- *Corresponding Author:
- K. Batcioglu
Department of Biochemistry, Faculty of Pharmacy, University of İnönü, Malatya, 44280, Turkey
E-mail: kadir.batcioglu@inonu.edu.tr
| Date of Submission | 27 December 2016 |
| Date of Revision | 18 August 2017 |
| Date of Acceptance | 02 April 2018 |
| Indian J Pharm Sci 2018;80(3):496-502 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Acute pancreatitis is an inflammatory disease of the pancreas associated with high mortality but without a specific therapy. Intrapancreatic protease activation appears to be an early event in the development of acute pancreatitis but its association with pathogenesis has not been established. Recently it has been demonstrated that an enormous rise in the cytoplasmic Ca2+ levels of pancreatic acinar cells could be associated with the development of acute pancreatitis, thereby suggesting that Ca2+ channel could be a potential target for treatment. In this study, the protective effects of calcium channel blockers such as 2-aminoethoxydiphenyl borate, dantrolene and verapamil were investigated in cerulein-induced acute pancreatitis in vivo. Rats were divided into five groups. Group 1 (control), group 2 (cerulein, 100 µg/kg), group 3 (cerulein+ 2-aminoethoxydiphenyl borate, mg/kg), group 4 (cerulein+dantrolene, 10 mg/kg) and group 5 (cerulein+verapamil, 2.5 mg/kg). Activities of amylase, lipase, cathepsin B, pancreatic secretory trypsin inhibitor and trypsin and levels of trypsinogen activation peptide and trypsinogen were measured addition to histological examination of the sections of pancreas. The results showed that cerulein increased the amylase, lipase and trypsin activities and trypsinogen activation peptide levels significantly. Administration of calcium channel blockers significantly protected the pancreas from histological damage (p<0.05). The results of this study demonstrated that calcium channel blockers can mitigate early protease activation and pancreas injury. It was suggested regulation of calcium channels would be relevant to maintain pancreatic acinar cell homeostasis and further research is necessary to understand the protective effects of calcium channel blockers.
Keywords
Acute pancreatitis, cerulein, 2-aminoethoxydiphenyl borate, dantrolene, verapamil
Acute pancreatitis is an acute inflammatory disease of the pancreas with variable involvement of other regional tissues and remote organs. It is characterised by abdominal pain and increased levels of serum amylase and lipase [1]. It is associated with high mortality and has no specific therapy [2]. Many causes of acute pancreatitis have been found, but gallstones and alcoholism are the most common. Early zymogen activation is known to lead to the onset of acute pancreatitis, although pathogenetic mechanisms of the disease are still unclear [1]. Many pancreatic digestive enzymes are inactive zymogen precursors within the acinar cell. When they reach the duodenum, trypsinogen is converted to trypsin by brush border hydrolase. Then, trypsin activates other pancreatic zymogen precursors [3]. Zymogen granules have some protective mechanisms against early zymogen activation in the acinar cell such as pancreatic secretory trypsin inhibitor (PSTI), keeping up optimum pH for many enzymes, and proteases that degrade activated enzymes [4]. It is proposed that the pathogenesis of acute pancreatitis is due to early over activation of trypsinogen and progressing proteolytic cascade [5]. Although several theoretical mechanisms for early trypsinogen activation have been suggested, but trypsinogen autoactivation to trypsin and trypsinogen activation by lysosomal hydrolase cathepsin B are the most widely accepted [3]. It has been indicated that excessive cytosolic calcium levels cause cellular changes in different experimental models of in vivo acute pancreatitis [6]. Furthermore, sustained intracellular elevation of calcium leads to intra-acinar enzyme activation, vacuolization and necrosis that play major roles in the initiation of acute pancreatitis pathogenesis [7]. Both enzyme secretion and the intracellular activation of zymogens require an increase in acinar cell cytosolic Ca2+ [8]. Cholecystokinin (CCK) and acetylcholine (ACh), primary pancreatic secretagogues, open Ca2+ channels in the endoplasmic reticulum membrane to release Ca2+. There are at least two known types of Ca2+ channel that regulate release from Ca2+ stores; an inositol(1,4,5)-trisphosphate (IP3)-sensitive channel (Ins(1,4,5)P3 receptor, IP3R) and a ryanodinesensitive channel (ryanodine receptor, RyR) [9]. CCK and ACh bind a specific 7-transmembrane domain receptor in the plasma membrane that couples to the heterotrimeric G protein. Activation of G protein results in the increased activity of phospholipase C, which cleaves phosphatidylinositol(4,5)-bisphosphate in the plasma membrane and subsequently generates the second messengers IP3 and diacylglycerol. IP3 opens the IP3R, localized in the apical region of the acinar cell, and is used as a second messenger in many cells to release Ca2+ [10,11]. Effects of inhibition of IP3- mediated Ca2+ release by 2-aminoethoxydiphenyl borate (2-APB) on acute pancreatitis were investigated in this study.
