Japanese ethnic groups. These results suggested that Taiwanese subjects showed genetic variation in fexofenadine pharmacokinetics that was associated with differences in organic anion transporting polypeptide 1A2 activity.
Keywords
Fexofenadine, OATP1A2, organic anion transporting polypeptide 1A2, pharmacokinetics, Taiwanese
Introduction
Organic anion transporting polypeptide (OATP) transporters are expressed in organs and tissues that are involved in pharmacokinetics, where they mediate cellular uptake of endogenous substances, drugs, and xenobiotics [1]. Thirty-nine members of the OATP superfamily have been identified in human and mammalian species; they are capable of facilitating the cellular uptake of endogenous substances, drugs, and xenobiotics in the intestine and liver, and of transporting these substances across the blood-brain barrier [2,3]. Among the OATP superfamily members identified in humans, OATP1A2 and OATP2B1 (encoded by SLCO1A2 and SLCO2B1, respectively) are the only examples associated with drug transport in the enterocytes [2,4].
Consistent with inhibition of OATP1A2 at the apical membrane of enterocytes, in vivo studies have shown that the AUC of fexofenadine decreased by approximately 25% by naringin, a low-strength OATP1A2 inhibitor, and decreased by 40–70% by consumption of grapefruit juice or orange juice, which are strong OATP1A2 inhibitors [5,6]. Similar studies were conducted using oral talinolol, in which inhibition of OATP1A2 decreased talinolol bioavailability by 44% [7,8]. These studies suggest that OATP1A2 plays an important pharmacokinetic role, particularly in determining absorption capability.
Fexofenadine is a selective antihistamine that is used to relieve symptoms of allergic rhinitis, and which has fewer anticholinergic effects than other H1-antihistamines [9,10]. It undergoes minimal metabolism in humans (less than 5%) and is excreted unchanged in urine and faeces after oral administration [11-13]. In vitro and in vivo studies have shown fexofenadine to be a substrate of OATP1A2 and OATP2B1. However, Shimizu et al. conducted in vitro tests that showed that OATP2B1 did not transport fexofenadine significantly, and that OATP1A2 was a major mediator of fexofenadine uptake [14]. These properties make fexofenadine an optimal choice as a probe drug to evaluate OATP1A2 activity in humans [11,15,16].
Some SNPs in the SLCO1A2 gene have been shown to decrease OATP1A2 transport activity In vitro for substrates such as estrone-3-sulfate and imatinib [17,18]. In vitro studies on OATP1A2 variants A516C and A404T showed markedly reduced transport of estrone-3-sulfate. Variants G559A and C2003G also appeared to possess altered transport activities [17]. The effects of SLCO1A2 polymorphisms on imatinib pharmacokinetics were investigated in healthy volunteers and patients, and results showed that the pharmacokinetics of imatinib were altered in patients with the 1105G>A/1032G>A and 361GG SLCO1A2 genotypes [18]. These findings suggest that SLCO1A2 polymorphisms significantly affect the pharmacokinetics of OATP1A2 substrates such as imatinib and estrone-2-sulfate. Moreover, OATP1A2 variants were observed to be dependent on ethnic background, and such differences may result in varied AUC of OATP1A2 in particular populations [19].
Based on these previously published results, fexofenadine was used as a probe drug to evaluate the function of OATP1A2 in Taiwanese subjects, and clustering methods were used to identify distinct pharmacokinetic phenotypes based on pharmacokinetic results.
Materials and Methods
Chromatographic conditions and analytical methods
The analysis methods used in all studies reported herein were previously described by Chen et al [20]. The chromatographic instrument consisted of a highperformance liquid chromatography system (1100 Series; Agilent Technologies, Wilmington, DE, USA) equipped with a tandem mass spectrometry detection system. The calibration curves for the ratio of peak area and concentration of losartan versus those of fexofenadine showed a linear relationship over a concentration range of 1–1000 ng/ml. The mean relative error ranged from 3.0–6.0%, and the coefficients of variance were all within acceptable ranges.
Subjects
This study examined 104 healthy male Taiwanese subjects from 3 pharmacokinetic studies conducted at a single clinical facility in Taiwan, of which 103 subjects completed their respective studies. The study subjects were fasted overnight and administered a single dose of Allegra® (Sanofi-Aventis U.S. LLC) at a dose of 180 mg with 240 ml of water. Venous blood samples (10 ml) were drawn in heparinised tubes before dosing (0 h) and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 10, 12, 24, 36, 48, and 60 h after drug administration. These study protocols were approved by the Institutional Review Board of Mackay Memorial Hospital (Taipei, Taiwan) and conducted in Formotract Health Clinics (Taipei, Taiwan).
