- *Corresponding Author:
- M. A. Saleem
Luqman College of Pharmacy, Behind P and T Quarters, Old Jewargi Road, Gulbarga - 585 102, India
E-mail: ssaleempharm@rediffmail.com
| Date of Submission | 30 November 2009 |
| Date of Revision | 23 September 2010 |
| Date of Acceptance | 12 November 2010 |
| Indian J Pharm Sci 2010, 72 (6): 710-718 |
Abstract
The ability of β-cyclodextrin, hydroxypropyl-β-cyclodextrin, polyvinyl pyrrolidone and urea to influence the percutaneous absorption of meloxicam through isolated rat skin was evaluated. Carrier complex were prepared by kneading method in 1:1 and 1:2 in molar ratios for β-cyclodextrin and hydroxypropyl-β-cyclodextrin and in 1:1, 1:3 and 1:5 in weight ratios for polyvinyl pyrrolidone and urea. The complexes were characterized by IR, DSC and evaluated for solubility, dissolution and skin permeability. The solubility, dissolution and permeability of meloxicam were enhanced by using the carriers. The influence of cyclodextrins, polyvinyl pyrrolidone and urea on in vitro permeation of meloxicam through rat skin was investigated by incorporation of prepared carrier complex in 1% carbopol gel. The prepared gel was evaluated for drug content, pH and viscosity and in vitro permeation. All the percutaneous parameters like flux (Jss), amount permeated (Q 6 ), diffusivity (D), permeability coefficient (K p ), partition coefficient (K) and release rate constant (k) were calculated statistically. In vitro permeation study showed the trend that the penetration flux and enhancement factor increases with increasing concentration of β-cyclodextrin and hydroxypropyl-β-cyclodextrin and then decrease dramatically in case of hydroxypropyl-β-cyclodextrin gel formulation with the increase to 1:2 ratio. Similar changes in pattern of permeation were also observed with polyvinyl pyrrolidone and urea carrier complex. These findings concluded that the carriers cyclodextrins, polyvinyl pyrrolidone and urea could be used as transdermal permeation enhancer in topical preparation of meloxicam.
Keywords
β-cyclodextrin, hydroxypropyl-β-cyclodextrin, in vitro release, meloxicam, polyvinyl pyrrolidone, permeation, urea
Transdermal drug delivery system provides the objective of systemic medication through topical application to the intact skin surface. The skin is generally regarded in scientific literature as an impermeable barrier. Several drugs have been successfully delivered for local and systemic action. However the transdermal drug transport is greatly limited by stratum corneum permeation characteristics, so attempts at improving topical absorption have been reported. In recent years, numerous studies have been conducted in the area of penetration enhancement [1]. The various penetration enhancers such as phospholipids, ethanol, alcohols, n-octanol, cyclic monoterpenes, nonionic surfactants, propylene glycol, isopropyl myristate, dimethyl sulphoxides, dimethyl formamide have been used in many studies to increase the percutaneous absorption of drugs [2]. The rate and extent of drug release from the base describe the effectiveness of topical preparations [3].
Cyclodextrins is a cyclic polysaccharide form complexes with many hydrophobic drugs there by increasing the solubility and have been extensively reported in topical formulations as transdermal absorption promoter [4]. The different chemically modified cyclodextrins like hydroxylpropyl β-cyclodextrin and dimethyl β-cyclodextrin used as penetration enhancers which act by solubilizing lipophilic drugs and constantly supplying the dissolved drug molecules to the skin surface where they partition into the skin barrier [5]. The cyclodextrins also alleviate the drug induced primary irritation of skin by complexation [6]. Urea is a physiological component of NMF (natural moisturizing factor) of the skin, has mild keratolytic effect and hence is used in the topical drug formulations. Urea and their derivatives were studied as percuteneous permeation enhancers [7]. Pyrrolidones and their derivatives have been investigated as potential penetration enhancers [2].
Meloxicam is a potent nonsteroidal antiinflammatory drug (NSAIDs) of the enolic acid class of oxicam derivative act by inhibition of cyclooxygenase-2 (COX-2) and prostaglandin synthesis. It is used in the treatment of rheumatoid arthritis, osteoarthritis and other joint disorder. The melting point of meloxicam is 254º, it is practically insoluble in water, slightly soluble in acetone, soluble in dimethyl formamide, very slightly soluble in ethanol (96%) and in methanol [8]. Naidu et al [9] have prepared meloxicamcyclodextrin binary system both in solution and solid state to improve the dissolution properties of meloxicam via complexation with, b, g cyclodextrin. El-Badry et al. reported enhanced dissolution and permeation rate of meloxicam by formulation of its freeze-dried solid dispersion in polyvinyl pyrrolidone K-30 [10]. Janatharaprapap et al [11] have prepared meloxicam gel by using hydroxypropyl cellulose and studied the effect of different combination of co-solvent on meloxicam permeability. In the present work, solubility and permeability of meloxicam was improved through formation of inclusion complex with different carriers like cyclodextrins, polyvinyl pyrrolidone, urea and the effect of these carriers on in vitro permeation of meloxicam through rat skin was studied after incorporation in carbopol 940 as gellent.
