- *Corresponding Author:
- S. B. Kolla
Department of Analytical Research and Development, Formulations, GVK Biosciences Private Limited, Mallapur, Hyderabad, Telangana 500076
E-mail: sudheerchowdary1979@gmail.com
| Date of Received | 09 April 2021 |
| Date of Revision | 13 July 2021 |
| Date of Acceptance | 02 June 2022 |
| Indian J Pharm Sci 2022;84(3):683-702 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Tripartite synergistic model of solid core technology, dual organic modifiers and combined mixture design was implemented to achieve combined assay and related substances method by reverse phasehigh performance liquid chromatography with short run time, enhanced sensitivity and improved resolution between multiple impurity peaks. pH of mobile phase, ternary mobile phase composition and high performance liquid chromatography column temperature are experimented as variable parameters. Acetonitrile and isopropyl alcohol mixture was experimented as dual organic modifier. Special focus was given to detailed methodology of dealing with elution order changes by assigning negative sign for resolution. Separation of etoricoxib and related impurities was evaluated as a case study to prove this concept. The method was developed with Ascentis® Express C18, 150×4.6 mm, 2.7 μ column. Mobile phase comprised of buffer (0.1 % v/v ortho phosphoric acid, pH 3.6), acetonitrile and isopropyl alcohol (65.3:29:5.7 v/v) with a flow rate of 1.0 ml/min and ultraviolet detection at 285 nm. Forced degradation studies revealed that the method was stability indicating, suitable for both assay and impurities of drug product. The recoveries for impurities and assay were found to be in the range of 94.0 %-111.0 % and 97.9 %-101.8 %, respectively. Linearity was established for impurities and assay in the range of 0.25-2.0 μg/ml and 125-750 μg/ml, respectively. The method was validated as per international conference on harmonisation guidelines. The method can be successfully employed for determination of assay and impurities of etoricoxib in bulk drugs and formulations.
Keywords
Design of experiments, solid core technology, dual organic modifier, assay and impurities method, impurity profile swap, etoricoxib, stability indicating, high performance liquid chromatography
Pharmaceutical dosage forms are to be tested for critical quality attributes like assay and Related Substances (RS) to ascertain potency and purity[1,2]. Most of the assay and RS methods are developed by Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) technique with either isocratic or gradient elution of mobile phase depending on the number of analytes to be analysed. RP-HPLC methods for assay have an average run time of 15 min and above and may not be suitable for RS, but not in all cases[3]. If multiple analytes are difficult to separate by isocratic method, a gradient change of organic modifiers such as buffer and organic solvent are applied over a constant time for impurities methods along with Ultra-High Performance Liquid-Chromatography (UHPLC) to achieve shorter run times[4-7]. Gradient elution mode of RP-HPLC employs aqueous buffer solvent in one channel and organic solvent in another channel at standard flow rate but with varying compositions over time to generate a gradient program for elution. Aqueous solvents used for the mobile phase are usually buffered solvent with addition of inorganic salt as an organic modifier to impart sufficient buffer strength and maintain constant pH. Acetonitrile (ACN) and Methanol (MeOH) are often the organic solvents of choice as they can solubilize many small molecules, offers low back pressure and miscible with most of the aqueous buffers used in RP-HPLC[8]. Solvent elution strength order for commonly used solvents is MeOH<ACN<Isopropyl Alcohol (IPA)[9]. Selectivity (alpha (α)) and retention factor (k prime as capacity factor) can be altered with introduction of a second organic modifier to compensate the part of volume of first organic modifier, so that same elution strength of mobile phase is maintained with ternary mobile phase and MeOH, ethanol and IPA are common solvents of choice in RP-HPLC[10,11]. IPA is the solvent of choice to consider as second organic modifier over MeOH and ethanol because MeOH exerts high back pressure and the solvent strength is less, hence required in large proportions in mobile phase. Ethanol on the other hand is having high elution strength but is highly volatile and is also a controlled category solvent and hence is not of primary choice as mobile phase component. IPA as organic modifier is well conversed in the past in separation of complex analytical resolutions in micellar liquid chromatography[12] in glycol-peptide enrichment[13] with Hydrophilic Interaction Liquid Chromatography (HILIC) columns, in aqueous normal phase chromatography[14] and as washing solvent in column regeneration with ACN at 25 % volume[15].
Solvochromatic properties like elution strength (εº), Polarity (P), dipole character (Π), acidity (α) and basicity (beta (β)) play an important role in selectivity of analytes. IPA, because of high viscosity (2.04 mPa) and higher elution strength (3.9 polarity) is not a primary choice in chromatography as sole organic modifier[8]. Many articles in past discussed ethanol as substitution solvent to ACN and MeOH[16]. IPA has two main drawbacks which can hinder its use in HPLC. The first drawback is Ultraviolet (UV) cut off (205 nm) and second, high viscosity in combination with water, which exerts high back pressure on to HPLC columns[12]. IPA is not widely used as solo organic modifier because of high elution strength which causes the analytes to merge and distort increasing the band broadening of peaks[17]. IPA can be experimented as second organic modifier as elution strength of IPA (8.3) is much higher than ACN (3.1), MeOH (1.0) and Tetrahydrofuran (THF) (3.7). Acidity and basicity of IPA are almost same (0.36 and 0.40), which makes this solvent suitable for separation of both acidic and basic molecules[8]. Because of all above reasons, IPA scores over MeOH, ethanol and THF as co-solvent of choice. THF also has higher elution strength compared to other common solvent used in RP-HPLC, however, THF is relatively unstable and is prone to oxidation. Additives to stabilize the THF solvent are also incompatible with several inorganic salts of mobile phase. THF also has significant absorptive properties in UV range and is not a solvent of choice when higher sensitivities are expected at lower sample concentration. Loss of sensitivity requires the analytes to be injected at higher concentrations, which further will cause band broadening. Moreover, miscibility of THF with ACN and buffer solvents is less compared to IPA. Hence THF is not considered as tertiary solvent. Several literature articles discuss use of IPA as second organic modifier and the elution strength is two to three times higher than MeOH, ethanol and THF. Higher elution strength entails less volume of solvent requirement to elute late eluting/ low polar analytes. MeOH has less elution strength and is required in large volumes to compensate part of primary organic modifier, which in turn will increase the viscosity of mobile phase excreting high back pressure. Ethanol on the other hand is highly volatile and is also a controlled substance and hence is not of primary choice. A blend of ACN and IPA are used in the past to resolve co-elution of non-polar analytes[18]. IPA is also studied as an alternate organic modifier during ACN shortage[19] and also as green chromatography solvent, because it is environmentally benign in nature[20].
Current trends in HPLC method development use solid core technology columns to arrive at benefits of UHPLC technologies[21,22]. These particles operate at elevated mobile phase linear velocities to affect dramatic increase in the efficiency. Solid core particles are 2.7 μm in diameter with a 1.7 μm solid core and 0.5 μm porous shell that results in superior mass transfer kinetics and high efficiency in chromatography[23]. Superficially porous particle columns offer reduced plate height (h), reduced diffusion length which in turn improves plate number (n); enhancing selectivity for multiple analytes with improved performance for separation goals and also offer reduced back pressure that will allow to accommodate high viscosity organic modifiers like IPA[24-26]. Design of Experiments (DoE) technique employs randomization of selected variables with statistical algorithms to plan controlled experiments. When multiple responses are to be optimized involving both numeric and mixture variables, combined mixture design is used to determine the optimum combinations of factors, that deliver a desired response by using a minimum number of experimental runs[27-29].
Etoricoxib (ETO) is a Cyclooxygenase-2 (COX-2) selective inhibitor and crystalline in nature with acid dissociation constant (pKa) value of 4.5 and having chromophores[30]. ETO is soluble in organic solvents like ACN, MeOH and insoluble in water and is available in tablet dosage form. Many studies are reported in literature for assay and impurities for ETO[31-36]. Yet there is no study, which separates all impurities in isocratic method with runtime of less than 15 min. One of the literature reported method discussed the separation of about 13 impurities for ETO Active Pharmaceutical Ingredient (API), with gradient elution and also specified elution order reversal is observed with change in pH from 2.5 to 3.7[36]. Majority of the literature reported methods has pH of mobile phase around 3.0 to 5.0 except for one article which operates at pH 7.0. However, the current study focused on separation of 5 major impurities which are relevant for the tablet dosage form. Moreover, no supporting literature exists on the application of combined benefits of ternary isocratic mobile phase system, solid core technology and DoE to achieve fast and efficient method for assay and RS. In this study, tripartite concepts of solid core technology columns combining the benefits of dual organic modifiers in mobile phase and DoE concepts of combined mixture design are implemented to achieve sharp and well resolved peaks in combined assay and impurities method for ETO. The approach presented here was successfully implemented to reduce 45 min runtime of a literature reported gradient method to a 15 min isocratic method.