Data analysis
Individual fexofenadine plasma concentration data were analysed by a non-compartmental method using the WinNonlin® 6.3 (Pharsight, Cary, NC) program. Pharmacokinetic parameters including the maximum observed concentration (Cmax), the time to peak concentration (Tmax), the area under the concentration-time curve from time 0 to the last quantifiable concentration (AUC0–t), the area under the concentration-time curve from time 0 to infinity (AUC0–∞), plasma half-life (t1/2), and oral clearance (CL/F) were reported. All data are expressed as mean±standard deviation.
To evaluate AUC and Cmax phenotypes and their frequencies in the Taiwanese population, the normal distribution was estimated using the Kolmogorov-Smirnov method. The quantile-quantile plots (QQ plots), agglomerative hierarchical clustering and non-hierarchical K-means clustering were used to identify each phenotype. The pharmacokinetic parameters of fexofenadine among the identified phenotypes were compared using one-way ANOVA.
An extensive literature search was performed to identify published fexofenadine pharmacokinetic studies in other populations to compare corresponding pharmacokinetic parameters. The data were collected and summarized as arithmetic means and standard deviations. In published investigations, the pharmacokinetic properties of fexofenadine were similar after administration of single and multiple doses and appeared to be dose-proportional over a range of 10–800 mg [21,22]. With respect to linear pharmacokinetics, it was reasonable to compare the data after normalizing doses.
For relief of symptoms of seasonal allergic rhinitis, the most common fexofenadine dose is 120 mg, and the most common dose for the treatment of chronic idiopathic urticaria is 180 mg. The recommended maximum daily dose of fexofenadine is 240 mg. Thus, further discussion is focused on commonly used doses of fexofenadine, which ranged from 120 mg to 240 mg [15]. To minimize variation in AUC0–t, AUCs were adjusted and recalculated based on equivalent duration. Thus, AUCs truncated to 12, 24 and 48 h values were used to compare our data with published data. The two-sample t-test was used to determine the statistical significances of difference between our data and published data.
Results
Of the 104 subjects initially enrolled, 103 subjects completed the study. One subject was withdrawn voluntarily. The mean age was 23.7±3.5 years, the mean body weight was 64.7±6.4 kg, and the mean BMI was 21.9±1.7 kg/m2. Nine adverse events were reported from 8 subjects. All adverse events were reported to be mild in intensity. No serious adverse events were observed.
The pharmacokinetic parameters of a single dose of 180 mg fexofenadine in 103 healthy Taiwanese volunteers are summarized in Table 1. After oral 180 mg fexofenadine administration, the Cmax, AUC0–t, AUC0–∞, CL/F, and t1/2 were 682.49±301.20 ng/ml, 4323.75±1688.84 h×ng/ml, 4379.57±1691.94 h×ng/ml, 47.7±19.0 l/h, and 10.83±4.59 h, respectively. The mean ratio of AUC0–t/AUC0–∞ was greater than 80%, which indicated a suitable sampling schedule.
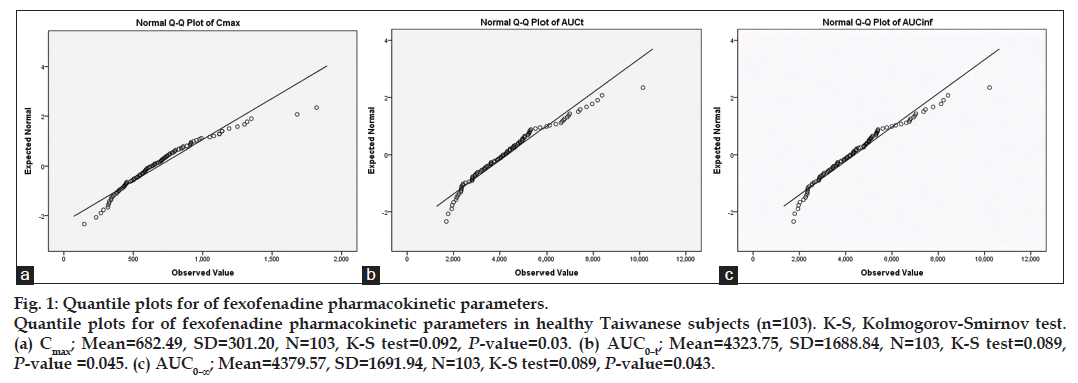
Fig. 1 shows quantile-quantile plots (QQ plots) with the results of the K-S tests for fexofenadine Cmax, AUC0–t, and AUC0–∞. Based on agglomerative hierarchical clustering and non-hierarchical K-means clustering for AUC0–t, 3 distinct phenotypes were identified and denoted as high absorption/exposure (HA), immediate absorption/exposure (IA), and poor absorption/exposure (PA), with frequencies of 15.6, 43.1, and 42.3%, respectively.