Material and Methods
Meloxicam was kind gift sample from Alcon Bioscience Pvt. Ltd. (Vapi, Gujarat, India). β-cyclodextrin and hydroxypropyl-β-cyclodextrin was provided by Indian Herbs and Chemicals, Indore and Gangwal Chemicals Pvt. Ltd. (Mumbai, India). Polyvinyl pyrrolidone was purchased from Loba Chemie Ltd. (Mumbai, India) and urea from SD Fine Chem Ltd. (Mumbai). All other chemicals used were of analytical grade.
Development, characterization and evaluation of carrier complexes
The binary systems of meloxicam and β-cyclodextrin, hydroxypropyl-β-cyclodextrin were prepared in 1:1 and 1:2 molar ratios in water: Methanol (1:1 v/v) solution by kneading method. Solid dispersion of meloxicam were prepared with polyvinyl pyrrolidone in weight ratios of 1:1, 1:3 and 1:5 using kneading method with a small volume of methylene chloride. The solid dispersions of meloxicam with urea were also prepared in weight ratios of 1:1, 1:3 and 1:5 using mixture of ethanol/chloroform as solvent. The prepared slurry obtained in all above methods were dried, crushed and sieved.
The infrared (IR) spectra and differential scanning calorimetery (DSC) thermogram were recorded for selected carrier complexes, such as B2, H2, P3 and U3. The IR spectra was recorded for selected carrier complexes by FT-IR (Perkin Elmer Instrument USA 16PC) using KBR disc method. For DSC studies each selected sample weighing in the range of 3 to 5 mg were scanned at rate of 10º/min on Shimadzu, DSC TA 60 WS Thermal Analyzer between 30º to 300º.
Solubility study was performed by adding 3 g of drug complex in 3 ml of phosphate buffer solution pH 7.4 and distilled water to make saturated solution and agitated for 72 h. The suspension was filtered and analyzed spectrophotometrically (Shimadzu 1700 UV/ Visible spectrophotometer) at 362 nm.
The dissolution study was conducted using dissolution apparatus USP XXIII (Electro lab). The dissolution medium was 900 ml phosphate buffer pH 7.4 maintained at 37±0.5º temperature and 50 rpm stirring rate. A weighed amount of the sample (equivalent to 10 mg meloxicam) was tied by using muslin cloth. The dissolution study was carried out for 120 min. At appropriate interval 5 ml sample was withdrawn, filtered and the concentration of meloxicam was determined spectrophotometrically at 362 nm.
The permeation study was conducted by using rat abdominal skin, obtained from Wistar albino male rat weighing 140-160 g after approval from the institutional animal ethics committee (No. 346/ CPCSEA). Skin (0.025 cm) was stretched over one end of an open-ended glass tube. The tube was immersed in 100 ml beaker containing 100 ml phosphate buffer pH 7.4 and kept in vertical position so that the membrane touches (1-2 mm deep) the surface of the buffer solution. The surface area available for the diffusion was 1.76 cm2 and maintained at 37º using hot plate magnetic stirrer maintained at 50 rpm. A 3 ml aliquot of saturated phosphate buffer pH 7.4 solutions of pure drug (3 g) and drug-carrier complex (equivalent to 3 g of meloxicam) was inserted into the tube. After 6 h, 5 ml sample was removed from receptor compartment and analyzed spectrophotometrically at 362 nm.
Formulations of meloxicam-carrier complex gel
The prepared drug-carrier complexes were weighed accurately and soaked overnight with 1% carbopol 940 in required amount of water and then neutralize with sufficient quantity of triethalonamine and stirred to prepare the gel. The composition of meloxicam gel used in this study is shown in Table 1.
| Ingredient (% w/w) | Formulation code | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MG | B1G | B2G | H1G | H2G | P1G | P3G | P5G | U1G | U3G | U5G | |
| Meloxicam | 0.30 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| *carrier-complex | -- | 1.26 | 2.23 | 1.77 | 2.66 | 0.60 | 1.20 | 1.80 | 0.60 | 1.20 | 1.80 |
| Carbopol | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Triethalonamine(q.s) | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s |
| Water (q.s) | 98.57 | 97.61 | 96.64 | 97.11 | 96.22 | 98.27 | 97.67 | 97.87 | 96.22 | 98.27 | 97.07 |
Table 1: Formulation Of Meloxicam Gel With Different Meloxicam- Carrier Complexes
Evaluation of gels for pH, drug content and viscosity
One gram of the gel formulation was dispersed in 10 ml of distilled water and the pH was determined using digital pen pH meter. Drug content was determined by taking 3.33 g of gel formulation equivalent to 10 mg of meloxicam in 100 ml of volumetric flask containing phosphate buffer pH 7.4, allowed to sonicate and filtered, from which 1 ml of aliquot was pipette out and diluted to 10 ml with phosphate buffer pH 7.4. The content of meloxicam was determined by using Shimadzu UV/Visible spectrophotometer at 362 nm against blank. The tests were carried out in triplicate. The viscosity was determined by taking one gram of gel with the help of Brookfiled LVDV-III ultra programmable rheometer using spindle No. CP- 52 with an optimum speed of 2 rpm.