Materials and Methods
Materials:
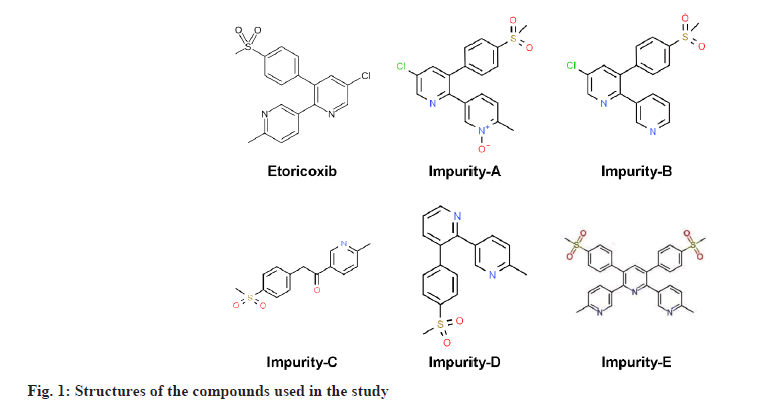
ETO of pharmaceutical grade was procured from Inogent Laboratories (A GVK Bio Company, Hyderabad, India). ETO Immediate Release (IR) tablets were supplied by GVK Biosciences (FDS, Hyderabad, India). Impurity-A (5-{5-chloro-3-[4-(methylsulfonyl) phenyl] pyridin-2-yl}-2-methylpyridine 1-oxide), Impurity-B (5-chloro-3-[4-(methylsulfonyl)phenyl]- 2,3'-bipyridine), Impurity-C (1-(6-Methylpyridin- 3-yl)-2-[4-(methyl sulfonyl)phenyl]ethanone), Impurity-D (6'-methyl-3-[4-(methylsulfonyl)phenyl]- 2,3'bipyridine (or) 3-(4-methyl sulfonyl)phenyl-2- (2-methyl-5-pyridinyl)-pyridine)]) and Impurity-E (6"-dimethyl-3',5'-bis[4-(methylsulfonyl)phenyl]- 3,2':6',3"-terpyridine) (fig. 1) were procured from Glenmark pharmaceuticals (Gujarat, India) and Mylan Laboratories (Hyderabad, India). Ortho Phosphoric Acid (OPA) (analytical grade), IPA (HPLC grade), Sodium hydroxide (NaOH) pellets (Emplura® grade), Hydrochloric acid (HCl) (Laboratory Reagent (LR) grade), ACN (HPLC grade) were purchased from Merck Ltd (Mumbai, India). Buffer salts and all other chemicals were of Emplura® grade from Merck India. Ultra-pure water was obtained from a Milli-Q® purification system (Millipore, Mumbai, India).
Instrumentation and software:
HPLC studies were carried out with Agilent™ instrument (Model 1200 Series), which was equipped with a photo diode array detector. The software used to operate the instrument and data processing was Waters
Chromatographic conditions and sample preparation:
The separation was achieved using Ascentis® Express C18 (150×4.6 mm, 2.7 μm) column and UV detector at 285 nm. Mobile phase buffer is OPA at a concentration of 0.1 % v/v and pH for initial trails is selected as 3.2, which is beyond the range of ±1 of pKa of ETO i.e. 4.5. Buffer pH across the selected range is adjusted with dilute phosphoric acid (30 %) or with dilute NaOH solution (0.1 M). The mobile phase consisted of OPA buffer, ACN and IPA in different proportions as suggested by experimental design at isocratic flow rate of 1.0 ml/min. Individual impurity solutions were prepared at 100 μg/ml concentration and all impurity mixture solutions were spiked to ETO (250 μg/ml) at a concentration of 1.0 μg/ml. Buffer, ACN and IPA in the ratio of 60:25:15 v/v is used as diluent. Final validated HPLC method for the separation of ETO in tablet dosage form for estimation of process and degradant impurities was performed on Ascentis® Express C18 (150×4.6 mm, 2.7 μm) column. The flow rate was 1.0 ml/min and column temperature was 35°. The ternary mobile phase consisted of component A, an aqueous solution of OPA at a concentration of 0.1 % v/v and pH for initial trails is selected as 3.6, component B, ACN and component C, IPA in the ratio of 70:23.5:6.5 v/v. Sample of ETO were dissolved in buffer, ACN and IPA in the ratio of 60:25:15 v/v is used as diluent at concentration of 500 μg/ml. Detection was by UV at 285 nm and UV spectra was collected by photodiode array detector for all forced degradation samples across the range of 200 to 400 nm.
Quality by Design (QbD) concepts and analytical target profile:
Entire method development programme was executed by aligning QbD principles to develop a quality method to consistently deliver indented results. QbD based method development employs more systematic approach to method development by including prior knowledge, results of One Factor at a Time (OFAT) studies, use of quality risk assessment and knowledge management throughout lifecycle management. QbD approach to development involves defining analytical target profile, identification of critical method attributes and evaluation of critical method parameters with quality risk management principles[37-39]. All the primary objectives of the method were methodically evaluated through analytical target profile. Table 1 presents a brief overview of analytical target profile. Combined assay and RS method, ternary mobile phase, solid core technology HPLC column and isocratic separation with targeted short run time were considered as the critical method quality attributes and were built in by design. Further to develop the method by QbD principle a structured, organized technique of determining relationship between factors and responses is adopted by means of experimental design.
| ATP element | Target | Justification |
|---|---|---|
| Assay and RS method | Measurement of potency and purity | To monitor the drug assay and related substance characteristics of drug product |
| Design and mode of method | RP-HPLC, isocratic elution and ternary mobile phase with ACN, IPA as dual organic modifier | To target run time of less than 15 min with ternary mobile phase system to offer improved selectivity. To evaluate efficiency of secondary organic modifier. To separate multiple impurities with short run time |
| Mode of detection and stationary phase | UV, Solid core technology with C18 stationary phase | The molecule has chromophore. Solid core technology offers superior porosity, resolution and sensitivity. C18 stationary phase is used in multiple literature references |
| Analytical method validation criteria | ||
| Specificity | Placebo interference should not be observed | As the method is for tablet dosage form, results shall not be affected by presence of excipient matrix |
| Selectivity | To separate impurities and degradants | To prove peak purity and stability indicating nature |
| Precision | To establish repeatability | As per requirements of International Conference on Harmonisation (ICH) Q2R1 guidelines and to obtain consistent and reproducible results |
| Accuracy | To offer accurate results | The % recovery of ETO and impurities in tablet matrix shall meet predefined criteria |
| Linearity | Establish linearity across concentration range | Linearity at different concentration levels should be obtained |
| Filter interference | Prove filter compatibility | To choose suitable filter membrane |
| Robustness | Method shall be reliable to critical variable of the method | Results shall not be affected by deliberate changes and analytical solutions shall be stable for known time and temperature conditions |
Table 1: Analytical Target Profile (ATP)
Chromatography optimization:
It was observed that all impurities are structurally related with minor differences in specific functional groups for each impurity. Impurity A to impurity D is differing in structure by either addition or deletion of chlorine, methyl and oxygen group, whereas impurity E is a dimer of ETO. Impurity-A was formed by attacking oxygen group at nitrogen in methyl pyridine ring, impurity-B was formed by desmethylation, Impurity-C was formed by oxidation of carbon in chloro pyridine ring, impurity-D by removal of chlorine group and impurity-E with addition of methyl pyridine as an additional molecule. Preliminary screening experiments were conducted with OFAT approach by studying independently, the effect of different variables namely mobile phase composition, different proportions of organic modifiers (ACN and IPA) and pH of mobile phase. Different types of columns were evaluated to arrive at a single isocratic method for elution of all impurities. Zorbax Eclipse C18 and symmetry C18 (250×4.6 mm, 5 μ) columns are selected based on literature review, for all initial experiments, as most of the literature reported methods discussed use C18 based columns. Later the column is changed to Ascentis® Express C18 (150×4.6 mm, 2.7 μ) to integrate benefits of solid core technology. In each case the focus was to arrive at good resolution between all impurities and to have good peak shape with tailing factor around 1.0.
By the process of QbD, solid core technology columns are introduced to have sharp and symmetric peaks. Introduction of solid core technology columns helped us to reduce the peak width for all analytes which further enhanced the resolution. All preliminary experiments of mobile phase composition optimization were carried out with 0.1 % v/v OPA buffer adjusted to pH 3.2. Further trials were performed at mobile phase composition of 60 % buffer, 25 % ACN and 15 % IPA for influence of pH at 3.0, 3.2, 4.0 and 6.0. Peak symmetries were achieved by the method design with selection of solid core technology columns. All the observed values for United States Pharmacopeia (USP) tailing factor were observed around 1.1 and peaks were relatively sharp. It was observed that selectivity of the peaks was varying drastically with variation in chromatography conditions and hence theoretical plate count was considered as one of the responses. Objective during OFAT studies was confined to minimum possible retention time of ETO and maximum possible resolution between all analytes. All sample solutions of ETO are prepared by dissolving the crushed tablet powder in diluent. Diluent selection is arrived based on observed solubility of the molecule. ETO is insoluble in water but highly soluble in all organic solvents. The tablet formulation has calcium dihydrogen phosphate, magnesium stearate, microcrystalline cellulose and croscarmellose sodium as excipients. Majority of the excipients except for microcrystalline cellulose are soluble in water. Hence a combination of water and ACN in equal ratio is selected for extraction of drug from tablet dosage form. Later the diluent is modified to buffer, ACN and IPA in the ratio of 60:25:15 v/v to maintain solvent similarities with that of the mobile phase. A combined, split plot, D-optimal design was employed to develop combined assay and impurities method with 5 factors and 12 responses. The ranges for variables were selected with the knowledge gained from OFAT experiments. The variables are mixture components along with individual numerical variables with some variables treated as hard-to-change factors. To experiment both mixtures and individual components, a combined mixture design is the suitable option. pH is selected as hard-to-change factor and hence split-plot design is selected. Split-plot designs are useful to control the number of times a hard-tochange factor is randomised and still provide adequate power to the design model. D-optimal designs are more suitable when the design has mixture of variables which are mixtures, continuous, discrete and hard-to-change components and still offer better randomization with block effects. Mobile phase composition with different proportions of buffer, ACN and IPA were selected as mixture variables. pH of buffer and column temperature were selected as numeric variable with discrete as subtype for column temperature. The design consisted of 41 experiments, including the combinations of factors at different levels. The ranges studied for the five factors were 50.0 %-70.0 % of buffer, 5.0 %-40.0 % of ACN, 0 %-25.0 % of IPA, 3.0-5.5 for the buffer pH and 25°-35° for the column temperature. pH of mobile phase was selected as hard-to-change factor in order to limit variation of this factor to 3 different pre-specified levels (3.0, 4.3 and 5.5) with 4.3 selected as center point to deal with curvature effect, if any. The order of experiments was randomized to minimize systematic error and the experiments were divided into five blocks. A composite sample with mixture of all impurities and ETO was employed in the optimization of experiments. Experimental runs as obtained from DoE software are presented in Table 2.
