- Corresponding Author:
- T. E. G. K. Murthy
Department of Pharmaceutics, Bapatla College of Pharmacy, Bapatla - 522 101, India
E-mail: gopalakrishnatalasila@yahoo.com
| Date of Submission | 2 September 2006 |
| Date of Revision | 2 July 2007 |
| Date of Acceptance | 21 September 2007 |
| Indian J. Pharm. Sci., 2007, 69 (5): 646-650 |
Abstract
In the present work, cellulose acetate and ethyl cellulose films were prepared and evaluated as rate controlling membrane for transdermal drug delivery systems. In each case films were prepared using solutions of the polymer in various solvents to evaluate the influence of the solvent used on the mechanical and permeability properties of the films. Acetone-methanol (8:2), chloroform-methanol (8:2), dichloromethane-methanol (8:2) and ethyl acetate-methanol (8:2) were used as solvents in the preparation of cellulose acetate and ethyl cellulose films. Dibutyl phthalate or propylene glycol at a concentration of 40% w/w of the polymer was used as a plasticizer in the preparation films. The method of moulding was found to be giving thin uniform films. The dry films were evaluated for physical appearance, thickness uniformity, folding endurance, water vapour transmission, drug diffusion and permeability coefficient. Both water vapour transmission and Drug diffusion rate followed zero order kinetics. The mechanism of drug release was governed by Peppas model. The diffusion exponent of release profiles (slope) has a value of 1.0360-1.3147 (n>1), which indicates non anomalous transport diffusion. The results obtained in the present study thus indicated that the polymers and solvents used in the preparation of films have shown significant influence on the water vapour transmission, drug diffusion and permeability of the films. Area of patches ranging from 1.29- 4.53 cm 2 were found to yield the desired release rate of propranolol hydrochloride. Cellulose acetate films employed with ethyl acetate:methanol in 8:2 ratio as casting solvent yielded low area (1.29 cm 2 ) of patch with desired release rate.
Keywords
Drug diffusion and permeability coeffi cient, polymer, solvents, water vapour transmission
The development of transdermal drug delivery systems using polymeric materials has become popular for various reasons. Among the various types of transdermal drug delivery systems developed, membrane controlled type utilizes a thin polymeric film as rate controlling membrane, which delivers the drug from the drug reservoir to the systemic circulation for an extended period of time. The permeability of drug through polymeric film is dependent on characteristics of the polymer [1],[2], casting solvent [3],[4] and plasticizer [5],[6] used. In the present work ethyl cellulose (EC) and cellulose acetate (CA) films were prepared and evaluated as rate controlling membranes for transdermal drug delivery systems. Propranolol hydrochloride [7] which requires controlled release due to its short biological half life (3.9 h), was used as model drug. Moulding technique was employed in the present work for the preparation of films.
Materials and Methods
Propranolol hydrochloride was obtained as a gift sample from Natco Pharma, Hyderabad. Ethyl cellulose (with an ethoxyl content of 47.5-53.5% by weight and a viscosity of 14 cps in a 5%w/w 80:20 toluene:ethanol solution at 250, (S. D. Fine Chem), Cellulose acetate (viscosity of 6% solution in 95% acetone-water mixture at 200 having 140 CS viscosity, G. S. Chemical Testing Lab and Allied Industries) Acetone, chloroform, dichloromethane and ethyl acetate (Qualigens), dibutyl phthalate (Ranbaxy Laboratories) and propylene glycol (S. D. Fine Chem) were obtained commercially. All materials were used as received.
