- *Corresponding Author:
- Tulasi Uma Rani Nutakki
Department of Pharmacology, Hindu College of Pharmacy, Guntur, Andhra Pradesh 5522007, India
E-mail: nturani35@gmail.com
| Date of Received | 27 November 2023 |
| Date of Revision | 15 May 2023 |
| Date of Acceptance | 20 August 2024 |
| Indian J Pharm Sci 2024;86(4):1307-1315 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present aim of our study was to assess drug related problems among patients with cardiovascular diseases. A prospective observational design was used to conduct this study. 1158 patients with cardiovascular are recruited in the study who visits the cardiology departments in Guntur. The classification of drug related problems was done by using the Pharmaceutical Care Network Europe drug related problem classification tool V9.1. A total of 743 drug-related problems were identified among 1158 patients, averaging 0.6 drug related problems per patient. The primary drug-related problem identified was in pulmonic closure treatment safety, with 60.1 % of adverse drug events possibly occurring. The most prevalent cause was drug selection, accounting for 71.19 %. During the study period, 148 adverse drug reactions were observed. On causality assessment, 41.89 % were classified as probable, 30.41 % as definite, and 27.7 % as possible. The most common adverse drug reactions encountered in this study were electrolyte imbalance, giddiness, headache, and cough (8.78 %). Treatment safety and drug selection were the major reasons for the development of drug related problems among patients with cardiovascular conditions. Majority of the adverse drug reactions are probable and mainly causing electrolyte imbalance.
Keywords
Prospective observational study, Pharmaceutical Care Network Europe, drug related problems, cardiovascular patients
Cardiovascular disorders are a prominent precipitant of mortality across the globe and a significant barrier to the ongoing progress of human development[1]. It is predicted that 23.6 million people will die due to Cardiovascular Disease (CVD) by 2030[2]. During the last few decades, there is a significant rise in the quantity of medications that are available in the market, managing medication therapy has become challenging, as a result of circumstances responsible for causing Drug Related Problems (DRPs)[3]. The majority of earlier research has focused on DRPs as a reason for hospitalization particularly in adults, ambulatory care, nursing homes and hospitalized patients[4]. DRPs negatively impact patient health outcomes, leading to worsened symptoms, prolonged hospital stays, increased healthcare expenditures and a reduced standard of living for cardiovascular patients[5]. Definition of DRPs according to Pharmaceutical Care Network Europe (PCNE), a DRPs is an event or circumstance involving drug therapy that actually or potentially interferes with desirable health outcomes[6].
DRPs are classified into numerous categories, but there is no single standardized classification system used globally[7,8]. PCNE version 9.1 is the current version, created in February 2020 following an expert workshop and validation round. PCNE classification version 9.1 includes 3 problem domains, 9 cause domains, 5 planned intervention domains, 3 acceptance domains and 4 problem status domains. Problems categorized more precisely fall into 7 sub-domains with 43 for causes, 17 for interventions and 10 for intervention acceptance[6]. DRPs can arise during medication administration, dispensing and patient use. A significant issue identified was the lack of monitoring and re-evaluation by physicians[5]. Cardiovascular medications are a common cause of DRPs. According to a study by Andreazza et al.[9], cardiac medications are identified as the root cause of all DRPs. Guntur has a very little information about DRPs among the CVD patients[9,10].
Materials and Methods
A data collecting form has been prepared with the necessary information for the study. This covers patient demographics, including religion, marital status, social habits, profession, education status and any co-morbid conditions. It also includes information on treatment charts, prescribed medications and therapy changes. PCNE (version 9.1) was used to classify DRPs. It includes specific domains for problems, causes, planned interventions, acceptance, problem status along with their sub domains.
