- *Corresponding Author:
- Xiangbing Jiang
Department of Obstetrics and Gynecology, Wuhan First Hospital, Wuhan, Hubei Province 430022, China
E-mail: gongkfayrt538967@163.com
| Date of Received | 08 December 2022 |
| Date of Revision | 17 October 2023 |
| Date of Acceptance | 29 March 2024 |
| Indian J Pharm Sci 2024;86(2):525-530 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cyclophilin A regulates the biological behavior of placental trophoblast cells in severe preeclampsia by regulating oxygen inducible factor (hypoxia-inducible factor-1 alpha) signal pathway. JEG3 cell line was selected and randomly divided into control group (minimum essential medium 1 l containing 10 % fetal bovine serum) (group A), low dose group (cyclophilin A protein concentration was 1 pg/ml) (group B) and high dose group (cyclophilin A protein concentration was 3 pg/ml) (group C). The apoptosis of JEG3 cells was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling method, the expression of tumor necrosis factor-alpha, interleukin-6 and interleukin-1 beta messenger ribonucleic acid in JEG3 cells was detected by quantitative read-time polymerase chain reaction method, and the expression of cleaved caspase-3, caspase-3, cleaved poly (ADP-ribose) polymerase 1, poly (ADP-ribose) polymerase 1, p53 and hypoxia-inducible factor-1 alpha protein was detected by Western bolting. Quantitative polymerase chain reaction assay showed that the expression levels of tumor necrosis factor-alpha, interleukin-6 and interleukin-1 beta in group B and group C were raised than those in group A, and the expression levels of tumor necrosis factor-alpha, interleukin-6 and interleukin-1 beta increased with the increase of cyclophilin A concentration. Western blotting detection showed that the cleaved caspase-3 expression level of JEG3 cells in group B and C was raised than that in group A, and increased with the increase of concentration, while the expression of cleaved poly (ADP-ribose) polymerase 1 in group C was raised than that in group A. The levels of hypoxia-inducible factor-1 alpha in JEG3 cells were higher in both the group B and C compared to the group A, and they further increased with the rise in concentration. Cyclophilin A can promote inflammation by increasing the expression of tumor necrosis factor-alpha, interleukin-6 and interleukin-1 beta in trophoblasts, and accelerate the apoptosis of trophoblasts by activating caspase-3 pathway, which is an important pathogenic factor of preeclampsia. This mechanism may be related to the regulation of hypoxia-inducible factor-1 alpha signal pathway.
Keywords
Cyclophilin A, hypoxia-inducible factor-1 alpha, signaling pathway, preeclampsia, placental trophoblast, pregnancy
An elevated blood pressure and albuminuria are the symptoms of Preeclampsia (PE) after 20 w of pregnancy, along with headache, nausea, vomiting, epigastric discomfort, and more[1]. At present, the etiology of PE is not completely clear, which can cause adverse outcomes such as fetal growth restriction and fetal death, and is one of the important causes of disease and death in clinical pregnant women and perinatal infants[2]. At present, there is no effective treatment except for the termination of pregnancy. Therefore, to find the pathogenesis of PE and understand the mechanism of severe PE placental trophoblast injury is of great significance to find clinical drugs and reduce maternal and infant mortality. Excessive activation of inflammation and immunity is one of the important mechanisms in the pathogenesis of PE. It was found that the increased expression of inflammatory factors such as Tumor Necrosis Factor-Alpha (TNF-α), Interleukin (IL)-6 and IL-1 Beta (IL-1β) were all involved in the pathogenesis of PE[3]. Hypoxia Inducible Factor-1 Alpha (HIF- 1α) is an important transcription factor, which can negatively regulate the expression of Vascular Endothelial Growth Factor (VEGF) and inhibit angiogenesis. The expression of HIF-1α was significantly increased in placenta of patients with severe PE[4]. Cyclophilin A (CypA) is the most widely distributed and richly expressed inflammatory factor in the Cyp protein family, which plays critical role in the progression of cardiovascular diseases[5]. The pathogenesis of PE and cardiovascular disease have a lot in common, so it is speculated that CypA may be involved in the occurrence and development of severe PE. The purpose of this study was to analyze the effect of CypA on the biological behavior of severe PE placental trophoblast cells by regulating HIF-1α signal pathway.