Fig. 1: Quantile plots for of fexofenadine pharmacokinetic parameters. Quantile plots for of fexofenadine pharmacokinetic parameters in healthy Taiwanese subjects (n=103). K-S, Kolmogorov-Smirnov test. (a) Cmax; Mean=682.49, SD=301.20, N=103, K-S test=0.092, P-value=0.03. (b) AUC0–t; Mean=4323.75, SD=1688.84, N=103, K-S test=0.089, P-value =0.045. (c) AUC0–∞; Mean=4379.57, SD=1691.94, N=103, K-S test=0.089, P-value=0.043.
Table 1 shows the mean pharmacokinetic parameters for these 3 identified phenotypes. Tmax and t1/2 showed no significant differences among the 3 identified phenotypes. For other pharmacokinetic parameters, there were statistically significant differences between each group. The lack of change in Tmax and t1/2 indicated that there were no differences in fexofenadine elimination rates among these 3 groups. Significant differences in CL/F among the groups indicated differences in bioavailability. These results show that polymorphisms influence fexofenadine pharmacokinetics exist in the Taiwanese population.
| Parameters | All subjects | HA | IA | PA |
|---|---|---|---|---|
| n(%) | 103 (100) | 16 (15.6) | 46 (43.1) | 41 (42.3) |
| Tmax (h)a,b | 2.11±1.16 | 2.13±0.74 | 1.99±1.20 | 2.25±1.26 |
| C max (ng/ml)a,c | 682.49±301.20 | 1130.38±327.46 | 744.67±164.53 | 437.93±121.14 |
| AUC 0–t (h×ng/ml)a,c | 4323.75±1688.84 | 7322.64±995.16 | 4672.07±549.65 | 2762.66±571.84 |
| AUC0–∞ (h×ng/ml)a,c | 4379.57±1691.94 | 7376.29±1005.64 | 4731.22±563.18 | 2815.57±567.54 |
| CL/F (l/h)a,c | 47.7±19.0 | 24.8±3.0 | 38.6±4.6 | 66.8±14.7 |
| t1/2 (h)a,b | 10.83±4.59 | 11.56±2.69 | 10.97±4.27 | 10.38±5.49 |
| Ratio | ? | 1.69 | 1.08 | 0.64 |
Pharmacokinetic parameters of fexofenadine in different phenotypes within Taiwanese subjects. Data are expressed as mean±SD. Ratio: compared to the mean. a: ANOVA tests within the HA, IA and PA groups; b: compared with the other 2 phenotypes, both P-values >0.05; c: compared with the other two phenotypes, both P-values <0.05. SD: standard deviation, HA: high absorption/exposure, IA: immediate absorption/exposure, PA: poor absorption/exposure, Cmax : maximum observed concentration, Tmax: time to peak concentration, AUC0–t: area under the concentration?time curve from time 0 to the last quantifiable concentration, AUC0–∞: area under the concentration?time curve from time 0 to infinity, t1/2: Plasma half?life, CL/F: Oral clearance
Table 1: Pharmacokinetics Of Fexofenadine In Three Phenotypes
Studies of fexofenadine pharmacokinetics dose ranged from 120 to 240 mg have been performed in healthy American [21,23], Chinese [24,25], German [26], Indian [27], Japanese [28-30], and Korean [31] volunteers. To minimize variation in AUC0–t, AUCs were adjusted and recalculated based on equivalent duration. Results from the present study on the pharmacokinetic parameters of fexofenadine at these common doses were shown in Table 2. Fexofenadine AUCs in Taiwanese subjects were similar to those in American, Indian, and German subjects, but were significantly different from those in Korean and Japanese subjects.