Methodology:
The purpose of this prospective observational study is to look at the DRPs among CVD patients in Guntur’s cardiology departments. The study conducted from July 2021 to July 2023, data collection from patient’s case sheets, lab reports and does not involve invasive techniques like collection of blood samples. Patients who met the criteria are included in the study. Inclusion criteria includes patients of either gender, aged over 18 y, diagnosed with CVDs such as hypertension or coronary heart disease, who use antihypertensive medication, visit the hospital regularly for checkups, have varied food habits, may or may not have co-morbid conditions like diabetes mellitus, and are conscious and willing to participate with written informed consent are eligible for the study. Exclusion criteria includes patients below 18 y of age, those with hepatic or renal failure, pregnant or breastfeeding women, unconscious patients (e.g., in a continuous coma state), individuals who have visited departments other than cardiology and those who are not part of the outpatient ward of the cardiology department or do not visit the hospital regularly for checkups are excluded from the study. Data was gathered from treatment charts, outpatients OPD cards, patients and their care takers interviews, interviewing nurse and cardiologist and any other relevant sources.
Statistical analysis:
All raw data collected and recorded in the data collection forms were entered into Microsoft Office Excel 2007 and statistical analysis was performed using Statistical Package for Social Sciences 28.
Results and Discussion
A total of 1158 subjects participated in the study, including 732 males and 435 females. This finding indicates that males were approximately 26 % more susceptible to cardiovascular events compared to females, as shown in Table 1. Majority of the patients belonged to 61-70 y of age, later occupies 51-60 y of age. As the percentages do not differ significantly, we conclude that individuals aged 51-70 y have a high incidence of cardiovascular events, with very few patients being younger than 40 y (Table 2). The highest incidence of CVD is observed in married patients at 88.3 %, followed by 11.4 % in single/widowed patients and 0.3 % in unmarried patients, who have the least incidence of CVD (Table 3). 53.5 % of the participants were considered literate. 27.2 % had completed SSC (10th class), 10.6 % had discontinued their education after intermediate, 9.6 % had obtained a degree and the remaining 5.8 % had postgraduate or higher education. In contrast, 46.4 % were illiterate, as shown in (Table 4). The majority of the patients, 33.2 %, were daily wage earners, including farmers in this category. Next, 23.6 % of the patients were housewives (females). 19.1 % owned their own businesses in various fields. 8.2 % worked in either the private or public sectors. 15.9 % were retired employees, including both pensioners and those who were not (Table 5). Social behaviors have a significant impact on disease risk. Among the population, 36.4 % use tobacco, 29.5 % do not and 9.4 % are alcoholic. Additionally, 24.7 % are both alcoholics and smokers (Table 6).
| Group | n | Procollagen products (%) |
|---|---|---|
| A | 6 | 99.19±10.06 |
| B | 6 | 142.93±20.38a |
| C | 6 | 171.53±32.26ab |
| F | 522.412 | |
| p | 0.000 |
Note: Comparison to group B, ap<0.05 and comparison to normal group C, bp<0.05
Table 1: The Level of Procollagen Products
| Group | n | COL1A1 | COL1A2 |
|---|---|---|---|
| A | 6 | 0.37±0.09 | 0.41±0.14 |
| B | 6 | 0.43±0.14a | 0.54±0.14a |
| C | 6 | 0.86±0.16ab | 0.78±0.12ab |
| F | 80.413 | 47.276 | |
| p | 0.000 | 0.000 |
Table 2: Cardiovascular Distribution as Per Patients Age
| Patient marital status | No. of patients | Percentage |
|---|---|---|
| Married | 1023 | 88.3 |
| Unmarried | 3 | 0.3 |
| Single/widow | 132 | 11.4 |
Table 3: Cardiovascular Distribution as Per Patients Marital Status
| Education profile | No. of persons | Percentage % |
|---|---|---|
| No schooling | 538 | 46.4 |
| X class | 316 | 27.2 |
| 10+2 | 123 | 10.6 |
| Degree | 114 | 9.8 |
| PG and higher | 67 | 5.7 |
Table 4: Cardiovascular Distribution as Per Education Status
| Occupation | No. of Patients | Percentage |
|---|---|---|
| Daily wages | 385 | 33.2 |
| Business | 221 | 19.1 |
| Job holder | 95 | 8.2 |
| House wife | 273 | 23.6 |
| Retried | 184 | 15.9 |
Table 5: Cardiovascular Distribution as Per Occupation
| Social habits | No. of patients | Percentage |
|---|---|---|
| Smoker/tobacco | 421 | 36.4 |
| Alcoholic | 109 | 9.4 |
| Both | 286 | 24.7 |
| None | 342 | 29.5 |
Table 6: Cardiovascular Distribution as Per Social Habits
The majority of the patients had both hypertension and diabetes mellitus with 35.6 % experiencing the highest incidence. Additionally, 31.8 % had coronary artery disease, while 13.5 % had hypertension (including arrhythmia, heart failure and left ventricular disease among other conditions). Dilated cardiomyopathy was present in 12.4 % of patients and 6.7 % had myocardial infarction, the least common cardiovascular event (Table 7). A total of 1158 patients were included in the study. The doctors either continued prescribing the same medication or made minor adjustments.