Materials and Methods
General information:
JEG3 cell line was selected and provided by Shanghai Aolu Biotechnology Co., Ltd.
Methods:
Cell culture and cryopreservation: Cell culture and cryopreservation including the JEG3 cell line was thawed rapidly in water bath at 37°, and the cell suspension was added to the centrifuge tube of 10 % Fetal Bovine Serum (FBS), and Methylsulfonylmethane (MSM) medium containing 3 ml. Centrifuge 3 min at the rotational speed of 800 rpm. The supernatant was discarded, MEM medium 3 ml was added, transferred to the culture flask, cultured at 37° and 5 % Carbon dioxide (CO2), and the fresh medium was replaced for 24 h. When the cell growth density reaches 80 %-90 %, the culture bottle is moved to the super-clean work table and Phosphate Buffer Solution (PBS) is washed three times; a few drops of trypsin are added and placed in the incubator at 37° for about 3 min, and the MEM medium of 2 ml is added to the 15 ml centrifuge tube to stop digestion, and then centrifuged at the speed of 800 rpm for 3 min; the cell precipitation is cultured at 37° and 5 % CO2 by adding 4 ml MEM medium. When the cell growth density reaches 80 %-90 %, the trypsin digests the cells, obtains the cell precipitation, adds the MEM medium of 4 ml, uses the blood cell counting plate to count, and adjusts the cell density; the cell suspension is slowly added along the pore wall and cultured at 37° and 5 % CO2. When the cells are in good condition and the growth density reaches 90 %, the cells are digested by trypsin to obtain cell precipitation; add the newly configured 8 % cell cryopreservation solution 3 ml, blow and resuspend, then sub-pack, each tube 1 ml, seal, and mark the cell type and freezing time; put it in the freezer and freeze overnight at -80°; take out the cryopreservation tube after 24 h and immediately store it in a liquid nitrogen tank.
Cell grouping: When the cell density reached about 60 %, the MEM medium was replaced and cultured for 24 h at 37° and 5 % CO2. The intervention groups of exogenous recombinant human CypA protein were as follows; the cells were randomly divided into control group (MEM medium 1 ml containing 10 % FBS) (group A), low dose group (CypA protein concentration was 1 pg/ ml) (group B) and high dose group (CypA protein concentration was 3 pg/ml) (group C).
Observation index:
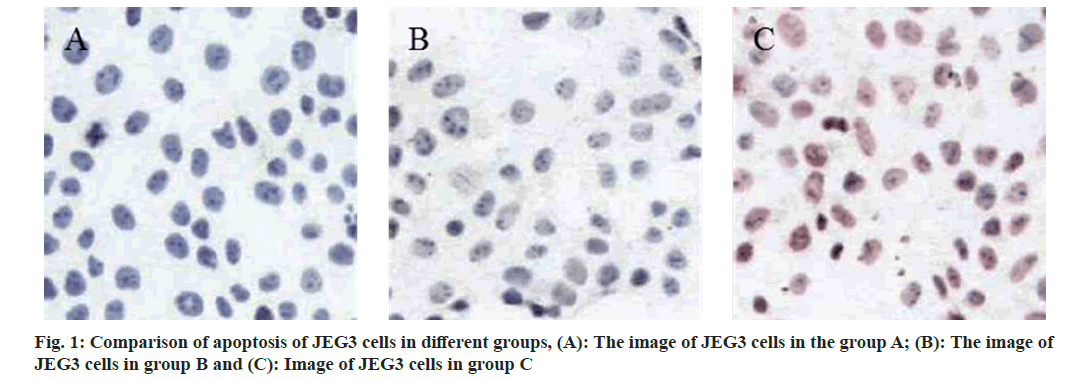
Detection of apoptosis of JEG3 cells by Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) method: JEG3 cells in good growth condition were added to MEM medium to prepare cell suspension and the concentration was adjusted to 5×104/ml. Open the 6-well plate, add a drop of FBS-free MEM medium to each hole, add MEM medium 2 ml; take 100 μl of fine cell suspension into each hole and culture at 37° and 5 % CO2 conditions; when the cell density reaches 60 %-70 %, add new MEM medium and add different concentrations of CypA protein intervention, each group is provided with 3 multiple holes and cultured at 37° and 5 % CO2conditions. Add 4 % paraformaldehyde, fix 30 min PBS washing for 5 min; add PBS washing containing 0.5 % TritonX-100; drip PBS, PBS washing containing 0.3 % Hydrogen peroxide (H2O2); add 50 μl/ tablet of biotin labeling solution, incubate 60 min PBS washing at 37° in a wet box; drip reaction termination solution 0.3 ml/tablet, PBS washing; drip Streptavidin-Horseradish Peroxidase (HRP) working solution 50 μl/tablet, PBS washing; drip 3,3′-Diaminobenzidine (DAB) chromogenic solution 0.5 ml/tablet, and PBS washing. Add 0.5 ml/tablets of hematoxylin dye solution, and wash them with PBS for 5 min, seal the tablets at 95% ethanol, 100 % ethanol and xylene, and observe and take pictures under the microscope.