In vitro skin permeation studies
The in vitro permeation study was conducted using rat abdominal skin (0.025 cm thick) obtained from Wistar albino male rat weighing 140-160 g. The prepared gel of about 3.33 g equivalent to 10 mg meloxicam was spread uniformly on the outer surface of skin and was fixed to one end of an openended glass tube, such that preparation occupies inner circumference of the tube. The glass tube was then immersed in diffusion medium of 100 ml phosphate buffer pH 7.4 so that it just touches the surface of diffusion medium, maintained at 37±2º and at 50±5 rpm. A quantity of 5 ml sample was withdrawn from receptor fluid at the time interval of 1, 2, 3, 4, 5 and 6 h and replace at each time with phosphate buffer pH 7.4. The release of drug was estimated by using Shimadzu UV/Visible spectrophotometer at 362 nm.
Skin retention study
Partitioning of meloxicam gel was carried out by mounting 3.33 g of gel formulation equivalent to 10 mg of meloxicam on the rat skin and allowed to equilibrate for 6 h and then gel was scrapped off and analyzed for drug content at 362 nm (in phosphate buffer pH 7.4) spectrophotometrically. Amount retained in skin was calculated [11].
Data analysis
Data obtained by UV spectrophotometry analysis were corrected for sampling effects. Cumulative quantity of meloxicam collected in the receiver (mg/cm2) was plotted as function of time. The flux value (Jss, mg/ cm2/h) for each experiment was obtained from the slope (steady state portion) of the linear portion of the data fitted by regression analysis. Lag time tL, was determined from the X-intercept of the regression line. The apparent permeability coefficient (D, cm/h) was obtained by dividing Jss by the donor concentration according to Fick’s first law of diffusion. For release data analysis cumulative permeation (mg/cm2) was plotted as a function of square root of time, where linearity is indicative of first order release (Higuchi, 1962). Release rate was estimated as the slope of such plots (mg/cm2/t½). Penetration enhancing activities were expressed as enhancement factor i.e., the factor of the flux value with enhancer to that obtained without enhancer. Mean, standard deviation and linear regression analysis were calculated using Microsoft Excel 2000.
Results and Discussion
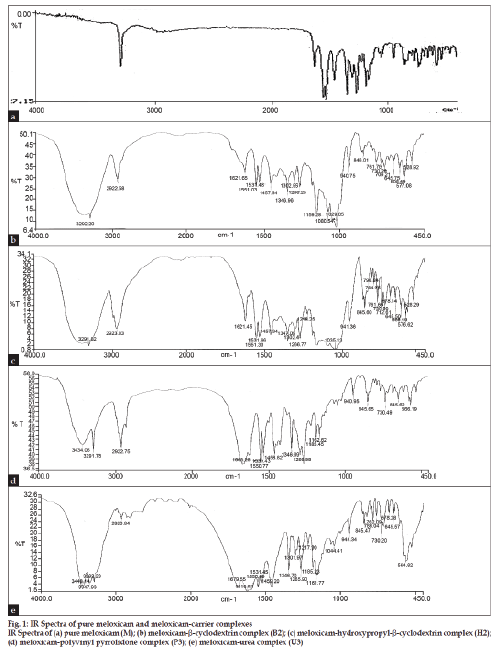
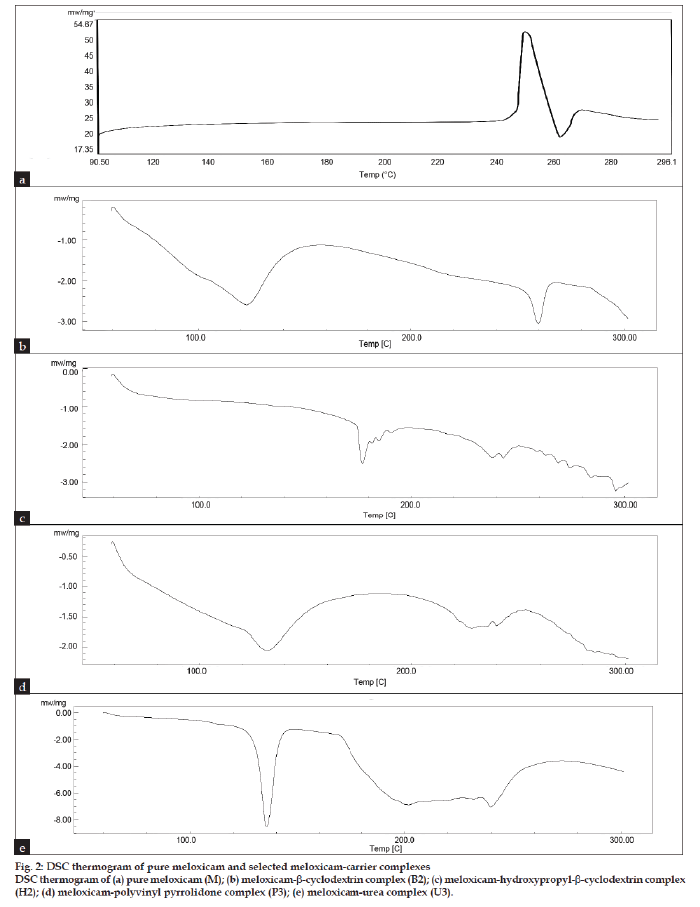