| Co-morbidities | No. of patients | Percentage |
|---|---|---|
| Coronary artery disease | 368 | 31.8 |
| Hypertension | 156 | 13.5 |
| Diabetic cardiomyopathy | 144 | 12.4 |
| Hypertension and diabetes mellitus | 412 | 35.6 |
| Myocardial infarction | 78 | 6.7 |
Table 7: Cardiovascular Distribution as Per Co-Morbidities
While some patients stayed on the same medication combination, others were switched from lower to higher dosages. As a result, each visit was considered a new prescription. Therefore, 1158×4 visits=4632 total number of prescriptions. Dual therapy was highly prescribed, accounting for 41.01 % of the 4632 prescriptions, due to its superior effectiveness and treatment outcomes compared to single-drug therapy, which was provided in 34.5 % of prescriptions. Dual therapy is generally preferred for patients who have not managed to reduce their high blood pressure to therapeutic levels. Triple therapy was prescribed in 19.2 % of the prescriptions. Quadruple therapy was prescribed in 4.3 % of the prescriptions, while penta therapy was given in only 0.86 %. Patients with severe illnesses were prescribed either quadruple or penta therapy (Table 8).
| Types of therapy | Total no. of prescriptions | Percentage |
|---|---|---|
| Monotherapy | 1600 | 34.5 |
| Dual therapy | 1900 | 41.01 |
| Triple therapy | 892 | 19.2 |
| Quadruple therapy | 200 | 4.3 |
| Penta therapy | 40 | 0.86 |
Table 8: Types of Therapy Prescribed
Among the 1158 patients, 743 experienced DRPs. These DRPs were determined and categorized based on the PCNE 9.1 version. The average number of DRPs per patient is calculated. Among all the DRPs, the most commonly found issues were treatment safety P2.1 (adverse drug events occurring) at 60.1 %, followed by treatment effectiveness P1 at 29.5 %, and others P3 at 10.24 % (Table 9). A total of 743 causes of DRPs were identified. The highest number of DRPs was due to drug selection (71.19 %), with the maximum causes within this domain being 'C1.3' at 39.6 %. This was followed by dispensing issues (9.55 %), with 'C5.2' showing the highest causes at 5.2 %. Other causes included 'C9.1' for no or inappropriate outcome monitoring (6.5 %), 'C6' for drug use process (2.9 %), patient-related issues (1.7 %), and the least common, patient transfer-related issues (0.4 %) (Table 10). Out of 1158 patients, 148 developed Adverse Drug Reactions (ADRs), experiencing at least one ADR. The medications most accountable for ADRs were diuretic drugs; furosemide (21.6 %), torsemide (12.1 %) and hydrochlorothiazide (8.1 %). They were followed by the angiotensin- II receptor antagonist telmisartan (10.1 %), the calcium channel blocker amlodipine (9.45 %), the beta blocker metoprolol (8.1 %) and the Angiotensin-Converting Enzyme (ACE) inhibitor ramipril (6.08 %) (Table 11).