| Dose (mg) | Population | n | Age (years) | Tmax (h) | Cmax (ng/ml) | AUC0–t (h×ng/ml) | AUC0–∞ (h×ng/ml) |
|---|---|---|---|---|---|---|---|
| 120 | American[21] | 24 | 19–45 | 1.44±0.68 | 427±170.8 | 2682±911.88b | |
| 120 | American[23] | 24 | 18–43 | 2.6±1.47 | 382.3±149.25 | 2524.25±788.07b | |
| 120 | Chinese[24] | 10 | 23.2±2.3 | 3.58±1.17 | 365.98±168.02 | 2404.3±885.5 | 2437.5±885.9b |
| 120 | Chinese[25] | 14 | 25–28 | 2.25±0.47 | 745.11±137.41* | 3894.27±923.45* | 3993.84±912.97*,a |
| 120 | Indian[27] | 60 | NA | 3.72±1.23 | 463±182 | 3109±1097 | 3175±1118b |
| 120 | Japanese[28] | 10 | 21–24 | 2.1±0.9 | 610±222* | 3808±1266*,b | |
| 120 | Japanese[29] | 12 | 25.2±5.6 | 1.5# | 611±206* | 3569±1222* | 3637±1199*,b |
| 120 | Japanese[30] | 8 | 22.8±1.4 | 1# | 699±366* | 4133±1776*,b | |
| 120 | Korean[31] | 12 | 22–30 | 2.2±1.1 | 304.4±139.6* | 2075.7±557.1* | 2159.2±573*,a |
| 180 | German[26] | 4 | NA | 1.5±0.6 | 734.5±261.3 | 4107.5±1837.4d | |
| 180 | Taiwanese | 103 | 20–39 | 2.11±1.16 | 682.49±301.20 | 4323.75±1688.84 | 4379.57±1691.94c |
| 240 | American[21] | 24 | 19–45 | 1.52±0.62 | 1119±548.31* | 6571±2299.85b |
Table 2: Pharmacokinetic Parameters Of Fexofenadine In Subjects
Discussion
In previous reports on OATP1A2 variation in human ethnic groups, the Chinese population was shown to possess a distinct distribution of polymorphisms, but the impact of OATP1A2 polymorphisms on fexofenadine pharmacokinetics has not been investigated [19]. Therefore, this study used fexofenadine as a probe drug to determine the influence of OATP1A2 polymorphisms on pharmacokinetics. However, OATP1A2 genotyping was not performed in these studies, and thus the fexofenadine pharmacokinetic results may not provide precise pharmacokinetic information for each group.
Shon et al. reported the pharmacokinetics of fexofenadine in Korean volunteers for 24 h after administration. The value of t1/2 was 5.0±1.7 h, which was less than that reported previously [32]. Boyle et al. investigated the pharmacokinetics of fexofenadine in Japanese volunteers, but the authors suggested that a sampling time of less than 24 h was not adequate to study elimination [33]. Guo et al. studied the pharmacokinetics of fexofenadine for up to 48 h and reported t1/2 of 9.29±3.61 h after administration of 120 mg of fexofenadine. The AUC0–48 and AUC0–∞ for 120 mg of fexofenadine were 978.19±411.06 h×ng/ml. The mean ratio of AUC0–48/AUC0–∞ was greater than 80%, which indicated a good fit with the sampling schedule [24]. These data indicated that the blood sampling duration for pharmacokinetic studies of fexofenadine should be at least 48 h to capture the complete plasma-concentration profile.
Pharmacokinetic parameters such as Tmax, t1/2 and AUC0–∞ have been highly variable among published studies. The reasons for this variability are complex and include the sensitivity of assay methods, blood sampling schedules, and sampling duration. Thus, this study focused on AUC0–t. Normality tests, agglomerative hierarchical clustering, and non-hierarchical K-means clustering were performed using AUC0–t data.
Three distinct phenotypes were identified, which was an unexpected result. According to recognized OATP1A2 SNP data in the Chinese population, no variants exist in the Chinese or Han populations. However, the results of the pharmacokinetic studies showed significant differences across 3 groups. The t1/2 was not changed, which indicated that the elimination rate was similar in the 3 identified phenotypes. Significant differences in CL/F indicated differences in bioavailability. These results suggested that variation in fexofenadine pharmacokinetics was attributed to differences in absorption capability, and that OATP1A2 played a key role in fexofenadine disposition.
Fexofenadine AUCs in Taiwanese subjects were similar to those in American, Indian, and German subjects, but were significantly differences from those in Korean and Japanese subjects. The fexofenadine pharmacokinetic parameters in Chinese subjects were highly variable between research reports, and thus we could not draw conclusions regarding difference between Chinese and Taiwanese populations [24,25]. These results indicated that the fexofenadine AUC in the Taiwanese population were significantly different from those of other Asian populations, which was a similar result to that obtained for OATP1A2 polymorphisms. Three phenotypes were observed; however, these observations conflicted with discovered frequencies of OATP1A2 alleles, and there no SNPs were discovered in the Taiwanese population. Thus, the reasons for the observed inter-subject differences should be further investigated.