The IR spectrum for pure meloxicam and with carriers is shown in fig. 1, indicated that there was only physical entrapment of meloxicam with the carrier molecule. DSC thermogram is represented in fig. 2. The DSC thermograph for pure meloxicam shows a sharp endotherm near 262º which is indicative of its melting point temperature followed by an exotherm which signifies that after melting the intensity of the endotherm at 262º diminishes in intensity indicating potential complex formation with β-cyclodextrins (B2) and hydroxypropyl-β-cyclodextrin (H2). In the thermogram of P3 formulation (with polyvinyl pyrolidone), no peak point was seen because of the amorphous structure of the polymer showing broad peak, suggesting that some crystal of pure meloxicam exist. The thermogram of U3 formulation (with urea) showed sharp endothermic peak at 135º corresponding to its melting point followed by exothermic peak which signifies that after melting, meloxicam decomposes indicating some crystalline nature of pure meloxicam. Solubility study was carried out in both distilled water and phosphate buffer pH 7.4 and the results are presented in Table 2.
Figure 2: DSC thermogram of pure meloxicam and selected meloxicam-carrier complexes
DSC thermogram of (a) pure meloxicam (M); (b) meloxicam-b-cyclodextrin complex (B2); (c) meloxicam-hydroxypropyl-b-cyclodextrin complex
(H2); (d) meloxicam-polyvinyl pyrrolidone complex (P3); (e) meloxicam-urea complex (U3).
| Formulation code | Drug content (%) | Solubility (mg/ml) | |
|---|---|---|---|
| Phosphate buffer pH7.4 |
Water | ||
| Puredrug | -- | 0.398 ± 0.35 | 0.011 ± 0.23 |
| B1 | 92.17 ± 0.15 | 2.653 ± 0.65 | 0.487 ± 0.33 |
| B2 | 101.50 ± 0.25 | 2.790 ± 0.45 | 1.046 ± 0.34 |
| H1 | 97.70 ± 0.37 | 3.683 ± 0.55 | 1.845 ± 0.37 |
| H2 | 98.50 ± 0.45 | 2.608 ± 0.70 | 1.636 ± 0.40 |
| P1 | 91.70 ± 0.66 | 1.251 ± 0.65 | 0.640 ± 0.45 |
| P3 | 91.93 ± 0.45 | 1.738 ± 0.45 | 1.329 ± 0.37 |
| P5 | 93.59 ± 0.53 | 1.768 ± 0.35 | 1.515 ± 0.43 |
| U1 | 99.46 ± 0.62 | 0.996 ± 0.43 | 0.284 ± 0.53 |
| U3 | 98.70 ± 0.18 | 1.056 ± 0.25 | 0.296 ± 0.62 |
| U5 | 101.36 ± 0.55 | 1.190 ± 0.15 | 0.355 ± 0.71 |
Table 2: Evaluation of meloxicam carrier Complexes
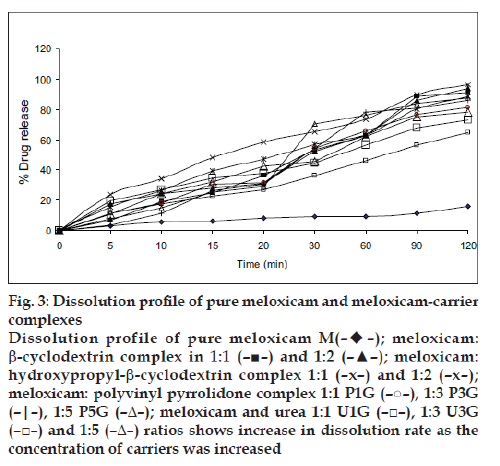
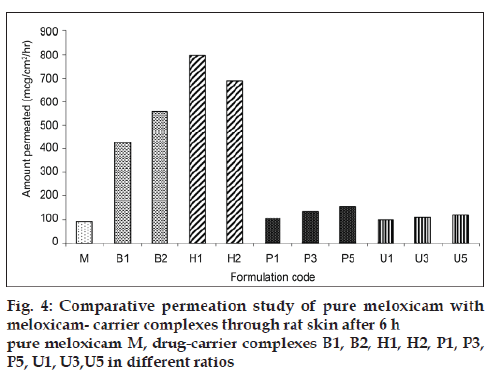
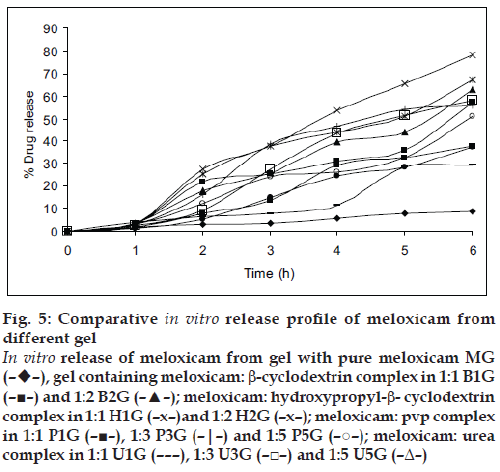