| Primary domain | Code | Problem | n % |
|---|---|---|---|
| Treatment effectiveness | P1.1 | No effect of drug treatment despite of correct use | 113 (15.1) |
| P1.2 | Effect of drug treatment not optimal | 78 (10.4) | |
| P1.3 | Untreated symptoms or indications | 30 (4.01) | |
| Treatment safety | P2.1 | Adverse drug event occurring | 450 (60.1) |
| Other | P3.1 | Unnecessary drug treatment | 26 (3.34) |
| P3.2 | Unclear problem/complaint | 52 (6.9) |
Table 9: DRPs Identification
| Primary domain | Code | Cause | n % |
|---|---|---|---|
| Drug selection | C1.1 | Inappropriate drug according to guideline/formulary | 16 (2.2) |
| C1.2 | No indication for drug | 21 (2.8) | |
| C1.3 | Inappropriate combination of drugs , or drugs and herbal medications, or drugs and dietary supplements | 294 (39.6) | |
| C1.4 | Inappropriate duplication of therapeutic group or active ingredient | 34 (4.6) | |
| C1.5 | No or incomplete drug treatment in spite of existing indication | 28 (3.8) | |
| C1.6 | Too many different drugs/ active ingredients prescribed for indication | 136 (18.3) | |
| Drug form | C2.1 | Inappropriate drug form/formulation | 25 (3.4) |
| Dose selection | C3.1 | Drug to low | 3 (0.4) |
| C3.2 | Drug dose of a single active ingredient too | 21 (2.8) | |
| C3.3 | Dosage regimen not frequent enough | 2 (0.3) | |
| C3.4 | Dosage regimen too frequent | 0 (0.0) | |
| C3.5 | Dose timing instructions wrong/unclear or missing | 0 (0.0) | |
| Treatment duration | C4.1 | Duration of treatment too short | 0 (0.0) |
| C4.2 | Duration of treatment too long | 7 (0.9) | |
| Dispensing | C5.1 | Prescribed drug not available | 25 (3.4) |
| C5.2 | Necessary information not provided or o incorrect advice provided | 39 (5.2) | |
| C5.3 | Wrong drug, strength or dose advised | 1 (0.1) | |
| C5.4 | Wrong drug or strength dispensed | 6 (0.8) | |
| Drug use process | C6.1 | Inappropriate timing of administration or dosing intervals by a health professionals | 0 (0.0) |
| C6.2 | Drug under administered by a health professional | 10 (1.3) | |
| C6.3 | Drug over administered by a health professional | 7 (0.9) | |
| C6.4 | Drug not administered at all by a health professional | 5 (0.7) | |
| C6.5 | Wrong administered by a health professional | 0 (0.0) | |
| C6.6 | Drug administered via wrong route by a health professional | 0 (0.0) | |
| Patient related | C7.1 | Patient intentionally uses/takes lessdrug than prescribed or does not take the drug at all for whatever reason | 2 (0.3) |
| C7.2 | Patient uses/takesmore drug than prescribed | 0 (0.0) | |
| C7.3 | Patient abuses the drug | 0 (0.0) | |
| C7.4 | Patient decides to use unnecessary drug | 3 (0.4) | |
| C7.5 | Patient takes food that interacts | 2 (0.3) | |