| Run | Variable | Response | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | R11 | R12 | |
| 1 | 50 | 40 | 10 | 5.5 | 25 | 0 | 0 | 7.17 | -2.04 | 5.13 | 4.1 | 6.14 | 4.1 | 16846 | 5.13 | 0 | 5.13 |
| 2 | 60 | 40 | 0 | 5.5 | 35 | 0 | -2.2 | 15.4 | 2.86 | 18.22 | 7.08 | 4.22 | 6.25 | 25131 | 16 | 2.23 | 16 |
| 3 | 70 | 5 | 25 | 5.5 | 25 | 0.74 | -2.7 | 13.2 | -4.17 | 9 | 6.81 | 10.98 | 5.88 | 13726 | 7.07 | 1.93 | 6.33 |
| 4 | 70 | 30 | 0 | 5.5 | 30 | 0 | -2.1 | 27 | 22.23 | 49.19 | 13.84 | -8.39 | 16.4 | 29373 | 47.1 | 2.09 | 47.1 |
| 5 | 50 | 25 | 25 | 5.5 | 25 | 0 | 0 | 4.41 | -3.3 | 1.11 | 2.43 | 5.73 | 3.12 | 12573 | 1.11 | 0 | 1.11 |
| 6 | 70 | 17 | 13 | 5.5 | 35 | 4.05 | 0 | 20.2 | 7.49 | 27.65 | 11.49 | 4 | 10.3 | 25828 | 31.7 | -4.05 | 27.7 |
| 7 | 50 | 25 | 25 | 5.5 | 35 | 0 | 0 | 4.44 | -3.15 | 1.29 | 2.39 | 5.54 | 3 | 14565 | 1.29 | 0 | 1.29 |
| 8 | 70 | 30 | 0 | 5.5 | 25 | 0 | -3.8 | 35.3 | 23.7 | 59.04 | 13.92 | -9.78 | 17.5 | 30084 | 55.3 | -3.76 | 55.3 |
| 9 | 50 | 40 | 10 | 3 | 35 | -2.15 | 2.15 | 7.13 | -7.13 | 0 | 0 | 7.13 | 3.25 | 15206 | 0 | 0 | 2.15 |
| 10 | 50 | 25 | 25 | 3 | 30 | 0 | -1.3 | 4.54 | -5.86 | -1.32 | 0 | 5.86 | 2.78 | 10801 | 0 | -1.32 | 0 |
| 11 | 70 | 30 | 0 | 3 | 25 | -12.6 | 17 | 20.2 | -25.93 | -5.74 | -11.6 | 14.36 | 6.37 | 21086 | -1.3 | -4.41 | 11.3 |
| 12 | 70 | 5 | 25 | 3 | 35 | -1.36 | 4.46 | 7.16 | -10.26 | -3.1 | -2.2 | 10.26 | 3.63 | 12632 | 0 | -3.1 | 1.36 |
| 13 | 50 | 40 | 10 | 3 | 25 | -2.5 | 2.5 | 5.94 | -5.94 | 0 | 0 | 5.94 | 3.27 | 13868 | 0 | 0 | 2.5 |
| 14 | 62 | 24 | 15 | 3 | 30 | -2.94 | 5.25 | 10.7 | -12.97 | -2.31 | -2.6 | 10.37 | 4.12 | 15304 | 0 | -2.31 | 2.94 |
| 15 | 60 | 40 | 0 | 3 | 35 | -5.24 | 6.57 | 13.1 | -14.39 | -1.33 | -3.48 | 10.91 | 4.08 | 18134 | 0 | -1.33 | 5.24 |
| 16 | 60 | 21 | 19 | 3 | 25 | -1.68 | 4.31 | 7.83 | -10.46 | -2.63 | -1.84 | 8.62 | 3.51 | 12726 | 0 | -2.63 | 1.68 |
| 17 | 50 | 25 | 25 | 4.3 | 35 | 0 | 0 | 4.39 | -3.43 | 0.96 | 2.33 | 5.76 | 3.01 | 13882 | 0.96 | 0 | 0.96 |
| 18 | 50 | 40 | 10 | 4.3 | 25 | 0 | 0 | 8.6 | -2.94 | 5.66 | 3.88 | 6.82 | 3.95 | 18746 | 5.66 | 0 | 5.66 |
| 19 | 70 | 19 | 11 | 4.3 | 30 | 2.29 | 3.18 | 24.8 | 6.2 | 30.95 | 8.9 | 2.7 | 11.8 | 23079 | 36.4 | -5.47 | 34.1 |
| 20 | 70 | 5 | 25 | 4.3 | 25 | 1.25 | 2.08 | 11.5 | -6.01 | 5.48 | 5.74 | 11.75 | 5.7 | 13246 | 8.81 | -3.33 | 7.56 |
| 21 | 62 | 25 | 14 | 4.3 | 35 | 1.21 | 0.88 | 13.2 | -1.33 | 11.83 | 6.99 | 8.32 | 5.78 | 21587 | 13.9 | -2.09 | 12.7 |
| 22 | 60 | 40 | 0 | 4.3 | 25 | -2.46 | -1.6 | 19.1 | 0 | 19.07 | 5.97 | 5.97 | 6.25 | 23967 | 15.1 | 4.01 | 17.5 |
| 23 | 70 | 30 | 0 | 4.3 | 35 | -1.06 | 0 | 32.9 | 16.95 | 49.87 | 11.56 | -5.39 | 15 | 28275 | 48.8 | 1.06 | 49.9 |
| 24 | 57 | 18 | 25 | 4.3 | 30 | 0.55 | 0 | 6.14 | -4.33 | 1.81 | 3.28 | 7.61 | 3.5 | 14617 | 2.36 | -0.55 | 1.81 |
| 25 | 60 | 23 | 17 | 5.5 | 35 | 1.49 | 0 | 9.66 | -2.17 | 7.49 | 6.07 | 8.24 | 4.84 | 18890 | 8.98 | -1.49 | 7.49 |
| 26 | 62 | 24 | 14 | 5.5 | 25 | 1.68 | 0 | 9.62 | 0.36 | 9.98 | 5.25 | 4.89 | 6.52 | 19467 | 11.7 | -1.68 | 9.98 |
| 27 | 60 | 40 | 0 | 5.5 | 25 | -1.15 | -3 | 20 | 2.61 | 22.61 | 7.4 | 4.79 | 6.39 | 26448 | 18.4 | 4.19 | 19.6 |
| 28 | 70 | 5 | 25 | 5.5 | 30 | 1.29 | 0.24 | 8.84 | -4.95 | 3.89 | 6.3 | 11.25 | 5.18 | 15769 | 5.42 | -1.53 | 4.13 |
| 29 | 70 | 30 | 0 | 5.5 | 35 | 1.31 | -3.6 | 33.9 | 23.46 | 57.32 | 14.45 | -9.01 | 15.9 | 31167 | 55 | 2.31 | 53.7 |
| 30 | 50 | 40 | 10 | 5.5 | 35 | 0 | 0 | 5.06 | -2.13 | 4.93 | 4.31 | 6.44 | 3.84 | 19164 | 4.93 | 0 | 4.93 |
| 31 | 70 | 5 | 25 | 5.5 | 35 | 2.06 | 0 | 10.4 | -4.79 | 5.61 | 6.9 | 11.69 | 5.2 | 17330 | 7.67 | -2.06 | 5.61 |
| 32 | 50 | 40 | 10 | 5.5 | 30 | 0 | 0 | 7.26 | -2.12 | 5.14 | 4.24 | 6.36 | 3.93 | 18923 | 5.14 | 0 | 5.14 |
| 33 | 60 | 40 | 0 | 3 | 30 | -5.62 | 4.34 | 9.56 | -8.53 | 1.03 | -2.48 | 6.05 | 4.27 | 19493 | -0.3 | 1.28 | 5.37 |
| 34 | 70 | 20 | 11 | 3 | 25 | -7.33 | 16.6 | 15.3 | -22.87 | -7.57 | -8.52 | 14.35 | 5.98 | 17562 | 1.65 | -9.22 | 8.98 |
| 35 | 70 | 5 | 25 | 3 | 25 | -0.94 | 5.24 | 7.77 | -13.95 | -6.18 | -3.37 | 10.58 | 3.47 | 10798 | -1.6 | -4.66 | -0.58 |
| 36 | 70 | 30 | 0 | 3 | 35 | -12.5 | 15.9 | 21.3 | -22.7 | -1.42 | -9.2 | 13.5 | 6.69 | 22906 | 2.01 | -3.43 | 14.5 |
| 37 | 60 | 40 | 0 | 3 | 25 | -7.27 | 5.71 | 13.6 | -15.37 | -1.79 | -5.13 | 10.24 | 3.91 | 16759 | -3.4 | 1.56 | 3.92 |
| 38 | 70 | 5 | 25 | 3 | 30 | -1.61 | 5.65 | 7.84 | -12.7 | -4.86 | -2.79 | 9.91 | 3.45 | 11642 | -0.1 | -4.04 | 0.79 |
| 39 | 62 | 24 | 14 | 3 | 35 | -3.1 | 6.12 | 10.5 | -12.58 | -2.04 | -1.85 | 10.73 | 4.08 | 15908 | 0.98 | -3.02 | 4.08 |
| 40 | 50 | 25 | 25 | 3 | 35 | 0 | 0.73 | 4.18 | -4.91 | -0.73 | 0 | 4.91 | 2.78 | 11036 | 0 | -0.73 | 0 |
| 41 | 50 | 25 | 25 | 3 | 25 | 0 | 1.26 | 4.31 | -5.57 | -1.26 | 0 | 5.57 | 2.8 | 10969 | 0 | -1.26 | 0 |
Table 2: DoE for screening
As shown in Table 2, A is buffer concentration; B is ACN concentration; C is IPA concentration; D is pH of mobile phase; E is column oven temperature; R1 is resolution between impurity-C and impurity-D; R2 is resolution between impurity-D and impurity-A; R3 is resolution between impurity-A and impurity-B; R4 is resolution between impurity-B and impurity-E; R5 is resolution between impurity-A and impurity-E; R6 is resolution between impurity-B and ETO; R7 is resolution between impurity-E and ETO; R8 is retention time of ETO; R9 is plate count of ETO; R10 is resolution between impurity-C and impurity-E; R11 is resolution between impurity-A and impurity-C and R12 is resolution between impurity-D and impurity-E.