Fexofenadine is a substrate for numerous transporters in addition to OATP1A2, including OATP1B1, OATP1B3, and OATP2B1. In a report by Niemi et al. on SLCO1B1 (encoding OATP1B1), the mean fexofenadine AUC in subjects with the 521CC genotype was 76.0% greater than that of subjects with the 521TC genotype, and 127% greater than that of subjects with the 521TT genotype. The SLCO1B1 521T>C polymorphism was shown to significantly influence the AUC, but not t1/2, of fexofenadine [34,35]. A similar phenomenon was observed in the present study. The AUC0–t of the HA group was 160% greater than that of the PA group and 56% greater than that of the IA group. However, the frequency of the 521T>C SNP in Taiwanese subjects was 0% [36]. For OATP2B1, no differences between ethnic groups were observed, and the frequencies of SCLO2B1 SNPs in Taiwanese subjects were similar to their frequencies in Japanese subjects [19,36,37]. Thus, OATP1B1 and OATP1B3 polymorphisms may not be critical factors influencing the pharmacokinetics of fexofenadine in the Taiwanese population. Based on well-known SNPs and allele frequencies, OATP1A2 seems to be a key transporter involved in the uptake and absorption of fexofenadine in humans, and variation in OATP1A2 function seems to contribute to differences in these parameters among the Taiwanese population and other Asian populations (e.g. Korean and Japanese) [19,36,37].
The ethnic population-based difference in OATP1A2 was the most important finding in this research. The phenomenon was also observed on BCRP (breast cancer resistance protein). BCRP-421C>A is the most prevalent allele in the Asian population (40.80%) and its allele frequency is much greater than in other populations. Volunteers with BCRP genotype 421CA and 421AA exhibited greater rosuvastatin bioavailability than that in volunteers with genotype 421CC, and thus plasma levels of rosuvastatin in Asians are generally twice those observed in people of European heritage [38].
The results of this study suggest that fexofenadine pharmacokinetics differ between the Taiwanese population and other Asian populations (Korean and Japanese), and are influenced by OATP1A2, which controls fexofenadine absorption in humans.
Acknowledgements
The authors thank Protech Pharmaservices Corporation for providing all raw data for this report.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 2009;158:693-705.
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: Phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 2004;447:653-65.
- Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica2008;38:778-801.
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. ClinPharmacolTher 2007;81:362-70.
- Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. ClinPharmacolTher 2002;71:11-20.
- Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. ClinPharmacolTher 2007;81:495-502.
- Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlisch E, Fromm MF, et al. Grapefruit juice ingestion significantly reduces talinolol bioavailability. ClinPharmacolTher 2005;77:291-301.
- Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J PharmacolExpTher 2010;332:181-9.
- Markham A, Wagstaff AJ. Fexofenadine. Drugs 1998;55:269-74.
- Shirasaka Y, Suzuki K, Nakanishi T, Tamai I. Differential effect of grapefruit juice on intestinal absorption of statins due to inhibition of organic anion transporting polypeptide and/or P-glycoprotein. J Pharm Sci 2011;100:3843-53.
- Simons FE, Simons KJ. Clinical pharmacology of new histamine H1 receptor antagonists. ClinPharmacokinet 1999;36:329-52.
- Chen C. Some pharmacokinetic aspects of the lipophilic terfenadine and zwitterionic fexofenadine in humans. Drugs R D 2007;8:301-14.
- Miura M, Uno T. Clinical pharmacokinetics of fexofenadine enantiomers. Expert Opin Drug MetabToxicol 2010;6:69-74.
- Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, et al.Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug MetabDispos 2005;33:1477-81.
- Simpson K, Jarvis B. Fexofenadine: A review of its use in the management of seasonal allergic rhinitis and chronic idiopathic urticaria. Drugs 2000;59:301-21.
- Smith SM, Gums JG. Fexofenadine: Biochemical, pharmacokinetic and pharmacodynamic properties and its unique role in allergic disorders. Expert Opin Drug MetabToxicol 2009;5:813-22.
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): Implications for altered drug disposition and central nervous system drug entry. J BiolChem 2005;280:9610-7.