The solubility of meloxicam was increased by using different carriers such as β-cyclodextrin, hydroxypropyl-β-cyclodextrin, PVP and urea. As the concentration of carrier increased either in molar ratio for cyclodextrins or in weight ratios for polyvinyl pyrrolidone and urea, the solubility increased, except in the formulation H2 which shows decreased in solubility due to increased viscosity and saturation of solution. The highest solubility was observed with cyclodextrins as compared to polyvinyl pyrrolidone and urea. All meloxicam carrier complexes and pure drug were subjected to in vitro dissolution study in phosphate buffer pH 7.4 and the results are shown in fig. 3. It was found that the dissolution of carrier complex was more than 60% within 120 min as compared to pure meloxicam which shows only 15.85% of drug dissolved. The highest percent drug dissolution was observed with cyclodextrin complexes (more than 90%) as compared to polyvinyl pyrrolidone and urea. The in vitro drug release was increased in the following order of pure drug<urea<PVP<βCD<HPβCD. The in vitro dissolution study followed the similar pattern of solubility study. The amount of drug permeated (mg/cm2/h) through rat skin during the time course of 6 h is shown in fig. 4.
Figure 3: Dissolution profile of pure meloxicam and meloxicam-carrier
complexes
Dissolution profile of pure meloxicam M(– –);
meloxicam:
b-cyclodextrin complex in 1:1 (–■–) and 1:2 (–▲–); meloxicam:
hydroxypropyl-b-cyclodextrin complex 1:1 (–x–) and 1:2 (–x–);
meloxicam: polyvinyl pyrrolidone complex 1:1 P1G (–○–), 1:3 P3G
(–|–), 1:5 P5G (–Δ–); meloxicam and urea 1:1 U1G (–□–), 1:3 U3G
(–□–) and 1:5 (–Δ–) ratios shows increase in dissolution rate as the
concentration of carriers was increased
The results indicated that complexation increased the overall meloxicam diffusion by increasing the amount of diffusion species in donor phase by enhancing drug solubility. Highest permeation of meloxicam was observed with H1 formulation as compared to other formulations. The amount permeated was increased with respect to each carrier as the concentration of carrier was increased except in H2 formulation which showed a decrease in permeation. The findings of permeation study fully supported the solubility and dissolution study. Based on the permeability study perform for the carrier complexes it was concluded that carrier used in the study results in increased permeation of meloxicam and hence the meloxicam carrier complexes were incorporated in carbopol 940 to prepare gel formulation as illustrated in Table 2.