The RyR is responsible for calcium-induced calcium release. The RyR owes its name to the plant alkaloid ryanodine, which binds to RyRs highly affinity. RYRs localize in the basal region of the acinar cells. They amplify Ca2+ signals that are triggered by apical IP3Rs [12]. In the current study, the RyR was inhibited with dantrolene, a drug that is clinically used to treat malignant hyperthermia.
Voltage-gated calcium channels provide calcium entry in response to membrane depolarization in many different cell types and regulate cellular processes such as contraction, secretion, neurotransmission, and gene expression. L-type channels play a role in the control of transmitter emission from endocrine cells and sensor neurons in heart and many kinds of smooth muscle contraction. L-type channels are the main target of Ca2+channel antagonist drugs such as verapamil, diltiazem. Acinar cells do not possess voltage-gated Ca2+ channels, and the cytosolic Ca2+ signals that activate exocytotic enzyme secretion are primarily caused by release from intracellular stores [13]. Although the protective mechanism of verapamil is not clearly known, it is suggested that it is related to the stabilization of the membrane. Increased pancreatic zymogen granule fragility is reported in conditions associated with pancreatic injury in the rat [14,15].
In the present study, since aberrant rise of Ca2+ plays a crucial role in the pathogenesis of acute pancreatitis, the effects of agents that are known calcium channel blockers were examined on protease activation and pancreatitis severity in vivo. The protective effects of calcium channel blockers, dantrolene, 2-APB, and verapamil on cerulein-induced acute pancreatitis in rats were investigated.
Materials and Methods
The study was undertaken at the Laboratory for Experimental Studies of İnönü University in accordance with the guidelines established in the Guide for the Care and Use of Laboratory Animals following the approval of the design by the Animal Ethics Committee of the İnönü University.
Sixty adult female Wistar rats weighing 250-300 g (age, 3-4 mo) were fed on standard rat chow with access to water ad libitum and housed in standard shoebox cages in a climate-controlled room with an ambient temperature of 23 ± 2° and a 12 h/12 h light-dark cycle. The animals were randomly divided into five groups of 12 rats each. Group 1: received two intra peritoneal (i.p.) injections of 0.9 % saline at 2 h intervals; served as control. Group 2: received two i.p. injections of cerulein (50 μg/kg; Sigma, USA) at 2 h intervals; served as acute pancreatitis group. Group 3: pre-treated with single dose i.p. injection of 2-APB (2 mg/kg; Sigma, USA) and received two i.p. injections of cerulein as described above; served as 2-APB group. Group 4: pre-treated with single dose of dantrolene (Sigma, USA; 10 mg/kg) and received two i.p. injections of cerulein as described above; served dantrolene group. Group 5: pre-treated with single dose i.p. injection of verapamil (2.5 mg/kg; Sigma, USA) and received two i.p. injections of cerulein as described above; served verapamil group. After 12 h, the rats were sacrificed by cervical dislocation under light ether anaesthesia. Blood samples were collected by cardiac puncture. The blood was centrifuged at 3500 g for 10 min at 4°, serum samples were drawn and kept at –20° until biochemical assays. Rat pancreases were dissected and perfused with phosphate-buffered saline (PBS; 50 mM, pH 7.4) and divided into two portions. The first portion of pancreas was homogenized in cold PBS (with a weight to volume ratio of 1 to 5) using a homogenizer (T25 Ultra-Turrax, IKA Werke GmbH, Staufen, Germany). The homogenates were sonicated four times for 10 and 20 s intervals using a VWR Bronson scientific sonicator (VWR Int. Ltd. Merck House Pool, UK). The homogenates were then centrifuged at 13250 g for 15 min at 4° (Centrifuge 5415R, Eppendorf AG, Germany). The supernatants were collected and kept at –80° until biochemical assays. Other portion of pancreas was kept in 10 % formalin until histological assays.