Quantitative real-time PCR: Collect the cells to be tested, add the Trizol lysate 1 ml/tube, transfer into the new Eppendorf (EP) tube, static 5 min. Add chloroform 200 μl/tube, shake violently for 30 s, place 3 min statically, centrifuge 15 min at 4° at the speed of 12 000 rpm, carefully transfer the supernatant to a new EP tube, add precooled isopropanol 200 μl/tube, blow and mix well, place 10 min statically, centrifuge 10 min at 4° at the speed of 12 000 rpm, and precipitate Ribonucleic Acid (RNA). Pour out the supernatant, add precooled 75 % ethanol 1 ml, centrifuge 5 min at 4° at the speed of 8000 rpm, pour out the supernatant, add 75 % ethanol 1 ml, centrifuge 5 min at the speed of 7500 rpm at 4°, add anhydrous ethanol 1 ml, centrifuge 5 min at 4° at the speed of 12 000 rpm, and determine and record the concentration of RNA by ultraviolet spectrophotometer. RNA sample solution was stored at -80° to prevent degradation. Reverse Transcription-Polymerase Chain Reaction (RT-PCR). According to the coding genes of TNF-α, IL-6 and IL-1β and complementary Deoxyribonucleic Acid (cDNA) sequence, primers were designed by PerlPrimer software. The Cycle threshold (Ct) value was recorded with β-actin gene as internal reference. The Ct value of β-actin was used as the standard to confirm the amplification and melting curve. The relative amount of the target gene of the sample was calculated.
Western blotting:
The cells to be tested were collected, and the precooled PBS washing cells were precipitated twice, then the mixed solution of Phenylmethylsulfonyl Fluoride (PMSF) and Radio-Immunoprecipitation Assay (RIPA) was added to fully lyse the cells and hatch 20 min on ice. 10 min was centrifuged at 40° at the rotational speed of 13 000 rpm, and the supernatant was stored at -20°. The protein standard and Bicinchoninic Acid (BCA) working solution were configured. The extracted sample protein 2 μl was added to the PBS volume of 18 μl. In the 96-well plate, add the standard sample and the sample to be tested in turn, then add the BCA working solution 200 μl/well, shake the table to mix 30 min, and store at room temperature for 2 h. The Optical Density (OD) value at A562 was detected by enzyme labeling instrument. The standard curve was drawn by the concentration and OD value of the standard sample, and the protein concentration of the sample was calculated and equipped with 12 % release glue and 5 % upper glue. According to the sample protein concentration, the sample volume was determined, loading buffer 4 μl, filled to 20 μl with RIPA, and denatured by metal bath 10 min at 99°. Electrophoresis, electroporation, sealing, incubation of the first antibody, incubation of the second antibody, Electrochemiluminescence (ECL) development, photographic preservation after development, and gray analysis of the developed bands by lab works image analysis system.