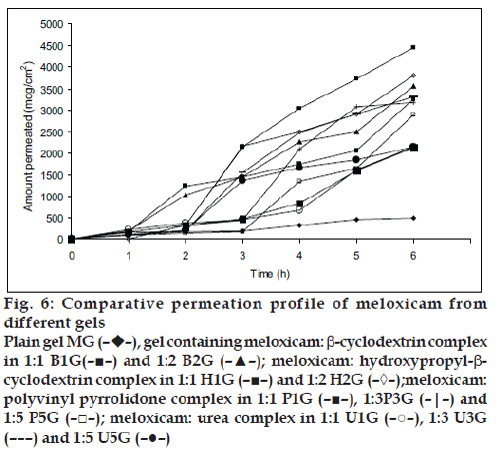
All the prepared gels were subjected to evaluation for pH, content uniformity, viscosity and the data is presented in Table 3. The pH of all formulation was more than 6.1±0.20, which lies in the normal skin pH of 4.5 to 7. The drug content was in the range of 91.4% to 99.24% indicating uniform dispersion of meloxicam in the gels. The formulated gels showed a decrease in the viscosity as the amount of carrier was increased in gel formulation containing β-cyclodextrin and decreased in gel with hydroxypropyl-β- cyclodextrin as concentration of carrier increased. Gel formulations containing urea and polyvinyl pyrolidone showed first decrease and then increased viscosity. The in vitro percent drug release of meloxicam through rat skin was highest for carrier complexed gels as compared to plain gel (without carrier) presented in fig. 5. This was due to more solubility and highest dissolution of drug-carrier complex as compared to pure meloxicam. The in vitro release was in the order of HPbCD gel>bCD gel>PVP gel>urea gel>plain gel. All the percutaneous parameters were calculated by plotting amount permeated versus time (fig. 6) and presented in Table 4. The B1G formulation showed flux of 510.81 mg/cm2/h with enhancement factor of 6.2 while B2G formulations have 583.85 mg/ cm2/ h with enhancement factor of 7.1. The gel H1G showed highest flux of 783.76 mg/cm2/h with enhancement factor of 9.5. The results suggested that permeation rate of meloxicam was increased significantly (P<0.05) for cyclodextrin gel formulation as compared to plain gel MG, which showed flux of only 82.04 mg/cm2/h. In the hydroxypropyl-β-cyclodextrin gel formulation, H2G showed decrease flux as the molar concentration of hydroxypropyl-β-cyclodextrin was increased.
| Formulation code | pH | Drug content (%) | Viscosity (cps) |
|---|---|---|---|
| MG | 6.3 ± 0.15 | 96.58 ± 0.54 | 7413.43 ± 102.13 |
| B1G | 6.9 ± 0.15 | 90.50 ± 0.35 | 5328.44 ± 113.14 |
| B2G | 6.9 ± 0.05 | 98.02 ± 0.70 | 5200.00 ± 161.24 |
| H1G | 6.1 ± 0.20 | 91.40 ± 0.20 | 3152.18 ± 113.12 |
| H2G | 6.3 ± 0.08 | 92.25 ± 0.40 | 4954.90 ± 104.13 |
| P1G | 6.2 ± 0.11 | 90.60 ± 0.38 | 6125.11 ± 126.11 |
| P3G | 6.1 ± 0.20 | 90.31 ± 0.07 | 5416.42 ± 115.12 |
| P5G | 6.2 ± 0.05 | 93.31 ± 0.15 | 7177.37 ± 112.3 |
| U1G | 6.3 ± 0.10 | 94.68 ± 0.23 | 7157.10 ± 107.13 |
| U3G | 6.4 ± 0.05 | 99.24 ± 0.34 | 5221.50 ± 115.10 |
| U5G | 6.4 ± 0.20 | 98.63 ± 0.15 | 7323.44 ± 116.14 |
Table 3: evaluation parameters of meloxicam Gel
Figure 5: Comparative in vitro release profile of meloxicam from
different gel
In vitro release of meloxicam from gel with pure meloxicam MG
(––),
gel containing meloxicam: b-cyclodextrin complex in 1:1 B1G
(–■–) and 1:2 B2G (–▲–); meloxicam: hydroxypropyl-b- cyclodextrin
complex in 1:1 H1G (–x–)and 1:2 H2G (–x–); meloxicam: pvp complex
in 1:1 P1G (–■–), 1:3 P3G (–|–) and 1:5 P5G (–○–); meloxicam: urea
complex in 1:1 U1G (– – –), 1:3 U3G (–□–) and 1:5 U5G (–Δ–)