Preparation of drug free films
Moulding technique was employed in the present work for the preparation of cellulose acetate and ethyl cellulose films. The films were prepared with ethyl cellulose and cellulose acetate (2% w/v) by employing different casting solvents namely acetone-methanol (8:2), chloroform-methanol (8:2), dichloromethane-methanol (8:2), and ethyl acetatemethanol (8:2). Dibutyl phthalate at a concentration of 40% w/w of the polymer was used as a plasticizer in the preparation of cellulose acetate and ethyl cellulose films. The cellulose acetate films made from its solution in dichloromethane-methanol (8:2) and ethyl cellulose films made from its solution in acetone-methanol (8:2), incorporating dibutyl phthalate were found to be brittle. In those cases propylene glycol at 40% w/w of the polymer was used as a plasticizer. Twenty millilitre of the polymer solution was poured in a Petri plate (9.4 cm diameter) placed on a horizontal flat surface. The rate of evaporation was controlled by inverting a funnel over the Petri plate. After 24 h the dried films were taken out and stored in a dessicator.
Evaluation of transdermal fi lms
All the films prepared were evaluated for physical appearance, uniformity of thickness, folding endurance, water vapour transmission and drug diffusion and permeability characteristics. The thickness of the films was measured using a Screw Gauge. The mean of the five observations were calculated. The folding endurance was measured manually for the prepared films. A strip of film (2×2 cm) was cut evenly and repeatedly folded at the same place till it broke. The number of times the film could be folded at the same place without breaking gave the exact value of folding endurance [8].
For the study of water vapour transmission (WVT) rate, vials of equal diameter were used as transmission cells. These cells were washed thoroughly and dried in an oven. About 1 g of calcium chloride was taken in the cell and the polymeric films measuring 3.14 cm2 area was fixed over the brim with the help of an adhesive. The cells were weighed accurately and initial weight is recorded, and then kept in a closed desiccator containing saturated solution of potassium chloride (about 200 ml). The humidity inside the desiccator was measured by a hygrometer and it was found to be in between 80-90% RH. The cells were taken out and weighed after 18, 36, 54 and 72 h.
From increase in weights the amount of water vapour transmitted and the rate at which water vapour transmitted were calculated by using the following formula [9], WVT rate= WL/S, where, W is water vapour transmitted in gms, L is thickness of the film in cm, S is exposed surface area in cm2
Drug diffusion study [10]
Drug diffusion study was conducted using Franz diffusion cell. The receptor compartment was filled with 15 ml of phosphate buffer having pH 7.4 as diffusion media. Polymeric film was mounted on the donor compartment with the help of an adhesive. An aliquot of 10 ml of the 0.25% W/V solution of drug (propranolol hydrochloride) was poured into the donor compartment. Magnetic stirrer was set at 50 rpm and whole assembly was maintained at 32±0.50. The amount of drug released was determined by withdrawing 1 ml of sample at regular time intervals for 3 h. The volume withdrawn was replaced with equal volume of fresh buffer solution. Samples were analyzed for drug content using a UV spectrophotometer at 290 nm [11].
Permeability coeffi cient
From the drug diffusion data the permeability co efficient for various films was calculated using the equation; Pm= (Kapp.H)/A, where, Kapp is the diffusion rate constant (mg/h) calculated from the slope of the linear drug (d/p) diffusion profiles , H is the thickness of the film (cm), A is the surface area of the film (cm2).
The rate and the mechanism of release of propranolol hydrochloride through the prepared films were analyzed by fitting the diffusion data into [12], zero-order equation, Q=Q0 -k0t, where Q is the amount of drug released at time t, and k0 is the release rate. First order equation, Ln Q=Ln Q0- k1t, where k1 is the release rate constant and Higuchi’s equation, Q= k2t1/2, where Q is the amount of the drug released at time t and k2 is the diffusion rate constant. The diffusion data was further analyzed to define the mechanism of release by applying the diffusion data following the empirical equation , Mt/Mα=Ktn, where Mt/Mα is the fraction of drug released at time t, K is a constant and n characterizes the mechanism of drug release from the formulations during diffusion process.