| C7.6 | Patient stores drug inappropriately | 0 (0.0) | |
| C7.7 | Inappropriate timing or dosing intervals | 2 (0.3) | |
| C7.8 | Patient unintentionally administers/uses the drug in wrong | 0 (0.0) | |
| C7.9 | Patient physically unable to use drug/form as directed | 0 (0.0) | |
| C7.10 | Patient unable to understand instructions properly | 3 (0.4) | |
| Patient transfer related | C.8 | Medication reconciliation problem | 3 (0.4) |
| Other | C9.1 | No or inappropriate outcome monitoring | 48 (6.5) |
| C9.2 | Other cause; specify | 0 (0.0) | |
| C9.3 | No obvious cause | 0 (0.0) |
Table 10: Causes Identified for DRPs
| Drug | ADR | Total ADR, n (%) |
|---|---|---|
| Furosemide | Hypovolemia(4), Dry mouth(5), Vertigo(6), Giddiness(10), | 32(21.6) |
| Hyponatremia(2), Anorexia(3), Hypokalaemia (2) | ||
| Torsemide | Polyuria(8), Hyponatremia(5), Dry mouth(5) | 18 (12.1) |
| Hydrochlorothiazide | Hyponatremia(4), Hypokalemia(2), Anorexia(8) | 12 (8.1) |
| Chlorothiazide | Hyponatremia(1), Hypokalemia(1) | 02 (1.35) |
| Spironolactone | Gastritis(3), Hyperkalemia(1) | 04 (2.7) |
| Metroprolol | Bradycardia(2), Headache(6), Insomnia(4) | 12 (8.1) |
| Labetalol | First dose hypotension(2) | 02 (1.35) |
| Carvedilol | Hypokalaemia(1), Insomnia(1), Fatigue(3) | 05 (3.37) |
| Enalapril | First dose hypotension(1), Cough(2) | 03 (2.02) |
| Lisinopril | Fatigue(3), First dose hypotension(1) | 04 (2.7) |
| Captopril | First dose hypotension(1) | 01 (0.67) |
| Ramipril | Cough(3), Generalized weakness(3), Giddiness(3) | 09 (6.08) |
| Losartan | Cough(1) | 01 (0.67) |
| Candesartan | Cough(1), Hypotension(1) | 02 (1.35) |
| Valsartan | Cough(2) | 02 (1.35) |
| Telmisartan | Cough(4), Hypotension(3), Insomnia(4), Hyperkalemia(2), Hyponatremia (2) | 15 (10.1) |
| Amlodipine | Pedal edema (3), Gingival overgrowth (1), Headache(5), Generalized weakness(4), Angioedema(1) | 14 (9.45) |
| Nifedipine | Bradycardia(1) | 01 (0.67) |
| Clindipine | Pedal edema (1), Giddiness(3), Headache(3) | 07 (4.72) |
| Total | 148 |
Table 11: Drugs Causing Adverse Drug Reactions
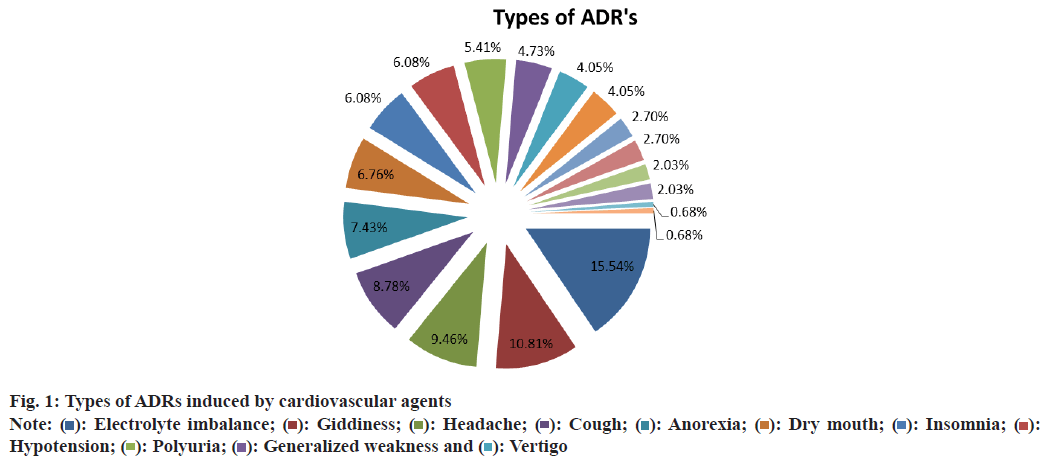
The most common ADRs observed were electrolyte imbalance (15.5 %), giddiness (10.8 %), headache (9.45 %) and cough (8.78 %) (Table 12 and fig. 1). Causality assessment using Naranjo’s algorithm was probable 41.89 %, definite 30.41 % and possible 27.7 % (Table 13). During the study period 743 DRPs were identified among 1158 patients. DRPs were relatively prevalent among these patients, with an average of 0.64 DRPs per patient. The average number of DRPs found in this study is lower compared to studies conducted in Ethiopia, which reported 1.2 DRPs per patient and Movva et al.[5], which reported 2.2 DRPs per patient.