| Formulation code | Amount permeated at 6 hr, Q6 (mg/cm2) | Flux, Jss (mg/cm2/h) | Enhancement factor | Lag time, tL (h) | Permeability coefficient, KP (cm/h x10-2) | Diffusion coefficient, D(cm2/h x 10-4) | Partition coefficient (K) | Release rate constant, k (mg/cm2/h0.5) |
|---|---|---|---|---|---|---|---|---|
| MG | 485.04 ± 0.25 | 82.04 ± 0.25 | 1.01 ± 0.27 | 0.11 ± 0.43 | 0.82 ± 0.33 | 8.90 ± 0.53 | 0.22 ± 0.23 | 199.49 ± 0.55 |
| B1G | 3261.54 ± 151.24 | 510.81 ± 0.56 | 6.20 ± 0.34 | 0.39 ± 0.65 | 5.10 ± 0.55 | 2.60 ± 0.45 | 4.88 ± 0.37 | 1226.19 ± 112.5 |
| B2G | 3565.69 ± 106.12 | 583.85 ± 0.24 | 7.10 ± 0.22 | 0.26 ± 0.23 | 5.80 ± 0.27 | 3.90 ± 0.65 | 3.67 ± 0.08 | 1419.34 ± 115.3 |
| H1G | 4445.79 ± 118.13 | 783.76 ± 0.55 | 9.55 ± 0.15 | 0.28 ± 0.45 | 7.80 ± 0.38 | 3.60 ± 0.70 | 5.39 ± 0.15 | 1901.30 ± 117.13 |
| H2G | 3811.60 ± 125.12 | 642.23 ± 0.28 | 7.82 ± 0.25 | 0.21 ± 0.34 | 6.40 ± 0.47 | 4.29 ± 0.42 | 3.20 ± 0.07 | 1572.70 ± 151.11 |
| P1G | 2135.53 ± 107.15 | 375.52 ± 0.74 | 4.57 ± 0.65 | 0.66 ± 0.20 | 3.70 ± 0.36 | 1.50 ± 0.63 | 5.90 ± 0.50 | 878.01 ± 0.114.35 |
| P3G | 3183.88 ± 128.17 | 611.16 ± 0.65 | 7.44 ± 0.35 | 0.30 ± 0.27 | 6.10 ± 0.23 | 3.40 ± 0.28 | 4.40 ± 0.53 | 1508.07 ± 103.15 |
| P5G | 2892.67 ± 105.13 | 488.81 ± 0.55 | 5.90 ± 0.70 | 0.40 ± 0.25 | 4.88 ± 0.39 | 2.57 ± 0.35 | 4.75 ± 0.62 | 1166.6 ± 117.34 |
| U1G | 1676.07 ± 126.14 | 279.97 ± 0.25 | 3.41 ± 0.43 | 0.64 ± 0.35 | 2.70 ± 0.40 | 1.60 ± 0.71 | 4.36 ± 0.46 | 651.06 ± 0.02 |
| U3G | 3306.84 ± 145.13 | 621.70 ± 0.36 | 7.50 ± 0.25 | 0.57 ± 0.45 | 6.21 ± 0.25 | 1.79 ± 0.25 | 8.64 ± 0.38 | 1474.09 ± 112.46 |
| U5G | 2148.477 ± 116.11 | 384.90 ± 0.29 | 4.69 ± 0.40 | 0.62 ± 0.38 | 3.80 ± 0.29 | 1.67 ± 0.45 | 5.70 ± 0.03 | 900.78 ± 0.26 |
Table 4: Percutaneous Permeation Parameters Of Meloxicam Gel Through Rat Skin
Figure 6: Comparative permeation profile of meloxicam from
different gels
Plain gel MG (––),
gel containing meloxicam: b-cyclodextrin complex
in 1:1 B1G(–■–) and 1:2 B2G (–▲–); meloxicam: hydroxypropyl-bcyclodextrin
complex in 1:1 H1G (–■–) and 1:2 H2G (–◊–);meloxicam:
polyvinyl pyrrolidone complex in 1:1 P1G (–■–), 1:3P3G (–|–) and
1:5 P5G (–□–); meloxicam: urea complex in 1:1 U1G (–○–), 1:3 U3G
(– – –) and 1:5 U5G (–●–)
In β-cyclodextrin gel formulation, the permeation rate was increased as the molar concentration of β-cyclodextrin was increased. The cyclodextrins such as β-cyclodextrin and hydroxypropyl-β-cyclodextrin might act as permeation enhancer by transferring the drug from the solution towards lipophilic surface of biological membrane, when the drug molecule distributed from the complex into the membrane. The complex does not penetrate the skin but the drug in the complex is in rapid dynamic equilibrium with drug in the aqueous phase, thus continuously supplying the drug molecule to skin surface in diffusible form. The lipophilic drug in the cavity of cyclodextrin, partition into membrane (skin) for which it has a greater affinity. In hydroxypropyl-β- cyclodextrin, gel formulation as molar concentration of hydroxypropyl-β-cyclodextrin increased there would be decreased in the amount of free drug and hence reduced the penetration flux through skin [12].