Selection of responses and elution pattern:
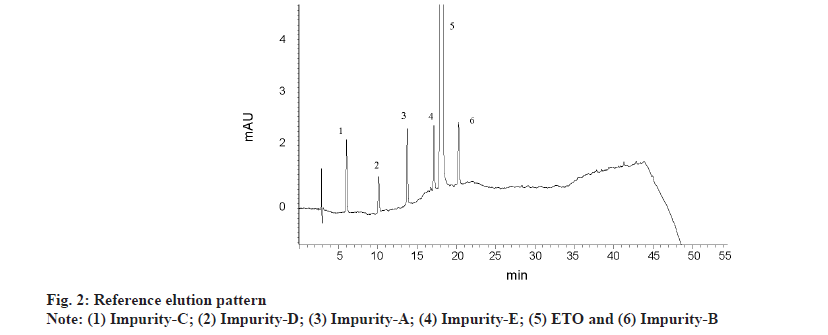
Major focus was set on resolution between all the six analytes (five impurities and ETO). With six analytes, there is a likelihood of five different responses as resolution criteria between adjacent peaks. However, it is pragmatic that elution order swap is observed with variation in pH and IPA concentration. As the entire method optimisation is performed by DoE, it is essential to capture all possible responses and feed the data to design for evaluation. Post execution of DoE, all the responses are thoroughly evaluated for elution order change, impact of variables on responses and then 12 responses were arrived by fixing a standard elution order. A reference elution pattern (Imp-C, Imp-D, Imp-A, Imp-E, ETO and Imp-B) observed with literature reported gradient method was fixed and for any different elution pattern other than the reference, a negative value was assigned to resolution between closely eluting peaks. Fig. 2 represents reference elution order. Therefore the twelve responses were resolution between impurity-C and impurity-D (R1), resolution between impurity-D and impurity-A (R2), resolution between impurity-A and impurity-B (R3), resolution between impurity-B and impurity-E (R4), resolution between impurity-A and impurity-E (R5), resolution between impurity-B and ETO (R6), resolution between impurity-E and ETO (R7), retention time of ETO (R8), plate count of ETO (R9), resolution between impurity-C and impurity-E (R10), resolution between impurity-A and impurity-C (R11) and resolution between impurity-D and impurity-E (R12).
Results and Discussion
Several trials were conducted by OFAT approach with ACN as solo organic modifier. Initial screening experiments were performed with gradient run of mobile phase by keeping pH 3.2 buffer as mobile phase A and ACN as mobile phase B. Quite a few experiments were conducted to predict the optimum concentration of ACN required for the isocratic experimentation. After testing several combinations, the gradient programme for final chromatography with buffer and ACN in mobile phase was kept as 78 % of buffer and 22 % of ACN at start of gradient with a linear change of ACN to 60 %, over 40 min. The mobile phase was then re-equilibrated to original condition over a period of 10 min. With the above conditions, ETO is eluting at 18 min and at an organic concentration of above 40 %. An exemplary chromatogram with buffer and ACN gradient programme as reference elution pattern is presented in fig. 2. Based on above experiments mobile phase composition for isocratic elution was studied at 60 %-70 % of buffer, 5 %-25 % of ACN and 15 %-25 % of IPA. It was observed that 40 % and above volume of ACN is required to elute all the impurities within 15 min. However, most of the analyte peaks are co-eluting and are merged with each other or with ETO peak. As a next step, isocratic runs were conducted with both ACN and IPA in the mobile phase with solvent ratio ranging from 5 %-25 % v/v, keeping buffer concentration constant at 70 % v/v. At all the combinations, observed retention time for ETO is 3-7 min and elution pattern change is observed for multiple impurities with poor resolution between closely eluting analytes. Evaluation of pH influence in the range of 3.0-6.0, by OFAT approach, revealed that elution pattern change is predominant with change in pH of mobile phase. As isocratic elution with OFAT trials is not conclusive. DoE as a tool for method optimization is selected.
Design adequacy was evaluated with Analysis of Variance (ANOVA) statistical parameters such as p-value, R-squared and adjusted R-squared (Table 3), p-values were observed below 0.01 which signifies adequacy of model. R-squared and adjusted R-squared values were above 0.90 for most responses indicating good correlation between variables and responses.
| Response | p value | R-Squared | Adjusted R-squared | |
|---|---|---|---|---|
| Subplot | Linear mixture | |||
| R1 | <0.0001 | <0.0001 | 0.98 | 0.97 |
| R2 | <0.0001 | 0.0013 | 0.97 | 0.95 |
| R3 | 0.0004 | 0.0174 | 1 | 0.94 |
| R4 | <0.0001 | <0.0001 | 0.97 | 0.96 |
| R5 | <0.0001 | <0.0001 | 0.98 | 0.97 |
| R6 | <0.0001 | 0.0046 | 1 | 0.99 |
| R7 | <0.0001 | <0.0001 | 0.94 | 0.91 |
| R8 | <0.0001 | <0.0001 | 0.98 | 0.97 |
| R9 | <0.0001 | <0.0001 | 0.99 | 0.98 |
| R10 | <0.0001 | <0.0001 | 0.98 | 0.98 |
| R11 | <0.0001 | <0.0001 | 0.82 | 0.74 |
| R12 | <0.0001 | <0.0001 | 0.99 | 0.98 |
Table 3: ANOVA test results
In Table 3, R1 is resolution between impurity-C and impurity-D; R2 is resolution between impurity-D and impurity-A; R3 is resolution between impurity-A and impurity-B; Ris resolution between impurity-B and impurity-E; R5 is resolution between impurity-A and impurity-E; R6 is resolution between impurity-B and ETO; R7 is resolution between impurity-E and ETO; R8 is retention time of ETO; R9 is plate count of ETO; R10 is resolution between impurity-C and impurity-E; R11 is resolution between impurity-A and impurity-C and R12 is resolution between impurity-D and impurity-E, p-value shall be less than 0.001 and difference between R-squared and adjusted R-squared values shall be less than 2 to indicate significance model to evaluated response.
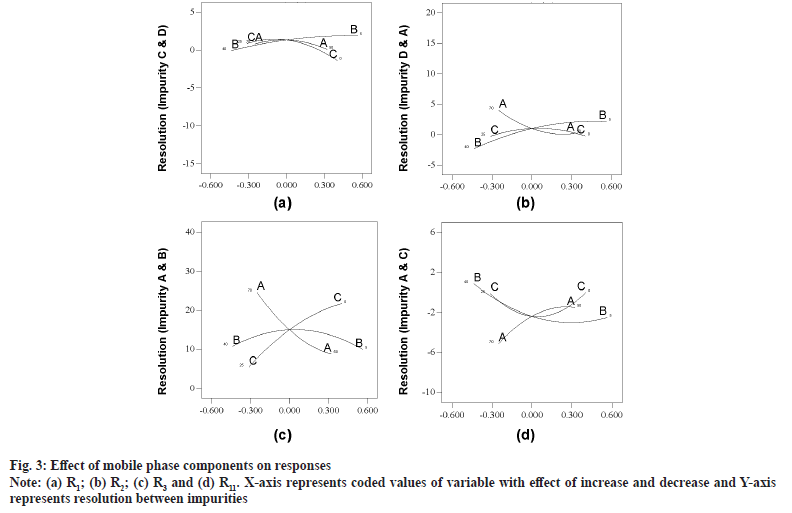
Model graphs evaluation revealed that all the responses were fitting into four different clusters. First cluster comprises R1 and R7 where resolution was improving with increase in volume of buffer and IPA from mid to highest value (fig. 3a). Second cluster was represented by R2 alone and all variables were positive at mid values of studied ranges and resolution was both in negative as well positive value, indicating shift in elution order (fig. 3b). Responses R3, R4, R5, R6, R8, R9, R10 and R12 fit into third cluster with positive response to increase in buffer and IPA concentration and minimal effect of ACN composition (fig. 3c). All the responses in cluster three were having positive response with an exception of R4 which exhibits elution order change. R11 alone was in fourth cluster and resolution was with negative value with increase in buffer and on positive value with increase in organic modifiers (fig. 3d).
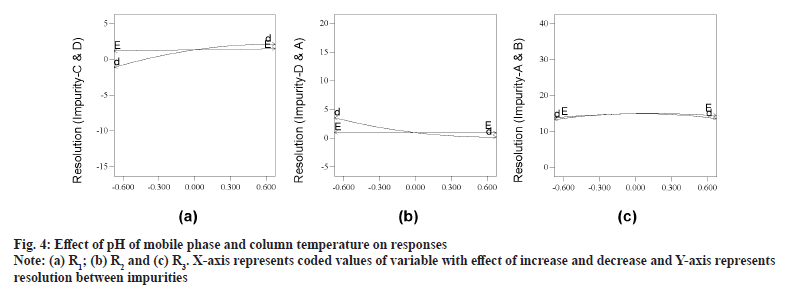
As an outcome of trace plots evaluation, it was found that R1, R2, R4 and R11 were exhibiting elution pattern change and were sensitive to both buffer and IPA concentration. Readers are suggested to make a note that archetypal plot for each cluster is presented for enhanced debate, as all other plots look analogous in pattern. Evaluation of independent variables (pH and column temperature) was exercised with perturbation plots. Column temperature was having negligible impact on all responses and was always of positive impact. pH of mobile phase had substantial influence on all responses with both positive and negative impact (selected range of pH is around reported pKa of ETO 4.5). Decrease in pH of mobile phase was refining resolution with negative values for R1, R4 and R11 responses (fig. 4a) and with positive value for R2 (fig. 4b). Any pH was found to be acceptable for remaining responses (fig. 4c). R5 and R6 are favoring mid to high pH, however the resolution was optimal in most instances.