Estimation of area of patch required for desired release rate
The mathematical description of drug release that follow zero order kinetics is based on the equation [13]; 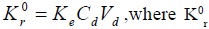 is zero order rate constant for drug release, Ke is the first order rate constant for overall drug elimination, Cd is the desired drug level in the body and Vd is the volume space in which drug is distributed. For propranolol hydrochloride [7], t½= 3.9 h, Vd= 4.3 l and Cd= 0.02 µg/ml and therefore the desired drug release rate can be calculated as Kr0= (0.693/3.9)×0.02×4.3×70 =1.064 mg/h. Hence, area of patch (A) required for desired release rate is A= Kr0/(Kapp/S), where K0 r is the required drug release rate (mg/h), Kapp is the diffusion rate constant (mg/h), S is the surface area of the film subjected to diffusion (cm2).
is zero order rate constant for drug release, Ke is the first order rate constant for overall drug elimination, Cd is the desired drug level in the body and Vd is the volume space in which drug is distributed. For propranolol hydrochloride [7], t½= 3.9 h, Vd= 4.3 l and Cd= 0.02 µg/ml and therefore the desired drug release rate can be calculated as Kr0= (0.693/3.9)×0.02×4.3×70 =1.064 mg/h. Hence, area of patch (A) required for desired release rate is A= Kr0/(Kapp/S), where K0 r is the required drug release rate (mg/h), Kapp is the diffusion rate constant (mg/h), S is the surface area of the film subjected to diffusion (cm2).
Results and Discussion
In the present work, cellulose acetate and ethyl cellulose films were prepared and evaluated as rate controlling membranes for transdermal drug delivery systems. Moulding technique was employed in the present work for the preparation of cellulose acetate and ethyl cellulose films. In each case films were prepared using solutions of the polymer in various solvents to evaluate the influence of the solvent used on the mechanical and permeability properties of the films. Acetone-methanol (8:2), chloroform-methanol (8:2), dichloromethane-methanol (8:2), and ethyl acetate-methanol (8:2) were used as solvents in the preparation of cellulose acetate and ethyl cellulose films. Cellulose acetate and ethyl cellulose films showed good film forming properties. The method of moulding was found to be giving thin uniform films. The films prepared with polymer alone were found to be brittle. To prevent embrittlement a plasticizer, dibutyl phthalate was tried at various concentrations ranging from10-50% w/w of the polymer. Preliminary experiments indicated that lower concentrations of dibutyl phthalate were found to give rigid and brittle films where as higher concentrations gave soft films. Dibutyl phthalate at a concentration of 40% w/w of the polymer was found to give good flexible films. Hence, dibutyl phthalate was included as a plasticizer in the preparation of cellulose acetate and ethyl cellulose films at a concentration of 40% w/w of the polymer (or 2% w/v of the polymer solution.).
The cellulose acetate films made from its solution in dichloromethane-methanol (8:2) and ethyl cellulose films made from its solution in acetone-methanol (8:2), incorporating dibutyl phthalate were found to be brittle. In those cases propylene glycol at 40% w/w of the polymer was found to give good flexible films. All the films prepared were evaluated for uniformity of thickness, folding endurance, water vapour transmission and drug diffusion and permeability characteristics. Thickness measurements of films prepared in various solvents are given in Table 1. Low standard deviation values in the film thickness measurements ensured uniformity of thickness in each film. The method of moulding was found to be given reproducible results with regard to film thickness. The folding endurance was measured manually and folding endurance was found to be high in ethyl cellulose films compared with cellulose acetate films. Folding endurance is decreased in the order of films in various solvents is as follows in both cases. Ethyl acetate-methanol (8:2)>acetone-methanol (8:2)> dichloromethane-methanol (8:2)>chloroform-methanol (8:2).