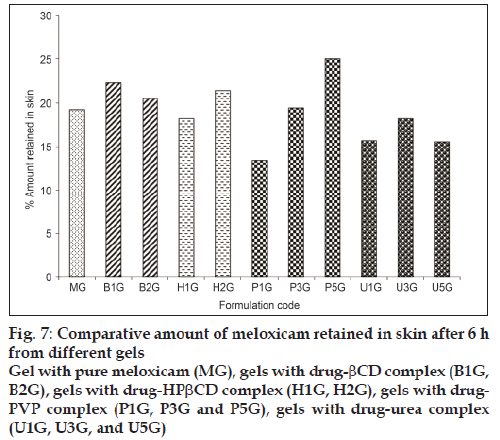
The flux obtained for polyvinyl pyrrolidone gel formulations P1G, P3G, and P5G was 375.52 mg/ cm2/h, 611.16 mg/cm2/h, 488.81 mg/cm2/h with the enhancement factor of 4.5, 7.4, and 5.9 respectively. The permeation rate was significantly increased (P<0.05) as compared to plain gel MG. It might be due to increased wettability and decreased the aggregation of drug by polyvinyl pyrrolidone [13]. The low flux observed in P5G formulation as the concentration of polyvinyl pyrrolidone increased might be due to increased in the viscosity of the solution in donor phase. The flux obtained for urea gel formulation U1G, U3G, U5G was found to 279.97 mg/cm2/h, 621.708 mg/cm2/h, 384.90 mg/cm2/h with the enhancement factor of 3.4, 7.5 and 4.6 respectively. The permeation rate was more as compared to plain gel MG. The flux obtained for U5G was less as compared to U3G. Urea act as a penetration enhancer as hydrating agent and keratolytic agent, which could affect the stratum corneum coenocytes, this action lead to believe that it would increase the penetration of drug through the skin [14]. The overall permeation rate was in the order of HPbCD gel>Urea gel >PVP gel>bCD gel >plain gel. Skin retention of meloxicam from gel to rat skin was studied and presented in fig.7. As we had measured the total thickness of rat skin and not the layer of skin subcutaneous and dermis layers, therefore non-recoverable fraction of meloxicam may be trapped in the skin. It was observed that there was not much difference between the control gel (without carrier) and carrier-complexed gel formulations. The amount retain was in the range of 13.50 to 25.05%. The skin retention of meloxicam was more which might be due to the physicochemical property of drug or due to its lipophilicity towards the skin.
These findings demonstrated that the carriers used in the study enhanced the solubility, dissolution and permeability of meloxicam. Hence cyclodextrins, polyvinyl pyrrolidone and urea could be used as potential permeation enhancer for percutaneous permeation of meloxicam.
Acknowledgements
The authors are very much thankful to M/s. Alcon Bioscience Pvt. Ltd. (Vapi, Gujarat, India), for providing gift sample of meloxicam. Authors are also thankful to Indian Herbs and Chemicals, Indore and Gangwal Chemicals Pvt. Ltd. (Mumbai, India) for providing the gift sample of β-cyclodextrin, hydroxypropyl-β-cyclodextrin.
References
- Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 2001;14:101-14.
- Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev 2004;56:603-18.
- Nishihata T, Kamada A, Sakai K, Takahashi K, Matsumoto K, Shinozaki K, et al. Percutaneous absorption of diclofenac in rats and humans: Aqueous gel formulation. Int J Pharm 1988;46:1-7.
- Loftsson T, Masson M. Cyclodextrins in topical drug formulations: Theory and practice. Int J Pharm 2001;225:5-30.
- Masson M, Loftsson T, Masson G, Stefansson E. Cyclodextrins as permeation enhancers: Some theoretical evaluations and in vitro testing. J Control Release 1999;59:107-8.
- Uekama K, Irie T, Sunada M, Otagiri M, Arimato Y, Nomura S. Alleviation of prochlorperazine induced primary irritation of skin by cyclodextrin complexation. Chem Pharm Bull 1982;30:3860-2.
- Wong O, Hungtinton J, Konichi R, Rytting JH, Higuchi T. Unsaturated cyclic urea, new nontoxic biodegradable penetration enhancers I: Synthesis. J Pharm Sci 1998;77:967-91.
- Sweetman SC, Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 51-2.
- Naidu NB, Chowdary KP, Murthy KV, Satyanarayana V, Hayman AR. Physicochemical characterization and dissolution properties of meloxicam–cyclodextrin binary system. J Pharm Biomed Anal 2004;35:75-86.
- El-Badry M, Fathy M. Enhancement of the dissolution and permeation rate of meloxicam by formulation of its freeze-dried solid dispersion in PVPK-30. Drug Dev Ind Pharm 2006;32:141-50.
- Janathanaprapap R, Stangi G. Effect of penetration enhancer on in vitro permeability of meloxicam gels. Indian J Pharm Sci 2007;343:26-33.
- Peng Z, Yang X, Huifen K, Yanswang Y, Yu L. Effect of HPbCD on solubility and transdermal delivery of capsaicin through rat-skin. Int J Pharm 2008;358:151-8.
- Tantishaiyakul V, Kaewonpparat N, Ingkatawornwong S. Properties of solid dispersion of piroxicam in polyvinyl pyrrolidone. Int J Pharm 1999;181:143-51.
- Godwin LD, Player RM, Sowell, Walter J, Michnaik BB. Synthesis and investigation of urea compound as transdermal penetration enhancer. Int J Pharm 1998;167:165-75.