Protein determination
Protein concentration was determined by the method of Lowry et al. [16] using bovine serum albumin as a standard. A Shimadzu 1601 UV/Vis spectrophotometer (Shimadzu, Japan) was employed for all spectrophotometric assays.
Assay of trypsin activity
Trypsin activity was measured by the procedure of the assay that is used in the Worthington laboratory with the rate of increase in absorbance at 247 nm during the hydrolysis of p-toluene-sulfonyl-L-arginine methyl ester (TAME). One unit hydrolyses 1 μ mol of TAME per minute 25°, pH 8.2, in the presence of 0.01 M calcium ion [17].
Assay of PSTI activity
The ability of the PSTI to prevent trypsin hydrolysis of benzoyl-L-arginine ethyl ester is measured spectrophotometrically according to the Worthington laboratory procedure. The activity of the inhibitor is expressed as the amount of trypsin inhibited by one milligram of inhibitor [18].
Assay of cathepsin B activity
Cathepsin B activity was detected by a fluorescencebased assay that utilizes the preferred cathepsin B substrate sequence R-R labelled with amino-4- trifluoromethyl coumarin (AFC; BioVision Research Products, USA). Samples that contain cathepsin B cleaved the synthetic substrate R-R-AFC to release free AFC. The released AFC was quantified using a fluorescence plate reader equipped with a 400 nm excitation filter and a 505 nm emission filter. Foldincrease in cathepsin B activity was determined by comparing the relative fluorescence units with the level of the negative control sample.
Determination of serum amylase and lipase levels
Serum amylase and lipase levels were determined by enzyme-linked immunosorbent assay (ELISA) method (Immundiagnostik, Bensheim, Germany and Alpha Diagnostic, San Antonio, TX, USA; respectively).
Determination of TAP and trypsinogen levels
Pancreas TAP and trypsinogen levels (USCNK, Wuhan, China, Cusabio Biotech, China) were determined by ELISA method in accordance with the manufacturer’s manual.
Histological examination of pancreas
Tissue sections were cut at 5 μm, mounted on slides, stained with hematoxylin-eosin and examined with a light microscope (Olympus BX 50, Japan). For each section, tissue alterations were assessed in 20 different fields by an experienced histologist who was unaware of the treatment. Pancreatic injury was scored (0-3) based on pancreatic oedema, leukocyte infiltration, acinar vacuolization and necrosis according to Dembin´ski et al. [19].
Statistical analysis
Statistical analysis was carried out using the SPSS 15.0 statistical program (SPSS Inc., USA). All data are reported as mean ± SE. Biochemical data and histological scores were analysed by the ANOVA and LSD tests except for trypsinogen. Kruskal-Wallis and Mann-Whitney U tests were used for trypsinogen data. Values of p<0.05 were regarded as significant.