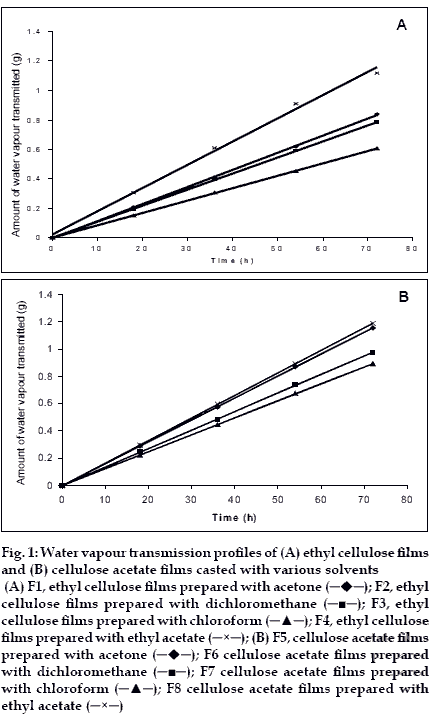
Water vapour transmission studies indicated that all the films prepared (both cellulose acetate and ethyl cellulose) were permeable to water vapour. Water vapour transmission through the films followed zero order kinetics. The results are given in Table 1 and shown in figs. 1A and B.
The water vapour transmission (Q) was more in the case of cellulose acetate films when compared to ethyl cellulose. Water vapour transmission values indicating that the cellulose acetate films were more permeable to water vapour. The rate of water vapour transmission was decreased in the order of films in various solvents is as follows in both cases. Ethylacetate-methanol (8:2)>acetone-methanol (8.2)> dichloromethanemethanol (8:2)>chloroform-methanol (8:2).
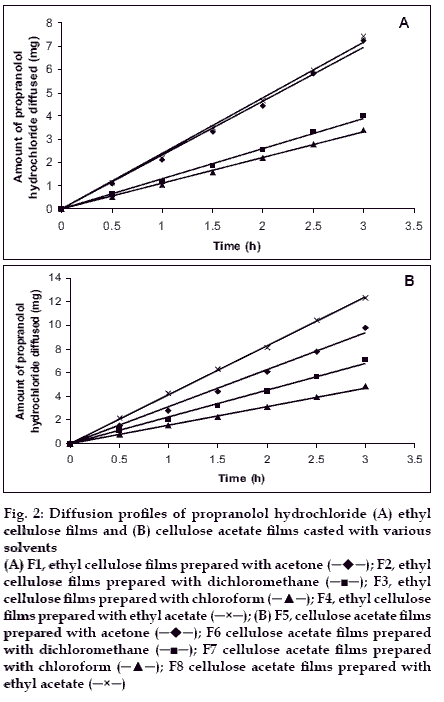
Drug diffusion through various films was studied with propranolol hydrochloride as a model drug by using Franz diffusion cell. All the films were found to be permeable to propranolol hydrochloride and diffusion profiles are shown in figs. 2A and 2B. Diffusion flux was calculated and same were shown in figs. 3A and 3B. Permeability coefficient values (Pm) of the films towards the propranolol hydrochloride was calculated from the drug diffusion data and the results were given in Table 1. The rate of permeability coefficient was decreased in the order of films in various solvents is as follows in both cases. Ethylacetate-methanol (8:2)>acetone-methanol (8:2)> dichloromethanemethanol (8:2)>chloroform-methanol (8:2).
| Formulation | Thickness (µm) | Folding endurance | Water vapour transmission (q×104 g/ cm2 24 h) | Permeability coefficient (pm×103 mg/cm. h) |
|---|---|---|---|---|
| F1(EC;A+M) | 45.95±0.15 | 198 | 4.01 | 2.28 |
| F2EC;DCM+M) | 45.30±0.17 | 223 | 3.779 | 1.27 |
| F3(EC;C+M) | 44.28±0.26 | 286 | 2.848 | 1.03 |
| F4(EC;EA+M) | 46.70±0.26 | 164 | 4.165 | 2.37 |
| F5(CA;A+M) | 49.88±0.65 | 129 | 6.115 | 3.36 |
| F6(CA;DCM+M) | 54.25±0.37 | 196 | 5.615 | 2.64 |
| F7(CA;C+M) | 56.75±0.15 | 234 | 5.38 | 1.86 |
| F8(CA;EA+M) | 51.40±0.28 | 132 | 6.498 | 4.27 |
Table 1: Properties Of Transdermal Films.