- Yamakawa Y, Hamada A, Shuto T, Yuki M, Uchida T, Kai H, et al. Pharmacokinetic impact of SLCO1A2 polymorphisms on imatinib disposition in patients with chronic myeloid leukemia. ClinPharmacolTher 2011;90:157-63.
- König J, Seithel A, Gradhand U, Fromm MF. Pharmacogenomics of human OATP transporters. NaunynSchmiedebergs Arch Pharmacol 2006;372:432-43.
- Chen YA, Chou HY, Chang WK, Hsu KY. Determination of fexofenadine in human plasma by LC-MS/MS and its application in pharmacokinetic study. J Chin Pharm Sci 2013;22:409-14.
- Robbins DK, Castles MA, Pack DJ, Bhargava VO, Weir SJ. Dose proportionality and comparison of single and multiple dose pharmacokinetics of fexofenadine (MDL 16455) and its enantiomers in healthy male volunteers. Biopharm Drug Dispos 1998;19:455-63.
- Russell T, Stoltz M, Weir S. Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride inhealthy male volunteers. ClinPharmacolTher 1998;64:612-21.
- Stoltz M, Arumugham T, Lippert C, Yu D, Bhargava V, Eller M, et al.Effect of food on the bioavailability of fexofenadine hydrochloride (MDL 16455A). Biopharm Drug Dispos 1997;18:645-8.
- Guo D, Zou J, Zhu Y, Lou S, Fan H, Qin Q. Measurement of fexofenadine concentration in micro-sample human plasma by a rapid and sensitive LC-MS/MS employing protein precipitation: Application to a clinical pharmacokinetic study. Biomed Chromatogr 2010;24:335-41.
- Zhou Q, Ye Z, Ruan Z, Zeng S. Investigation on modulation of human P-gp by multiple doses of Radix Astragali extract granules using fexofenadine as a phenotyping probe. J Ethnopharmacol 2013;146:744-9.
- Hofmann U, Seiler M, Drescher S, Fromm MF. Determination of fexofenadine in human plasma and urine by liquid chromatography-mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2002;766:227-33.
- Muppavarapu R, Guttikar S, Rajappan M, Kamarajan K, Mullangi R. Sensitive LC-MS/MS-ESI method for simultaneous determination of montelukast and fexofenadine in human plasma: Application to a bioequivalence study. Biomed Chromatogr 2014;28:1048-56.
- Uno T, Yasui-Furukori N, Takahata T, Sugawara K, Tateishi T. Liquid chromatographic determination of fexofenadine in human plasma with fluorescence detection. J Pharm Biomed Anal 2004;35:937-42.
- Yasui-Furukori N, Uno T, Sugawara K, Tateishi T. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. ClinPharmacolTher 2005;77:17-23.
- Shimizu M, Uno T, Sugawara K, Tateishi T. Effects of itraconazole and diltiazem on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Br J ClinPharmacol 2006;61:538-44.
- Kim KA, Park JY. Effect of metronidazole on the pharmacokinetics of fexofenadine, a P-glycoprotein substrate, in healthy male volunteers. Eur J ClinPharmacol 2010;66:721-5.
- Shon JH, Yoon YR, Hong WS, Nguyen PM, Lee SS, Choi YG, et al. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. ClinPharmacolTher 2005;78:191-201.
- Boyle J, Ridout F, Meadows R, Johnsen S, Hindmarch I. Suppression of the histamine-induced wheal and flare response by fexofenadine HCl60 mg twice daily, loratadine 10 mg once daily and placebo in healthy Japanese volunteers. Curr Med Res Opin 2005;21:1495-503.
- Niemi M, Kivistö KT, Hofmann U, Schwab M, Eichelbaum M, Fromm MF. Fexofenadine pharmacokinetics are associated with a polymorphism of the SLCO1B1 gene (encoding OATP1B1). Br J ClinPharmacol 2005;59:602-4.
- Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: A genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev 2011;63:157-81.
- Huang SW. Comparison of Antihypertensive Effect of Angiotensin II Type I Receptor Antagonists in Subjects with Genetic Polymorphism of OATP. Thesis, National Cheng Kung University; 2007.
- Laitinen A, Niemi M. Frequencies of single-nucleotide polymorphisms of SLCO1A2, SLCO1B3 and SLCO2B1 genes in a Finnish population. Basic ClinPharmacolToxicol 2011;108:9-13.
- Cropp CD, Yee SW, Giacomini KM. Genetic variation in drug transporters in ethnic populations. ClinPharmacolTher 2008;84:412-6.