Results and Discussion
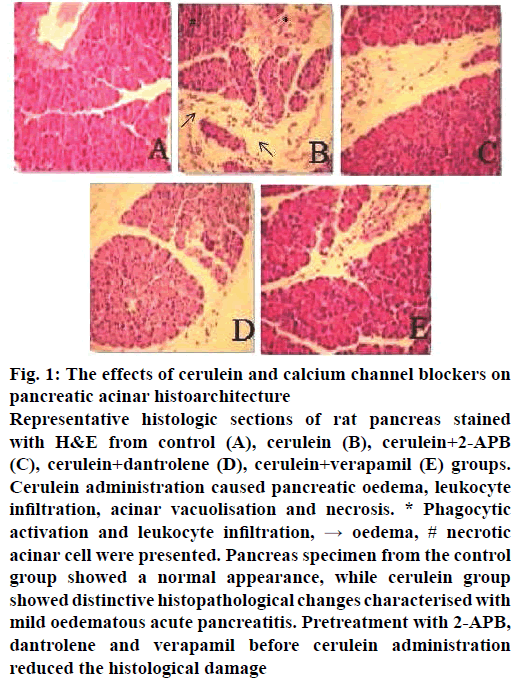
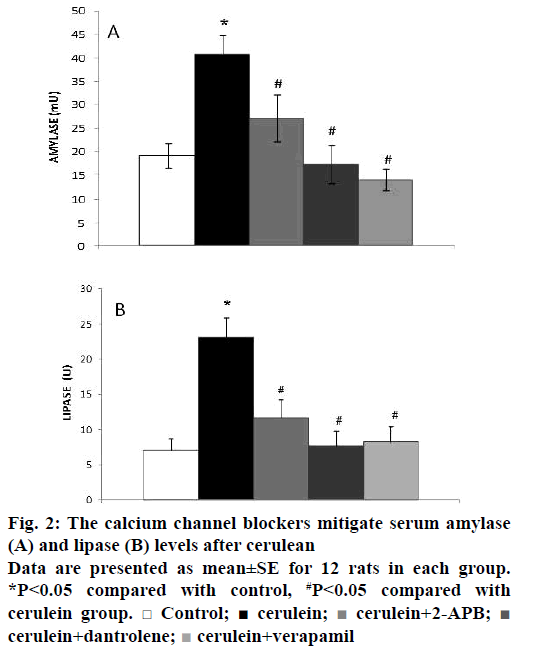
To examine the effects of calcium channel blockers on the development and severity of acute pancreatitis, which was induced by injection of cerulein (2×50 μg/kg), rats were pre-treated with 2-APB, dantrolene and verapamil (2, 10, 2.5 mg/kg, respectively). The severity of cerulein-induced acute pancreatitis was assessed by examining histological characteristics. While the pancreas specimens from control animals presented no histological alterations (Figure 1A), those from animals in the cerulein-treated group displayed prominent oedema, intracellular vacuolization and inflammatory infiltration (Figure 1B). The histological changes observed in the 2-APB, dantrolene and verapamil groups are illustrated in Figure 1C, D and E, respectively. The histopathological scores are summarised in Table 1. Pre-treatment with 2-APB, dantrolene and verapamil significantly protected the pancreas from histological damage induced by cerulein, as indicated by the lower histological scores. Serum amylase and lipase levels are the most commonly used biochemical markers of pancreatic diseases, particularly acute pancreatitis. Therefore, we assessed the severity of acute pancreatitis by measuring the enzyme levels. In the cerulein-treated group, the serum levels of lipase and amylase increased significantly (p˂0.05). However, 2-APB, dantrolene and verapamil reduced the levels of lipase and amylase significantly (p˂0.05; Figure 2A and B).