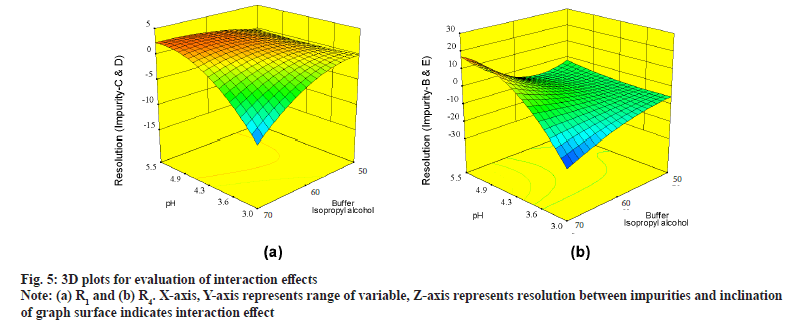
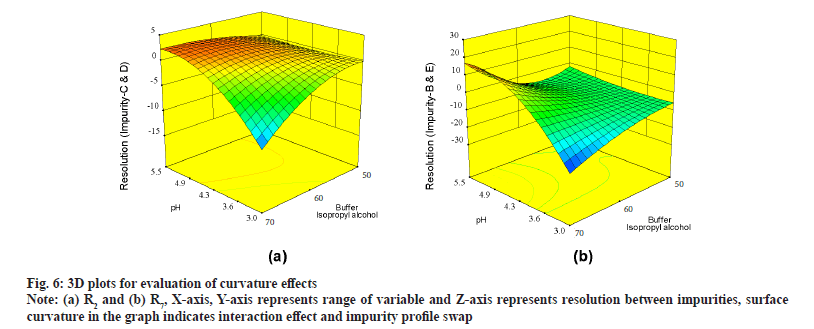
Further evaluation was deliberated with three Dimensional (3D) surface graphs to study interaction effects with focus on pH and concentration of buffer, IPA in mobile phase. Interaction effect was profoundly seen during evaluation of responses with 3D surface graphs. All responses were following majorly three different patterns. R1 is exhibiting good resolution at lower pH and higher buffer concentration and values were negative (fig. 5a). R4, R5, R6, R8, R10 and R12 were exhibiting mixed effects with both negative and positive values (fig. 5b) where resolution was negative with low pH and low IPA concentration and positive with increase in pH and IPA concentration. R2, R3, R7, R9 and R11 were not showing much of interaction effects and surface plot was mostly flat with an exemption of R2, displaying edge of failure effect at extreme low of pH and IPA concentration (fig. 6a) and R7 displaying curvature effect (fig. 6b) at mid-range of mobile phase composition.
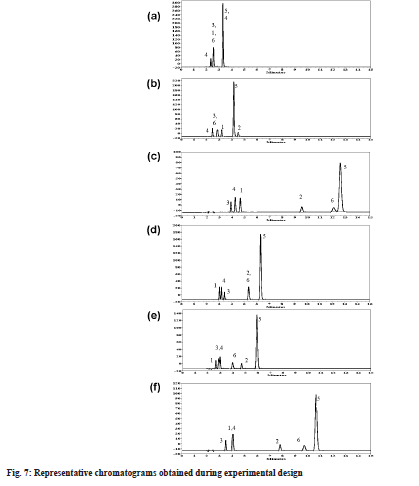
Elution pattern changes were depicted with representative chromatograms at different pH and mobile phase composition (fig. 7). Buffer at pH 3.0 was good for reduction in the retention time of all analyte peaks (below 5 min), with less than 60 % buffer concentration all peaks were merging (fig. 7a). With increase in buffer concentration above 60 %, keeping IPA concentration above 10 %, most of the peaks were separated except dimer and impurity-C (fig. 7b). Comparative evaluation of chromatographs at pH 4.3 revealed importance of IPA and elution order changes. As can be seen in fig. 7c at pH 4.3 elution order for first three peaks in the chromatogram was impurity-C, impurity-D and impurity-A. If IPA is removed from mobile phase, the elution order changed to impurity-A, impurity-D and impurity-C (fig. 7d). A similar observation on role of IPA can be made at pH 5.0. With increase in IPA concentration the resolution and elution pattern between first three peaks was drastically effected (fig. 7e). Column temperature is having marginal impact on resolution whereas IPA composition at an optimum of 20 % is positive for retention properties (fig. 7f).
As shown in fig. 7 chromatographic conditions used are (a) pH 3.0 buffer/ACN/IPA 50/40/10 (v/v), temperature 35° (b) pH 3.0 buffer/ACN/IPA 62/24/14 (v/v), temperature 30° (c) pH 4.3 buffer/ACN/IPA 70/19/11 (v/v), temperature 30° (d) pH 4.3 buffer/ACN/IPA 60/40/0 (v/v), temperature 35° (e) pH 5.5 buffer/ACN/ IPA 70/5/25 (v/v), temperature 25° (f) pH 5.3 buffer/ ACN/IPA 70/17/13 (v/v), temperature 35° . The peaks contained are represented as follows, (1) Impurity-A; (2) Impurity-B; (3) Impurity-C; (4) Impurity-D; (5) ETO and (6) Impurity-E
3D surface graph evaluation predicted that elution order change was predominant for R1, R2, R4, R5, R6, R7, R10 and R12 responses. Conversely low pH and low concentration of IPA was favorable for R1, R2, R4, R5, R6, R8 and R11, high pH and moderate concentration of IPA was favorable for R9, R3, R10 and R12, mid pH and moderate concentration of IPA was favorable for R7 and R9. Each response was fluctuating to different extremes of variable, majorly pH and IPA concentration, which were further critical for solution prediction and method operable region prediction becomes complicated as a result of this phenomenon[39-41]. Three elution patterns orderly, at pH 3.0 (impurity-D, impurity-C, dimer, impurity-A, ETO and impurity-B), at pH 4.3 (impurity-C, impurity-D, impurity-A, impurity-B, dimer and ETO) and pH 5.0 (impurity-C, impurity-D, impurity-A, dimer, impurity-B and ETO) were considered as reference for pivotal responses. Negative values were assigned to resolution between impurity-D and impurity-C, impurity-A and dimer, impurity-B and ETO and impurity-B and dimer with systematic elution pattern analysis with reference elution order as impurity-C, impurity-D, impurity-A, dimer and impurity-B followed by ETO. As most of the responses were having both negative and positive values as a result of elution order change, a novel approach of categorizing all observed responses against pH of mobile phase was experimented along with definitive remarks as basis for selection (Table 4). The article is mainly focused on dealing with impurity profile change with novel approach of response categorization, assigning negative values for resolution to deal with impurity profile change. Current research presented methodologies to deal with multiple responses to predict solutions/final chromatographic conditions from a complex separation goal, where multiple factors of the design are involved in interaction.
| Response* | Result | pH 5.5 | pH 3.0 | pH 4.3 | Remarks |
|---|---|---|---|---|---|
| R1 | - | 8 | 4 | 2 | pH 3.0 is favourable, Scattered effect at pH 5.5 and pH 4.3 |
| +ve | 6 | 0 | 4 | ||
| -ve | 2 | 13 | 2 | ||
| R2 | - | 9 | 3 | 4 | pH 3.0 is favourable, resolution not achieved at other pH |
| +ve | 2 | 14 | 3 | ||
| -ve | 5 | 0 | 1 | ||
| R3 | - | 1 | 0 | 0 | Any pH is suitable with positive response |
| +ve | 15 | 16 | 8 | ||
| -ve | 0 | 1 | 0 | ||
| R4 | - | 0 | 0 | 1 | pH 3.0 and pH 4.3 are favourable with negative response |
| +ve | 7 | 2 | 2 | ||
| -ve | 9 | 15 | 5 | ||
| R5 | - | 1 | 5 | 0 | Positive response is desirable at pH 4.3 and 5.0 |
| +ve | 15 | 2 | 8 | ||
| -ve | 0 | 10 | 0 | ||
| R6 | - | 0 | 6 | 0 | pH 4.3 and 5.0 are favourable with positive response |
| +ve | 16 | 0 | 8 | ||
| -ve | 0 | 11 | 0 | ||
| R7 | - | 0 | 1 | 0 | Positive response is observed at entire range of pH |
| +ve | 13 | 10 | 7 | ||
| -ve | 3 | 0 | 1 | ||
| R10 | - | 1 | 2 | 0 | Mixed response at pH 3.0. Positive response at pH 4.3 and 5.0 |
| +ve | 15 | 6 | 8 | ||
| -ve | 0 | 9 | 0 | ||
| R11 | - | 5 | 2 | 2 | Negative response is favourable at pH 3.0 and 4.3 |
| +ve | 5 | 2 | 2 | ||
| -ve | 6 | 13 | 4 | ||
| R12 | - | 0 | 3 | 0 | Positive response is favourable at entire range of pH |
| +ve | 16 | 13 | 8 | ||
| -ve | 0 | 1 | 0 |
Note: +ve represents resolution with positive sign and no impurity profile change; –ve represents resolution with negative sign and impurity profile change is observed; (-) represents no separation could be observed; *R8 and R9 are not critical for variable range selection and solution prediction
Table 4: Influence of butter pH and categorisation of responses
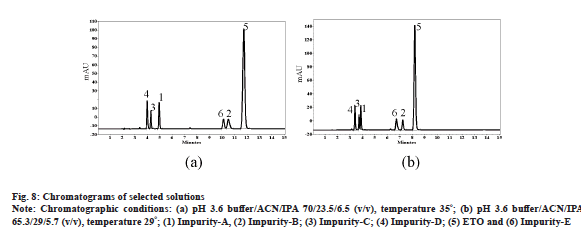
Observed responses for each run were coded with positive (+ve), negative (-ve) and neutral (-, no resolution). Based on most populated approach of observed responses, ranges and signs, desired responses were selected. For example R1 has 13 negative values at pH 3.0, which indicates that the desired outcome shall be in negative value and accordingly a range of -50 to -3.0 as resolution criteria was given. It is noteworthy that R8 and R9 were not part of response categorization exercise as values for these responses are always positive. After thorough evaluation of responses final chromatographic conditions were predicted with the help of software. For this purpose, numerical optimization option of DoE software is used. The software is given specific ranges for all variable and desired ranges for responses. Table 5 explains ranges for all variables and responses for solution prediction as an outcome of above exercise. DoE model was able to predict several solutions as desired combination of variables to yield chromatography with resolution between all analytes. Predicted solution-2 with DoE software was able to cut down ACN concentration to 23 %, but the total run time was extended to 15 min (fig. 8a). Solution-6 was able to cut down ACN concentration to 29 % and has excessive benefit of total run time less than 10 min with all impurities well resolved from each other and ETO as well (fig. 8b). All the resolution values for the responses are compared against design predictions for the selected two solutions and observed that the experimental results are in close agreement with DoE predictions (Table 6). Thus, experimental design for the targeted purpose is said to be validated.