Statistical method:
The data of this study are analyzed by Statistical Package for the Social Sciences (SPSS) 20.0 software package, all the measurement data are compared by (x̄ ±s). By using the t-test, counting data is expressed in percentages, and by using the test of proportions, we are able to express the comparison between two groups. The statistical results were statistically significant (p<0.05). A means that compared with the normal group, ap<0.05 and compared with the pneumonia group, bp<0.05.
Results and Discussion
TUNEL assay showed that the number of apoptosis in group B and C was raised than that in group A, and the number of apoptosis increased with the increase of CypA concentration (fig. 1). Q-PCR assay showed that the expression levels of TNF-α, IL-6 and IL-1β messenger RNA (mRNA) in group B and group C were raised than those in group A, and these were increased with the increase of CypA concentration (Table 1).
| Group | TNF-α | IL-6 | IL-1β |
|---|---|---|---|
| A | 1.02±0.04 | 1.03±0.03 | 1.01±0.02 |
| B | 1.85±0.12a | 1.95±0.11a | 2.41±0.21a |
| C | 2.48±0.21ab | 2.34±0.18ab | 3.76±0.30ab |
Note: Compared with the group B, ap<0.05 and compared with the pneumonia group C, bp<0.05
Table 1: Comparison of Expression Levels of Tnf-Α, Il-6 and Il-1β Mrna in Different Groups (X̄±S)
The Western blotting detection indicated that the cleaved caspase-3 expression level in JEG3 cells in group B and C was higher than in group A, and that the level increased with increasing concentration, while the expression of cleaved Poly (ADP-ribose) Polymerase-1 (PARP-1) in group C was raised than that in group A (fig. 2).
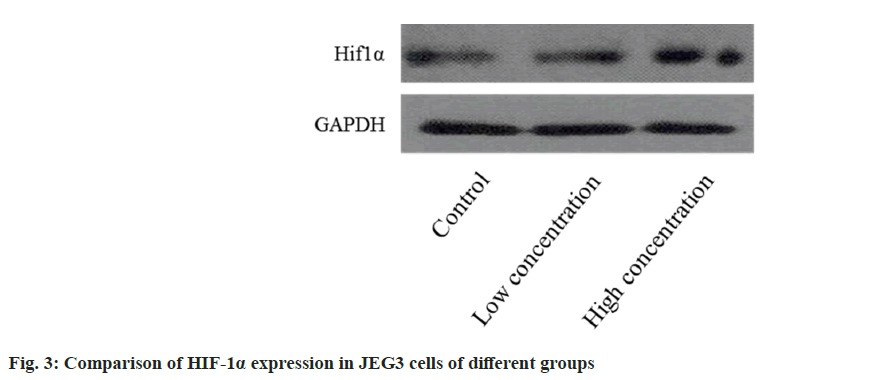
The Western blotting analysis revealed that the HIF-1α expression level in JEG3 cells was higher in both the group B and C compared to the group A. Furthermore, the expression level increased proportionally with the concentration (fig. 3).