| ADR’S | No (%) |
|---|---|
| Electrolyte imbalance | 23 (15.5) |
| Giddiness | 16 (10.8) |
| Headache | 14(9.45) |
| Cough | 13 (8.78) |
| Anorexia | 11 (7.43) |
| Dry mouth | 10 (6.75) |
| Insomnia | 9 (6.08) |
| Hypotension | 9 (6.08) |
| Polyuria | 8 (5.4) |
| Generalized weakness | 7 (4.72) |
| Vertigo | 6 (4.05) |
| Fatigue | 6 (4.05) |
| Pedal edema | 4 (2.7) |
| Hypovolemia | 4 (2.7) |
| Gastritis | 3 (2.02) |
| Bradycardia | 3 (2.02) |
| Gingival growth | 1 (0.67) |
| Angioedema | 1 (0.67) |
Table 12: Types of ADRs Induced by Cardiovascular Agents
| Category | n (%) |
|---|---|
| Definite | 45 (30.41) |
| Probable | 62 (41.89) |
| Possible | 41 (27.7) |
| Doubtful | 0 (0) |
Table 13: Causality Assessment by Naranjo’s Algorithm
DRPs are prevalent with P2 (treatment safety) 60.5 %, which reflects adverse drug event occurring and least with treatment effectiveness 29.23 %. Among the DRPs in this investigation, treatment safety accounted for more than half (60.5 %) this corresponds with the study done by Gokcekus et al., that showed most common DRP was ADR 54%[11]. In contrary to this finding, the study done by Movva et al.[5] which showed higher with treatment effectiveness (66.35 %) and least adverse reactions(19.47 %).
In our study 743 causes responsible for DRPs were identified. 71.1 % of all DRPs are observed to be produced by drug selection (C.1), which was determined to be the greatest DRP. This finding was consistent with earlier studies[5]. But in contradict to this study conducted by Gokcekus et al., showed causes of DRP was higher in others (C8) (54 %), second is drug selection (C1) (31 %) [11].
The most common DRPs are with P2 treatment safety 60.5 %, which reflects adverse drug event occurring. Our study revealed 148 adverse reactions were found during the study. The ADRs most frequently reported in our study were electrolyte imbalance (15.5 %), giddiness (10.8 %), headache (9.45 %) and cough (8.78 %). The aforementioned incidents are responsible by these drugs-furosemide, torsemide, chlorothiazide, hydrochlorothiazide, spironolactone, carvedilol, telmisartan, metoprolol, enalapril, ramipril and amlodipine.
USA asserted that the 4th most common prevalence of death is ADRs[12]. The ADRs most frequently reported in our study were electrolyte imbalance, headache; this finding are similar with Shanmugam et al.[13], with (14.8 %) electrolyte imbalance, (13.12 %) headache, (12.41 %) gastritis, but gastritis was very low ADR in our study. The most frequent ADR found in the studies done by Palaniappan et al., and Wankheda et al., was cough. Cough is one of the common ADRs in our study and these results are consistent with that[14,15].
Causality assessment of ADRs was done by using Naranjo’s algorithm, which showed probable 41.89 %, definite 30.41 % and possible 27.7 %. The study done by Sneha et al.,[16] 94 % of ADR are found to be possible and 6 % probable, which was inconsistent with our study. The largest ADRs was probable 61.19 %, which is consistent with our findings but different from possible 37.86 % and definite 1 %.
According to our study the drugs responsible for causing the ADRs are diuretics (33.7 %), calcium channel blockers (14.8 %), angiotensin II receptor blockers (13.5 %), ACEI (11.4 %), beta blockers (8.1 %) and α+β blockers (11.4 %). The most commonly prescribed drugs are diuretics and calcium channel blockers. Highest prescribed diuretics (33.7 %) drugs from this study, is in accordance with Datta et al., with diuretic’s (41 %)[17].
In conclusion treatment safety and drug selection are the major reasons for the development of DRPs among patients with CVDs in Guntur. Majority of the ADRs are Probable and mainly causing electrolyte imbalance.