| Name | Goalc | Lower limit | Upper limit | Importanced |
|---|---|---|---|---|
| Buffera | Is in range | 50 | 70 | 3 |
| ACNa | Is in range | 5 | 40 | 3 |
| IPAa | Is in range | 0 | 25 | 3 |
| pH | Is in range | 3 | 5.5 | 3 |
| Column temperatureb | Is in range | 25 | 35 | 3 |
| R1 | Is in range | -50 | -3 | 2 |
| R2 | Maximize | 2 | 50 | 1 |
| R3 | Maximize | 2 | 35.34 | 1 |
| R4 | Is in range | -50 | -3 | 3 |
| R5 | Maximize | 1.7 | 59.04 | 3 |
| R6 | Maximize | 1.7 | 14.45 | 3 |
| R7 | Maximize | 1.7 | 14.36 | 2 |
| R8 | Minimize | 3 | 17.5 | 1 |
| R9 | Maximize | 10798 | 31167 | 1 |
| R10 | Maximize | 1.7 | 55.28 | 3 |
| R11 | Is in range | -9.22 | -3 | 3 |
| R12 | Maximize | 1.7 | 55.28 | 2 |
Note: aPercentage (%) volume in mobile phase; bTemperature in ° ; cConstraint option to software; ‘in range’ indicates within specified values; ‘maximize’ and ‘minimize’ targets to increase or decrease in the value of prediction for response and dIndicates priority order in DoE software to predict solutions
Table 5: Constraints for optimisation and solutions prediction
In Table 6, S2 represents experimental conditions of OPA buffer adjusted to pH 3.6, ACN and IPA as mobile phase in the ratio of 70:23.5:6.5 (v/v) with 35° column temperature; S6 represents experimental conditions of OPA buffer adjusted to pH 3.6, ACN and IPA as mobile phase in the ratio of 65.3:29:5.7 (v/v) with 29° column temperature; R1 is resolution between impurity-C and impurity-D; R2 is resolution between impurity-D and impurity-A; R3 is resolution between impurity-A and impurity-B; R4 is resolution between impurity-B and impurity-E impurity; R5 is resolution between impurity-A and impurity-E; R6 is resolution between impurity-B and ETO; R7 is resolution between impurity-E and ETO; R8 is retention time of ETO; R9 is plate count of ETO; R10 is resolution between impurity-C and impurity-E; R11 is resolution between impurity-A and impurity-C and R12 is resolution between impurity-D and impurity-E.
| Response | S2a | S6b | ||
|---|---|---|---|---|
| Predicted | Observed | Predicted | Observed | |
| R1 | -3.2 | -2.4 | -3 | -2.6 |
| R2 | 8.0 | 7.5 | 5.1 | 3.19 |
| R3 | 25.7 | 27.4 | 18.2 | 21.94 |
| R4 | -3.0 | 1.3 | -4 | -3.77 |
| R5 | 21.0 | 28.7 | 15.5 | 19.28 |
| R6 | 4.4 | 5.1 | 2.5 | 2.96 |
| R7 | 6.2 | 3.8 | 6.8 | 4.14 |
| R8 | 10.2 | 11.7 | 7.8 | 8.1 |
| R9 | 23939 | 24704 | 21722 | 20815 |
| R10 | 25.9 | 33.8 | 17.8 | 14.18 |
| R11 | -4.0 | -5.1 | -3 | -2.22 |
| R12 | 29.1 | 36.3 | 20.4 | 18.62 |
Note: aPercentage (%) volume in mobile phase and bTemperature inº
Table 6: Design validation for selected solutions
Specificity of the method was carried out by evaluating different kinds of interferences, i.e. those produced by blank, placebo, known impurities and degradation products. For the first case, diluent as blank solution is tested. For placebo and known impurity interference, mixture of all excipients and all known impurities spiked to sample were evaluated. All impurities are well separated from ETO peak and no extra peaks were observed at retention of impurities as well ETO from diluent and placebo preparations[42]. Stability indicating nature of the method for degradation products was proven with forced degradation studies at different hydrolytic, oxidation, thermal and photolytic stress conditions. Several permutation and combinations of heat and additive concentrations were tested to arrive at suitable degradation conditions that will yield degradation in the range of 2 % to 20 %. Photolytic stress was performed both at visible stress (white fluorescent light-1.2 million lux h.), UV stress (200 Wh/m2) and the product is stable to light exposure and this observation is in contrary to the literature reported method observation of degradation to photostability and yielding two major degradants. One of the probable reasons can be that the literature method is applicable for ETO API, whereas current study majorly focuses on RS method for ETO tablets[36]. It is possible that the excipients and coating material of the tablet are giving extra protection from light exposure and hence no degradation is seen, while heat stress is not able to generate significant degradants at 80° for about 2 d. It was observed that acid (1 N HCl at 60° for 30 min), base (1 N NaOH at 60° for 30 min) and peroxide stress (3 % Hydrogen peroxide (H2O2) for 30 min) conditions were not able to generate considerable degradation and ETO tablets were found to be stable to all degradation conditions. Peak purity and mass balance studies confirm noninterference of degradation products and purity index for all degradation samples is observed above 1.0.
Linearity solutions, i.e. Limit of Quantitation (LOQ), 50 %, 100 % and 150 % levels for impurities and 50 %, 100 % and 150 % levels for assay method were injected into HPLC and chromatograms were recorded. The regression line analysis shows linear relationship between concentration and area response of ETO and all known impurities. Relative Response Factor (RRF) of each impurity against ETO diluted standard is calculated by comparing observed slope of linearity graph. Results of linearity and RRF are presented in Table 7. Readers shall make a note that impurity-E is not considered for method validation studies, because of insufficient quantity.
| Level | Area | |||||
|---|---|---|---|---|---|---|
| Impurity-A | Impurity-B | Impurity-C | Impurity-D | ETO | ||
| LOQ (25 %) | 3360 | 1668 | 1672 | 3454 | 3137 | |
| 50 % | 6558 | 3553 | 3307 | 6805 | 6540 | |
| 100 % | 13202 | 7041 | 6686 | 13697 | 12385 | |
| 150 % | 19805 | 10049 | 10046 | 20519 | 18565 | |
| 200 % | 26405 | 13491 | 13394 | 27390 | 24309 | |
| Slope | 12964.3 | 5992.74 | 6155.4 | 13162.9 | 11708.2 | |
| Intercept | 15.22 | 144.402 | -23.86 | -0.86 | 324.738 | |
| Correlation (r) | 1 | 0.9995 | 1 | 1 | 0.9998 | |
| RRF | 1.11 | 0.51 | 0.53 | 1.12 | 1 | |
| ETO assay | ||||||
| 25 % | 1490456 | |||||
| 50 % | 2986504 | |||||
| 75 % | 4455100 | |||||
| 100 % | 5924335 | |||||
| 150 % | 9083406 | |||||
| Slope | 12038.5 | |||||
| Intercept | -102066 | |||||
| Correlation (r) | 0.9996 | |||||
Note: RRF represents relative response factor for impurities calculated against ETO response at standard concentration and LOQ represents limit of quantitation
Table 7: Linearity, Range and RRF
As shown in Table 7, concentration range in related substances method for impurity-A is 0.254-2.035 μg/ ml; impurity-B is 0.279-2.230 μg/ml; impurity-D is 0.26-2.68 μg/ml; impurity-C is 0.273-2.18 μg/ml and ETO is 0.258-2.06 μg/ml. Concentration range for ETO in assay method is 127.5-756.0 μg/ml.
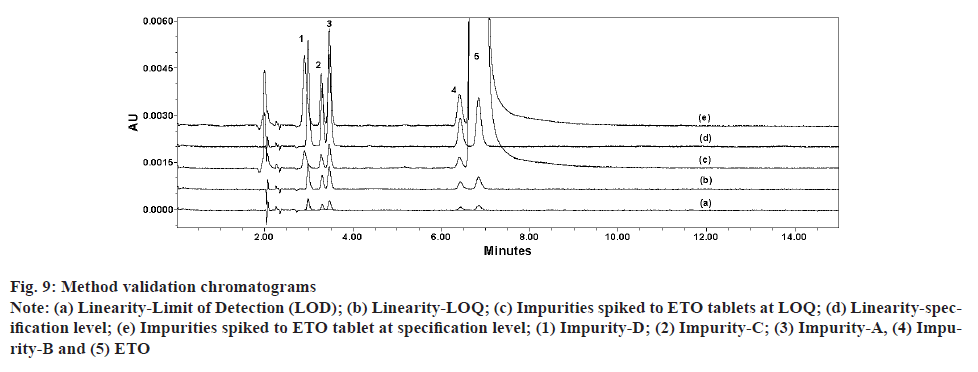
Accuracy of the method was performed at four different levels by spiking all known impurities at pre-determined concentration ranges to ETO tablets. Accuracy at 100 % of target impurities specification (0.2 % of sample concentration; 1.0 μg/ml) was performed in six replicates to evaluate method precision. Accuracy at LOQ level was also performed in six replicates to prove method range at low level. For all accuracy level, percentage (%) recovery of impurities and ETO were calculated along with % Coefficient of Variation (% CV) for replicate preparation at each level. All recoveries were calculated by applying RRF for impurities against an external standard prepared with ETO at concentration of 0.2 % with respect to sample preparation (1.0 μg/ml). All the tested levels were able to meet the acceptance criteria for recovery (85 %-115 %) and precision (% CV<15 %), indicating method suitability for routine use. It is sensible to make a note that, because of limitations in availability of sufficient quantity of impurity standards, the stock solutions prepared with all different impurities were stored at refrigerated condition and used for multiple days during the study. Storage of solutions for longer period and multiple passages of use for the stock solutions might have resulted in evaporation of solvent causing concentration of impurity. As a result of this, most of the recoveries for impurities during accuracy study are observed higher than 100 %. Accuracy for ETO for the purpose of assay test was proved at three levels ranging from 250 μg/ml to 750 μg/ml. Recovery ranges for ETO were in the range of 97.9 %-101.1 %. Observed % CV for replicate preparations at 50 % (n=3), 100 % (n=6) and 150 % (n=3) are 2.6, 1.0 and 1.2 respectively. Accuracy and precision results of RS test from the study are presented in Table 8. Chromatograms of finalized method conditions from validation study are shown in fig. 9. The present method uses short run time of 15 min with a Retention Time (RT) of ETO of about 8 min compared to a run time of 10-45 min of literature reported method. A detailed comparison of selected parameters with the present method is given in Table 9.