The normal function of trophoblasts is the first condition for the development of normal pregnancy[6]. Normally, trophoblasts participate in the process of spiral artery remodeling and play critical role in maintaining normal placental development and good blood supply[7]. When the invasive ability of trophoblasts is insufficient or apoptosis is abnormal, the reconstruction of uterine spiral artery is blocked, and the placenta is too shallow, thus activating oxidative stress and inflammation, destroying maternal-fetal immune tolerance, and finally leading to recurrent abortion, PE, limitation of fetal activity, placental abruption and even fetal death[8,9]. CypA exists widely in cells, which cannot only regulate the folding, transport and function of proteins, but also promote inflammation and proliferation, specifically participate in cell apoptosis, migration, matrix degradation and reactive oxygen species production, and may be involved in the progression of PE[10].
During normal pregnancy, the fetus, as a homologous semi-transplant, can stimulate the mother to produce a large number of inflammatory factors. The balance of pro-inflammatory and antiinflammatory cytokines plays critical role in the progression of placenta. In PE, the inflammatory reaction between maternal-fetal interface and systemic endothelial system was over-activated. Studies have shown that disorders of immune response and imbalance of anti-inflammatory/proinflammatory cytokines can lead to low placental blood perfusion and endothelial system injury, which lead to further deterioration of PE[11]. As common inflammatory factors in human body, TNF-α and IL-6 play a certain regulatory role in the development of PE. TNF-α cannot only damage the endothelial system of placenta, kidney and blood vessels, but also induce apoptosis by activating caspase-3 pathway[12]. IL-6 can regulate blood pressure and immunity by activating renin-angiotensin enzyme. IL-1β can promote the expression of TNF-α and IL-6, and regulate inflammatory response and oxidative stress to some extent. In this experiment, TUNEL assay showed that the number of apoptosis in group B and C was significantly higher than that in group A, and the number of apoptosis increased with the increase of CypA concentration. The expression levels of TNF-α, IL-6 and IL-1β in group B and C were raised than those in group A, and these were increased with the increase of CypA concentration. It is suggested that CypA may play a proinflammatory role by increasing the expression of TNF-α, IL-6 and IL-1β in trophoblasts, thus aggravating the development of PE.
Caspase-3 is the executor of apoptosis. When stimulated by the outside world, inactive caspase-3 is activated into cleaved caspase-3, which initiates the apoptosis process. Cleaved-PARP1 is the cutting substrate of caspase-3, participates in DNA repair and is a marker of apoptosis. P53 protein is also involved in the regulation of cell apoptosis[13]. In this experiment, Western blotting detection showed that JEG3 cells in the group B and group C expressed more cleaved caspase-3 than those in the group A, and the level increased with concentration; the expression of cleaved PARP1 was higher in the group C than in the group A. It is suggested that CypA can accelerate trophoblast apoptosis by activating caspase-3 pathway and is an important pathogenic factor of PE.
HIF-1α is an important transcription factor in the activity of the body, and its expression level is regulated by oxygen concentration. The expression of VEGF is negatively regulated under hypoxia stimulation, thus inhibiting angiogenesis. In the placenta of patients with PE, the expression level of HIF-lα is increased, which can further promote the expression of genes encoding soluble Endoglin (sEng), Endothelin-1 (ET-1) and soluble Fms-like tyrosine kinase-1 (sFlt-1). At present, it is believed that the placenta of PE is in a state of low blood perfusion and hypoxia, the related mechanism is not clear, and it has been found that the increase of HIF-1α level is independent of hypoxia stimulation[14]. During this experiment, the level of HIF-1α expression in JEG3 cells was found to be higher in both the group B and the C compared to the group A. Furthermore, the expression level increased as the concentration increased. It is suggested that the mechanism of CypA inducing the occurrence and development of PE may be related to its regulation of HIF-1α signal pathway, which is similar to the results of Rath et al.[15].
To sum up, CypA can promote inflammation by increasing the expression of TNF-α, IL-6 and IL-1β in trophoblasts, and accelerate trophoblast apoptosis by activating caspase-3 pathway, which is an important pathogenic factor of PE. This mechanism may be related to its regulation of HIF-1α signal pathway.
Author’s contributions:
Han Wang and Juan Du have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham Jr MW, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci 2016;130(6):409-19.