Figure 1:Water vapour transmission profiles of (A) ethyl cellulose films and (B) cellulose acetate films casted with various solvents (A) F1, ethyl cellulose films prepared with acetone ( ); F2, ethyl cellulose films prepared with dichloromethane (
); F2, ethyl cellulose films prepared with dichloromethane ( ); F3, ethyl cellulose films prepared with chloroform (
); F3, ethyl cellulose films prepared with chloroform ( ); F4, ethyl cellulose films prepared with ethyl acetate (
); F4, ethyl cellulose films prepared with ethyl acetate ( ); (B) F5, cellulose acetate films prepared with acetone (
); (B) F5, cellulose acetate films prepared with acetone ( ); F6 cellulose acetate films prepared with dichloromethane (
); F6 cellulose acetate films prepared with dichloromethane ( ); F7 cellulose acetate films prepared with chloroform (
); F7 cellulose acetate films prepared with chloroform ( ); F8 cellulose acetate films prepared with ethyl acetate(
); F8 cellulose acetate films prepared with ethyl acetate( ).
).
Figure 2:Diffusion profiles of propranolol hydrochloride (A) ethyl cellulose films and (B) cellulose acetate films casted with various solvents (A) F1, ethyl cellulose films prepared with acetone ( ); F2, ethyl cellulose films prepared with dichloromethane (
); F2, ethyl cellulose films prepared with dichloromethane ( ); F3, ethyl cellulose films prepared with chloroform (
); F3, ethyl cellulose films prepared with chloroform ( ); F4, ethyl cellulose films prepared with ethyl acetate (
); F4, ethyl cellulose films prepared with ethyl acetate ( ); (B) F5, cellulose acetate films prepared with acetone (
); (B) F5, cellulose acetate films prepared with acetone ( ); F6 cellulose acetate films prepared with dichloromethane (
); F6 cellulose acetate films prepared with dichloromethane ( ); F7 cellulose acetate films prepared with chloroform (
); F7 cellulose acetate films prepared with chloroform ( ); F8 cellulose acetate films prepared with ethyl acetate (
); F8 cellulose acetate films prepared with ethyl acetate ( ).
).
| Formulation | Correlation coeffcient(r) values | Zero order rate constant (k) value (mg/h) | Diffusion exponent value (n) | Area of patch for desired release rate (cm2) | |
|---|---|---|---|---|---|
| Zero order | Peppas model | ||||
| F1 (EC;A+M) | 0.9977 | 0.9994 | 2.4476 | 1.0644 | 2.14 |
| F2 (EC;DCM+M) | 0.9982 | 0.9983 | 1.3771 | 1.0524 | 3.8 |
| F3 (EC;C+M) | 0.9985 | 0.9986 | 1.1570 | 1.0416 | 4.53 |
| F4 (EC;EA+M) | 0.9800 | 0.9987 | 2.5218 | 1.3116 | 2.10 |
| F5 (CA;A+M) | 0.9978 | 0.9996 | 3.3127 | 1.0772 | 1.58 |
| F6 (CA;DCM+M) | 0.9978 | 0.9990 | 2.3945 | 1.0574 | 2.19 |
| F7 (CA;C+M) | 0.9989 | 0.9996 | 1.6164 | 1.0360 | 3.24 |
| F8 (CA;EA+M) | 0.9761 | 0.9971 | 4.0809 | 1.3147 | 1.29 |
Table 2: Diffusion Characteristics Of Propranolol Hydrochloride From Ethyl Cellulose And Cellulose Acetate Films Prepared With Various Organic Solvents.