Conflict of interests:
The authors declare no conflict of interest.
References
- Vijayakumar TM, Ananthathandavan P, Zago BA. Assessment of prescribing pattern and adverse drug reaction in patients receiving anticoagulant therapy: A prospective observational study. Health Sci Rep 2023;6(8):e1425.
[Crossref] [Google Scholar] [PubMed]
- Heart and stroke statistics 2018-at-a-glance. Dallas, TX: American Heart Association, American Stroke Association; 2018.
- Oki T, Ishii S, Furukawa K, Shono A, Akazawa M. Assessment of the potential impact of resolving drug-related problems by clinical pharmacists in Japan: A retrospective observational study. J Pharm Health Care Sci 2021;7:1-9.
[Crossref] [Google Scholar] [PubMed]
- Al-Azzam SI, Alzoubi KH, AbuRuz S, Alefan Q. Drug-related problems in a sample of outpatients with chronic diseases: A cross-sectional study from Jordan. Ther Clin Risk Manag 2016;12:233-9.
[Crossref] [Google Scholar] [PubMed]
- Movva R, Jampani A, Nathani J, Pinnamaneni SH, Challa SR. A prospective study of incidence of medication-related problems in general medicine ward of a tertiary care hospital. J Adv Pharm Technol Res 2015;6(4):190-4.
[Crossref] [Google Scholar] [PubMed]
- Classification for drug related problems. 9th edition: Pharmaceutical Care Network Europe Association; 2021:1-10.
- Basger BJ, Moles RJ, Chen TF. Development of an aggregated system for classifying causes of drug-related problems. Ann Pharmacother 2015;49(4):405-18.
[Crossref] [Google Scholar] [PubMed]
- Spollett G. Case study: A patient with uncontrolled Type 2 diabetes and complex comorbidities whose diabetes care is managed by an advanced practice nurse. Diabetes Spectrum 2003;16(1):32-6.
- Andreazza RS, de Castro MS, Köche PS, Heineck I. Causes of drug-related problems in the emergency room of a hospital in southern Brazil. Gaceta Sanitaria. 2011;25:501-6.
[Crossref] [Google Scholar] [PubMed]
- Garedow AW, Mamo MD, Tesfaye GT. Medication related-problems and associated factors among patients with hypertension at a tertiary care hospital in Ethiopia: A prospective interventional study. Integr Blood Press Control 2023;16:123-36.
[Crossref] [Google Scholar] [PubMed]
- Gokcekus L, Mestrovic A, Basgut B. Pharmacist intervention in drug-related problems for patients with cardiovascular diseases in selected community pharmacies in Northern Cyprus. Tropic J Pharm Res 2016;15(10):2275-81.
- Wiffen P, Gill M, Edwards J, Moore A. Adverse drug reactions in hospital patients. Bandolier Extra 2002:1-15.
- Shanmugam H, Panneerselvam N, Lawrence AA. Adverse drug reactions of cardiovascular drugs in intensive cardiac care unit in a tertiary care hospital: A prospective study. Biomed Pharmacol J 2019;12(3):1079-83.
- Palaniappan M, Selvarajan S, George M, Subramaniyan G, Dkhar SA, Pillai AA, et al. Pattern of adverse drug reactions reported with cardiovascular drugs in a tertiary care teaching hospital. J Clin Diagn Res 2015;9(11):FC01.
[Crossref] [Google Scholar] [PubMed]
- Wankhede SY, Pardeshi ML, Ghorpade VV, Ghongane BB. An assessment of pattern of adverse drug reactions of cardiovascular drugs in a tertiary care institute. Int J Basic Clin Pharmacol 2018;7(2):273-7.
- Sneha C, Anuradha HV, Karthik A. Assessment of adverse drug reactions in patients on cardiovascular drugs: A prospective study. J Pharmacol Pharmacother 2020;11(2):59-63.
- Datta S. Utilization study of antihypertensives in a South Indian tertiary care teaching hospital and adherence to standard treatment guidelines. J Basic Clin Pharm 2016;8(1):33-7.
[Crossref] [Google Scholar] [PubMed]