[Crossref] [Google Scholar] [PubMed]
- Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia screening: Evidence report and systematic review for the US preventive services task force. JAMA 2017;317(16):1668-83.
[Crossref] [Google Scholar] [PubMed]
- Han R, Zhang F, Wan C, Liu L, Zhong Q, Ding W. Effect of perfluorooctane sulphonate-induced Kupffer cell activation on hepatocyte proliferation through the NF-κB/TNF-α/IL-6-dependent pathway. Chemosphere 2018;200:283-94.
[Crossref] [Google Scholar] [PubMed]
- Kutscher C, Lampert FM, Kunze M, Markfeld-Erol F, Stark GB, Finkenzeller G. Overexpression of hypoxia-inducible factor-1 alpha improves vasculogenesis-related functions of endothelial progenitor cells. Microvasc Res 2016;105:85-92.
[Crossref] [Google Scholar] [PubMed]
- Nath PR, Dong G, Braiman A, Isakov N. In vivo regulation of human CrkII by cyclophilin A and FK506-binding protein. Biochem Biophys Res Commun 2016;470(2):411-6.
[Crossref] [Google Scholar] [PubMed]
- Gallo DM, Wright D, Casanova C, Campanero M, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 19–24 weeks’ gestation. Am J Obstet Gynecol 2016;214(5):72-9.
[Crossref] [Google Scholar] [PubMed]
- Du L, He F, Kuang L, Tang W, Li Y, Chen D. eNOS/iNOS and endoplasmic reticulum stress-induced apoptosis in the placentas of patients with preeclampsia. J Hum Hypertens 2017;31(1):49-55.
[Crossref] [Google Scholar] [PubMed]
- You WB, Wolf M, Bailey SC, Pandit AU, Waite KR, Sobel RM, et al. Factors associated with patient understanding of preeclampsia. Hypertens Pregnancy 2012;31(3):341-9.
[Crossref] [Google Scholar] [PubMed]
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am J Obstet Gynecol 2017;216(2):110-20.
[Crossref] [Google Scholar] [PubMed]
- Piechota-Polanczyk A, Włodarczyk M, Sobolewska-Włodarczyk A, Jonakowski M, Pilarczyk A, Stec-Michalska K, et al. Serum cyclophilin a correlates with increased tissue MMP-9 in patients with ulcerative colitis, but not with Crohn’s disease. Dig Dis Sci 2017;62(6):1511-7.
[Crossref] [Google Scholar] [PubMed]
- Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine 2016;83:189-92.
[Crossref] [Google Scholar] [PubMed]
- Li W, Wang X, Niu X, Zhang H, He Z, Wang Y, et al. Protective effects of nobiletin against endotoxic shock in mice through inhibiting TNF-α, IL-6, and HMGB1 and regulating NF-κB pathway. Inflammation 2016;39(2):786-97.
[Crossref] [Google Scholar] [PubMed]
- Qu Z, Jiang C, Wu J, Ding Y. Lenalidomide induces apoptosis and inhibits angiogenesis via caspase-3 and VEGF in hepatocellular carcinoma cells. Mol Med Rep 2016;14(5):4781-6.
[Crossref] [Google Scholar] [PubMed]
- Gomez-Roman N, Sahasrabudhe NM, McGregor F, Chalmers AJ, Cassidy J, Plumb J. Hypoxia-inducible factor 1 alpha is required for the tumourigenic and aggressive phenotype associated with Rab25 expression in ovarian cancer. Oncotarget 2016;7(16):22650.
[Crossref] [Google Scholar] [PubMed]
- Rath G, Aggarwal R, Jawanjal P, Tripathi R, Batra A. HIF-1 alpha and placental growth factor in pregnancies complicated with preeclampsia: A qualitative and quantitative analysis. J Clin Lab Anal 2016;30(1):75-83.
[Google Scholar] [PubMed]