Figure 3:Diffusion fl ux of propranolol hydrochloride through (A) ethyl cellulose films and (B) cellulose acetate films. Films prepared with various solvents (A) F1, ethyl cellulose films prepared with acetone ( ); F2, ethyl cellulose films prepared with dichloromethane (
); F2, ethyl cellulose films prepared with dichloromethane ( ); F3, ethyl cellulose films prepared with chloroform (
); F3, ethyl cellulose films prepared with chloroform ( ); F4, ethyl cellulose films prepared with ethyl acetate (
); F4, ethyl cellulose films prepared with ethyl acetate ( ); (B) F5, cellulose acetate films prepared with acetone (
); (B) F5, cellulose acetate films prepared with acetone ( ); F6 cellulose acetate films prepared with dichloromethane (
); F6 cellulose acetate films prepared with dichloromethane ( ); F7 cellulose acetate films prepared with chloroform (
); F7 cellulose acetate films prepared with chloroform ( ); F8 cellulose acetate films prepared with ethyl acetate (
); F8 cellulose acetate films prepared with ethyl acetate ( )
)
The correlation coefficient values (r) were reported in Table 2. These values revealed that the diffusion profiles follow zero order kinetics (fig. 2) and the mechanism of drug release was governed by Peppas model. The diffusion exponent of release profiles (slope) has a value of 1.0360-1.3147 (n>1), which indicates non anomalous transport diffusion14. The results obtained in the present study thus indicated that the polymers and solvents used in the preparation of films have been shown significant influence on the water vapour transmission, drug diffusion and permeability of the films. Area of patches required for desired release rate were calculated and are reported in Table 2. Area of patches ranging from 1.29-4.53 cm2 were found to yielding the desired release rate of propranolol hydrochloride. Cellulose acetate films employed with ethyl acetate:methanol in 8:2 ratio as casting solvent yielded low area (1.29 cm2) of patch with desired release rate.
References
- Lee SJ, Kim SW. Temperature and pH-response swelling behavior ofpoly (2-ethyl-2-oxazoline)/chitosan interpenetrating polymer network hydrogels. J Control Release 1987;82:3-6.
- Arwidson H, Johanson B. Application of intrinsic viscosity and interaction constant as a formulation tool for film coating. Int J Pharm 1991;76:91-7.
- Aziz AS Anderson W. The influence of casting solvent composition on structure and permeability of acrylic-methacrylic ester copolymer films. J Pharm Pharmcol 1976;28:801-5.
- Spitel J, Kinget R. Preparation and evaluation of free films: Influence of method of preparation and solvent composition upon the permeability. Pharma Act Helv 1977;52:47-50.
- Crawford RR, Esmerin OK. Effect of plasticizers on some physicalproperties of cellulose acetate phthalate films. J Pharm Sci1971;60:312-5.
- Spitael J, Kinget R. Preparation and evaluation of free films: Influence of plasticizers and filler upon the permeability. PharmaActaHelv 1977;52:106-8.
- Murad F. Goodman and Gilman’s Pharmacological basis of therapeutics. In; Gilman AG, Rall WT, Nies SA, Taylor P, editors. 9thed. New York:McGraw Hill Co., Inc.: 1996. p. 762.
- Khurana R, Ahuja A, Khar RK. Development and evaluation of mucoadhesive films of miconazole nitrate. Indian J Pharm Sci 2000;62:447-53.
- Kulkarni R, Doddayya H, Marihal S, Patil C, Habbu P. Comparative evaluation of polymeric films for transdermal applications. Eastern Pharmacist 2000;43:109-12.
- Paranjyothy KL, Thampi PP. Development of transdermal patches of verapamil hydrochloride using sodium carboxymethyl guar as a monolithic polymeric matrix and their invitro release studies. Indian J Pharm Sci 1997;52:49-54.
- Indian Pharmacopoeia, Vol. II, 4th ed. New Delhi: The Controller of Publications; 1996. p. 634.
- Salomon CJ, Bravo SA, Lamas MA. In vitro studies of diclofenac sodium controlled-release from biopolymeric hydrophilic matrices. J Pharm PharmaceutSci 2002;5:213-9.
- Robinson JR, Eriksen SP. Theoretical formulation of sustained-releasedosage forms. J Pharm Sci 1966;55:1254-8.
- Wise DL. Hand book of pharmaceutical controlled release technology, 1st ed. Cambridge: Marcel Dekker, Cambridge ScientificInc: 2005. p. 187-8.