| Analyte name | Level | Nominal (µg/ml) | Predicted (µg/ml) | Average area | % Recovery | % CV |
|---|---|---|---|---|---|---|
| Impurity-A | LOQ | 0.251 | 0.276 | 3412 | 110.0 | 4.6 |
| 50 | 0.502 | 0.526 | 6502 | 104.8 | 4.4 | |
| 100 | 1.004 | 1.114 | 13776 | 111.0 | 0.6 | |
| 150 | 1.506 | 1.584 | 19580 | 105.2 | 3.8 | |
| Impurity-B | LOQ | 0.262 | 0.298 | 1691 | 113.8 | 3.8 |
| 50 | 0.524 | 0.493 | 2799 | 94.0 | 2.7 | |
| 100 | 1.048 | 1.107 | 6288 | 105.7 | 1.4 | |
| 150 | 1.572 | 1.643 | 9330 | 104.5 | 1.5 | |
| Impurity-C | LOQ | 0.268 | 0.292 | 1680 | 109.0 | 2.8 |
| 50 | 0.536 | 0.568 | 3227 | 106.0 | 4.8 | |
| 100 | 1.072 | 1.164 | 6610 | 108.6 | 5.0 | |
| 150 | 1.608 | 1.691 | 9608 | 105.2 | 4.8 | |
| Impurity-D | LOQ | 0.253 | 0.263 | 3276 | 103.8 | 2.8 |
| 50 | 0.506 | 0.531 | 6626 | 104.9 | 3.1 | |
| 100 | 1.012 | 1.059 | 13203 | 104.6 | 3.3 | |
| 150 | 1.518 | 1.664 | 20749 | 109.6 | 4.1 | |
| ETO assay | 50 | 249.0 | 256.5 | 2978365 | 103.0 | 2.6 |
| 241.0 | 243.5 | 2827109 | 101.0 | |||
| 253.0 | 247.8 | 2876625 | 97.9 | |||
| 100 | 503.4 | 507.6 | 5893625 | 100.8 | 1.0 | |
| 503.4 | 508.8 | 5907286 | 101.1 | |||
| 503.4 | 502.3 | 5831657 | 99.8 | |||
| 503.4 | 507 | 5886038 | 100.7 | |||
| 511.9 | 515.1 | 5980152 | 100.6 | |||
| 506.0 | 498 | 5781834 | 98.4 | |||
| 150 | 743.0 | 756.4 | 8782134 | 101.8 | 1.2 | |
| 753.0 | 748.9 | 8694312.66 | 99.5 | |||
| 735.0 | 741.3 | 8606491.32 | 100.9 |
Note: Nominal represents amount of impurity spiked; predicted represents amount of impurity recovered; % recovery is ratio of predicted and nominal; % CV is co-variance of observed recoveries for n=3 at LOQ, 50 %, 150 % levels and n=6 at 100 % level, All concentrations are represented in µg/ml
Table 8: Accuracy and Precision
| S. no. | Column, elution process, mobile phase, flow rate, injection volume | Sample linear range, detection | Run time (RT of ETO) | Intended use, no. of impurities covered in study | Reference numbers |
|---|---|---|---|---|---|
| 1 | RP-C18 (250×4.6 mm, 5 µ), isocratic, 0.05 M ammonium acetate buffer pH 5.0:ACN (50:50 v/v), 1.0 ml/min, 20 µl | 25.68-73.5 µg/ml, UV at 235 nm | 10 min (5.5 min) | For quantification of ETO in bulk and tablets, Nil | [31] |
| 2 | Inertsil Octadecyl Silica (ODS)-4 (250×4.6 mm, 5 µ), isocratic, 0.01 M sodium perchlorate monohydrate buffer pH 5.0:ACN (48:52 v/v), 1.5 ml/min, 10 µl | 4.67-63.96 µg/ml, UV at 235 nm | 10 min (4.2 min) | For quantification of ETO in tablets and in vitro release determination, Nil | [32] |
| 3 | Xterra-RP-18 (150×3.5 mm, 5 µ), isocratic, 0.2 M phosphate buffer pH 5.0:ACN (60:40 v/v), 0.8 ml/min, 20 µl | 1-6 µg/ml, UV at 242 nm | 10 min (4.5 min) | For quantification of paracetamol and ETO in tablets, Nil | [33] |
| 4 | Zorbax-SB-CN (250×4.6 mm, 5 µ), isocratic, 0.02 M disodium hydrogen phosphate buffer pH 7.2: ACN (60:40 v/v), 0.8 ml/min, 10 µl | 0.003-500 µg/ml, UV at 235 nm | 30 min (11.2 min) | For quantification of impurities, 3 | [34] |
| 5 | Inertsil ODS-3V, (250×4.6 mm, 5 µ), Gradient, mobile phase A-0.01 M potassium dihydrogen phosphate buffer and ACN, 1.0 ml/min, 10 µl | 0.02-1000 µg/ml, UV at 238 nm | 45 min (21.5 min) | For quantification of impurities, 2 | [35] |
| 6 | YMC AQ-ODS (150×4.6 mm, 3 µ), Gradient, mobile phase A-0.01 M potassium dihydrogen phosphate buffer, pH 3.1 and B- ACN, 1.0 ml/min, 10 µl | 0.02-1000 µg/ml, UV at 220 nm | 45 min (12.5 min) | For quantification of impurities, 13 | [36] |
| 7 | Ascentis Express-C18 (150×4.6 mm, 2.7 µ), isocratic, 0.1 % OPA buffer pH 3.6:ACN:IPA (65.3:29:5.7 v/v), 1.0 ml/min, 10 µl | 0.25-750 µg/ml, UV at 285 nm | 15 min (11 min) | For quantification of content and impurities in tablet formulation, 5 | Current method |
Note: RT: Retention Time; RP: Reverse Phase, ODS: Octadecyl Silica, SB-CN: Stable Bond Cyano packing: UV: Ultraviolet spectrophotometer; Nil: Not covered; M: Molar concentration of buffer; IPA: Isopropyl Alcohol
Table 9: Comparison of selected analytical methods of ETO
Fig. 9: Method validation chromatograms
Note: (a) Linearity-Limit of Detection (LOD); (b) Linearity-LOQ; (c) Impurities spiked to ETO tablets at LOQ; (d) Linearity-specification level; (e) Impurities spiked to ETO tablet at specification level; (1) Impurity-D; (2) Impurity-C; (3) Impurity-A, (4) Impurity- B and (5) ETO
Information gained from entire method development cycle is used for risk management of method and to define method design space. Risk assessment is sciencebased approach to identify potential critical aspects of method that help in identifying which method attributes and method parameters have an impact on the outcome of the method. Solvent purity and reagent interference are considered as critical material attributes for risk assessment. pH, composition of mobile phase, column temperature, flow rate and different types of filter membranes are considered as critical method parameters of evaluation. As the mobile phase is ternary mixture, IPA composition is considered as critical variable in mobile phase composition because IPA is in minor proportion. Robustness of the method was evaluated by showing the impact changes to critical method parameters, such as mobile phase composition, temperature, flow rate, pH of mobile phase, filter validation and solution stability. IPA variation is studied for mobile phase composition as the same is less proportional when compared to other two solvents. Different types of filters (PVDF, Nylon) are studied for filter validation and the results are well within the acceptance criteria. Assay, purity of ETO along with tailing factor and critical resolution between closely eluting impurities are the responses studied for robustness. All analytical solutions are stable for a period of 48 h at controlled room temperature condition. Robustness study results are presented in Table 10. Through implementation of risk management and robustness testing resulted in defining control strategy for the method. Control strategy is designed to ensure that the method of required quality will be produced consistently. Primary element of control strategy is identified as mobile phase composition. As the mobile phase is ternary solvent system it is decided to keep all the three solvents (buffer, ACN and IPA) in three different channels of HPLC system and the mixing is arranged through gradient valve. Another key element of control strategy is to maintain the pH of mobile phase in the range of ±0.10 units of targeted pH 3.6, as any variation beyond the defined range will lead to impurity profile swap. All other method variables are found to be not susceptible to variations and still can deliver a rugged chromatography even if, minor variations happen in the controlled method parameters.
| Method parameter | ETO assay (% LC) | Purity (% w/w) | Tailing factor | Critical resolution |
|---|---|---|---|---|
| % IPA (% v/v) | ||||
| 6.0 | 99.4 | 99.92 | 1.02 | 1.6 |
| 6.5 | 100.5 | 99.92 | 1.07 | 1.5 |
| 7.0 | 98.2 | 99.91 | 1.01 | 1.6 |
| Column temperature (°) | ||||
| 30 | 101.6 | 99.92 | 1.15 | 1.5 |
| 35 | 100.5 | 99.92 | 1.07 | 1.5 |
| 40 | 100.7 | 99.91 | 1.02 | 1.7 |
| Flow (ml/min) | ||||
| 0.95 | 100.2 | 99.92 | 1.05 | 1.6 |
| 1.00 | 100.5 | 99.92 | 1.07 | 1.5 |
| 1.05 | 99.3 | 99.92 | 1.02 | 1.5 |
| Mobile phase pH | ||||
| 3.5 | 101.9 | 99.90 | 1.06 | 1.6 |
| 3.6 | 100.5 | 99.92 | 1.07 | 1.5 |
| 3.7 | 98.9 | 99.92 | 1.17 | 1.6 |
| Filter study (Type) | ||||
| No filtration | 101.2 | 99.89 | 1.11 | 1.7 |
| PVDF | 100.5 | 99.92 | 1.07 | 1.5 |
| Nylon | 99.5 | 99.91 | 1.17 | 1.6 |
| Solution stability (Time in h) | ||||
| 0 | 100.5 | 99.92 | 1.07 | 1.5 |
| 24 | 99.8 | 99.93 | 1.12 | 1.7 |
| 48 | 98.9 | 99.89 | 1.11 | 1.6 |
Note: LC: Label Claim
Table 10: Robustness of Method
Acknowledgements:
The authors wish to thank the management of GVK Biosciences Private Limited, Hyderabad, India for giving us an opportunity to carry out the dissertation work.
Conflict of interests:
The authors declared no conflict of interests.
References
- United States Pharmacopoeia/National Formulary online. <1151> Pharmaceutical dosage forms, general considerations, USP43-NF28 2S, United States Pharmacopoeial Convention Inc.; 2020.
- United States Pharmacopoeia/National Formulary online. <2> Oral drug products-Product quality tests. USP43-NF28 2S, United States Pharmacopoeial Convention Inc.; 2020.
- Viana OD, Medeiros FP, Grangeiro-Júnior S, Albuquerque MM, Soares MF, Soares-Sobrinho JL, et al. Development and validation of a HPLC analytical assay method for efavirenz tablets: A medicine for HIV infections. Braz J Pharm Sci 2011;47(1):97-102.
[Crossref] [Google Scholar] [PubMed]
- Snyder LR, Kirkland JJ. Introduction to modern liquid chromatography. 2nd ed. New York: Wiley-Interscience; 1979. p. 261-3.
- Wren SA, Tchelitcheff P. Use of ultra-performance liquid chromatography in pharmaceutical development. J Chromatogr A 2006;1119(1):140-6.
[Crossref] [Google Scholar] [PubMed]
- Joshi VS, Kumar V, Rathore AS. Role of organic modifier and gradient shape in RP-HPLC separation: Analysis of GCSF variants. J Chromatogr Sci 2015;53(3):417-23.
- Landge SB, Jadhav SA, Vishwambar SP, Solanki PV, Bembalkar SR, Mathad VT. Development and validation of RP-UPLC method for the determination of iloperidone, its related compounds and degradation products in bulk and dosage form. Am J Analyt Chem 2014;5(14):969-81.
- CHROMacademy. The theory of HPLC. Reverse phase chromatography e-learning module. CHROMacademy, LC-GC, crawfordscientific; 2021.
- Gilar M, Jaworski A, McDonald TS. Solvent selectivity and strength in reversed-phase liquid chromatography separation of peptides. J Chromatogr A 2014;1337:140-6.
- Snyder LR, Kirkland JJ. Introduction to modern liquid chromatography. 2nd ed. New York: Wiley-Interscience; 1979. p. 283-88.
- Tanaka N, Goodell H, Karger BL. The role of organic modifiers on polar group selectivity in reversed-phase liquid chromatography. J Chromatogr A 1978;158:233-48.
- Yabré M, Ferey L, Somé IT, Gaudin K. Greening reversed-phase liquid chromatography methods using alternative solvents for pharmaceutical analysis. Molecules 2018;23(5):1-25.
[Crossref] [Google Scholar] [PubMed]
- Alagesan K, Khilji SK, Kolarich D. It is all about the solvent: On the importance of the mobile phase for ZIC-HILIC glycopeptide enrichment. Anal Bioanal Chem 2017;409(2):529-38.
[Crossref] [Google Scholar] [PubMed]
- Wikipedia. Aqueous Normal-Phase Chromatography. Wikipedia: The free encyclopedia; 2020.
- Majors RE. The cleaning and regeneration of reversed-phase HPLC columns. LC GC N Am 2003;21(1):19-27.
- Shaaban H. New insights into liquid chromatography for more eco-friendly analysis of pharmaceuticals. Anal Bioanal Chem 2016;408(25):6929-44.
[Crossref] [Google Scholar] [PubMed]
- Du CM, Valko K, Bevan C, Reynolds D, Abraham MH. Characterizing the selectivity of stationary phases and organic modifiers in reversed-phase high-performance liquid chromatographic systems by a general solvation equation using gradient elution. J Chromatogr Sci 2000;38(11):503-11.
[Crossref] [Google Scholar] [PubMed]
- Hewitson HB, Wheat TE, Diehl DM. Alternative solvents for the reversed-phase separation of proteins. Massachusetts, USA: Waters Corporation; 2009.
- Desai AM, Andreae M, Mullen DG, Holl MM, Baker Jr JR. Acetonitrile shortage: Use of isopropanol as an alternative elution system for ultra/high performance liquid chromatography. Anal Methods 2011;3(1):56-8.
[Crossref] [Google Scholar] [PubMed]
- Shaaban H, Gorecki T. Current trends in green liquid chromatography for the analysis of pharmaceutically active compounds in the environmental water compartments. Talanta 2015;132:739-52.
[Crossref] [Google Scholar] [PubMed]
- Preti R. Core-shell columns in high-performance liquid chromatography: Food analysis applications. Int J Anal Chem 2016;2016:1-9.
[Crossref] [Google Scholar] [PubMed ]
- Hayes R, Ahmed A, Edge T, Zhang H. Core-shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. J Chromatogr A 2014;1357:36-52.
[Crossref] [Google Scholar] [PubMed]
- Kirkland JJ, Langlois TJ, DeStefano JJ. New fused-Core™ particles for very fast HPLC separations. Wilmington, Delaware: Advanced Materials Technology; 2021.
- Broeckhoven K, Cabooter D, Desmet G. Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7 μm and 5 μm: Intrinsic evaluation and application to a pharmaceutical test compound. J Pharm Anal 2013;3(5):313-23.
[Crossref] [Google Scholar] [PubMed]
- Kirkland JJ, Schuster SA, Johnson WL, Boyes BE. Fused-core particle technology in high-performance liquid chromatography: An overview. J Pharm Anal 2013;3(5):303-12.
[Crossref] [Google Scholar] [PubMed]
- Montemurro M, de Zan MM, Robles JC. Optimized high performance liquid chromatography-ultraviolet detection method using core-shell particles for the therapeutic monitoring of methotrexate. J Pharm Anal 2016;6(2):103-11.
[Crossref] [Google Scholar] [PubMed]
- Manranjan VC, Yadav DS, Jogia HA, Chauhan PL. Design of experiment (DOE) utilization to develop a simple and robust reversed-phase HPLC technique for related substances estimation of omeprazole formulations. Sci Pharm 2013;81(4):1043-56.
[Crossref] [Google Scholar] [PubMed]
- Solvason CC, Chemmangattuvalappil NG, Eljack FT, Eden MR. Efficient visual mixture design of experiments using property clustering techniques. Ind Eng Chem Res 2009;48(4):2245-56.
- Kurmi M, Kumar S, Singh B, Singh S. Implementation of design of experiments for optimization of forced degradation conditions and development of a stability-indicating method for furosemide. J Pharm Biomed Anal 2014;96:135-43.
[Crossref] [Google Scholar] [PubMed]
- Development and validation of a HPLC analytical assay method for efavirenz tablets: A medicine for HIV infections
- Haque M, Nasrin S, Monir MM, Rahman MM, Chowdhury S. Method development and validation of RP-HPLC method of etoricoxib in bulk and its tablet dosage forms. Am J PharmTech Res 2012;2(6):275-83.
- Singh B, Santhakumar R, Bala I, Prasad SB, Verma S. Development and validation of RP-HPLC method for the dissolution and assay of etoricoxib in pharmaceutical dosage forms. Int J Pharm Qual Assur 2014;6(1):1-7.
- Baheti KG, Shaikh S. Stability indicating RP-HPLC method for simultaneous estimation paracetamol and etoricoxib in tablet formulation. Int J PharmTech Res 2011;3(3):1719-27.
- Venugopal S, Tripathi UM, Devanna N. Validated Reverse Phase HPLC method for the determination of impurities in etoricoxib. E-J Chem 2011;8(S1):119-26.
- Susmetha K, Nataraj KS, Rao AS, Sai BDMSP. Analytical method development and validation of related substances in etoricoxib (API) by using RP-HPLC. Chem Sci Trans 2019;8(2):249-55.
[Crossref]
- Hartman R, Abrahim A, Clausen A, Mao B, Crocker LS, Ge Z. Development and validation of an HPLC method for the impurity and quantitative analysis of etoricoxib. J Liq Chromatogr Relat Technol 2003;26(15):2551-66.
- Schmidt AH, Molnár I. Using an innovative Quality-by-Design approach for development of a stability indicating UHPLC method for ebastine in the API and pharmaceutical formulations. J Pharm Biomed Anal 2013;78:65-74.
[Crossref] [Google Scholar] [PubMed]
- Murthy MV, Krishnaiah C, Srinivas K, Rao KS, Kumar NR, Mukkanti K. Development and validation of RP-UPLC method for the determination of darifenacin hydrobromide, its related compounds and its degradation products using design of experiments. J Pharm Biomed Anal 2013;72:40-50.
- US Food and Drug Administration. EMA-FDA pilot program for parallel assessment of Quality-by-Design applications: Lessons learnt and Q&A resulting from the first parallel assessment. European Medicines Agency and U.S. Food and Drug Administration; 2013.
- Dai S, Xu B, Luo G, Li J, Xue Z, Shi X, et al. Application of design of experiment and design space (DOE-DS) methodology for the HPLC separation of Panax notoginseng saponins. Open Chem Eng J 2015;9(1):47-52.
- Borman P, Roberts J, Jones C, Hanna-Brown M, Szucs R, Bale S. The development phase of an LC method using QbD principles. J Sep Sci 2010;2:2-8.
- International Conference on Harmonization. ICH guidance on analytical method validation. Toronto, Canada: Proceedings of the international convention on quality for the pharmaceutical industry; 2002.