Figure 1: The effects of cerulein and calcium channel blockers on pancreatic acinar histoarchitecture
Representative histologic sections of rat pancreas stained with H&E from control (A), cerulein (B), cerulein+2-APB (C), cerulein+dantrolene (D), cerulein+verapamil (E) groups. Cerulein administration caused pancreatic oedema, leukocyte infiltration, acinar vacuolisation and necrosis. * Phagocytic activation and leukocyte infiltration, → oedema, # necrotic acinar cell were presented. Pancreas specimen from the control group showed a normal appearance, while cerulein group showed distinctive histopathological changes characterised with mild oedematous acute pancreatitis. Pretreatment with 2-APB, dantrolene and verapamil before cerulein administration reduced the histological damage
Figure 2: The calcium channel blockers mitigate serum amylase (A) and lipase (B) levels after cerulean
Data are presented as mean ± SE for 12 rats in each group. *P˂0.05 compared with control, #P˂0.05 compared with cerulein group. □ Control; ■ cerulein;  cerulein+2-APB;
cerulein+2-APB;  cerulein+dantrolene;
cerulein+dantrolene;  cerulein+verapamil
cerulein+verapamil
| Groups | Oedema (0-3) | Leukocyte infiltration (0-3) | Acinar vacuolisation (0-3) | Necrosis (0-3) | Total pancreatic damage |
|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 |
| Cerulein | 2 | 2 | 2 | 0.8 ± 0.2 | 6.8 ± 0.2a |
| Cerulein+2-APB | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.6 ± 0.2 | 4.2 ± 0.2a,b |
| Cerulein+dantrolene | 1.6 ± 0.2 | 1.2 ± 0.2 | 1.4 ± 0.2 | 0.6 ± 0.2 | 4.8 ± 0.6a,b |
| Cerulein+verapamil | 1.2 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 | 0.6 ± 0.2 | 4.4 ± 0.4a,b |
Table 1: Pancreatic Damage Score (Oedema, Leukocyte Infiltration, Acinar Vacuolisation and Necrosis) for the Groups
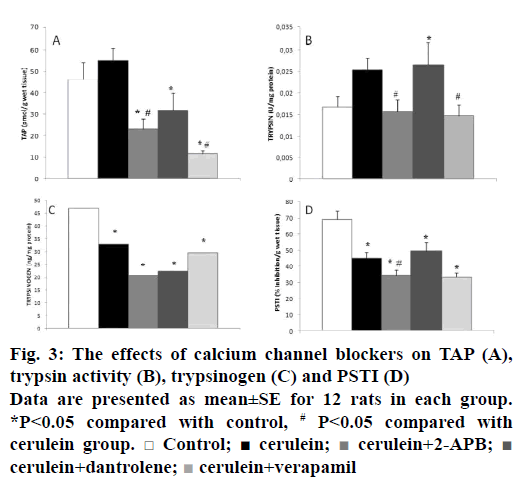
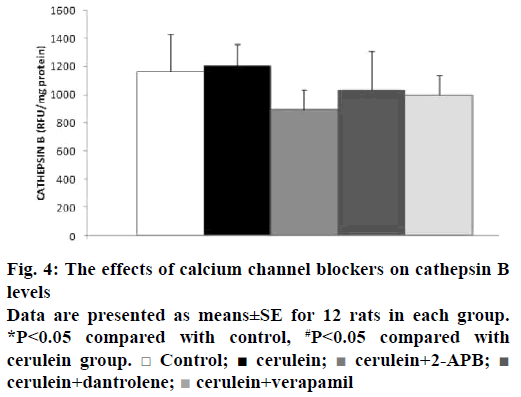
Intra-acinar activation of zymogens is a key event in the initiation of acute pancreatitis [20]. Therefore, we also examined whether calcium channel blockers could prevent early trypsinogen activation. TAP corresponds to the N-terminal region of the peptide released by the conversion of trypsinogen into active trypsin [21]. We have detected that elevated TAP levels after cerulein administration were significantly decreased by calcium channel blockers, except for dantrolene, according to the cerulein group (p˂0.05; Figure 3). Decreased trypsinogen levels after cerulein hyperstimulation were found in the cerulein group (p˂0.05). There were no significant differences in trypsinogen levels of calcium channel blocker groups compared with the cerulein group (p>0.05; Figure 3). Trypsin activity was measured in this study, as trypsin triggers cascade of digestive protease activation. As shown in Figure 3, trypsin activity increased in the cerulein-induced group insignificantly (p>0.05). Reduced trypsin activities were observed in the 2-APB and verapamil groups when compared to the cerulein-induced group significantly (p˂0.05). PSTI is one of the key defensive mechanisms against prematurely activated trypsin within the pancreatic acinar cells. When the inhibitory capacity of PSTI is overwhelmed by excessive trypsinogen activation, control of trypsin activity is lost and pancreatitis develops [22]. In our present study, trypsin inhibitory activity of PSTI decreased in the cerulein-induced group when compared to the control group (p˂0.05; Figure 3). Cathepsin B levels were slightly reduced with the calcium channel blockers-treated groups according to the cerulein group, this score was non-significant (Figure 4).
Figure 3: The effects of calcium channel blockers on TAP (A), trypsin activity (B), trypsinogen (C) and PSTI (D)
Data are presented as mean±SE for 12 rats in each group. *P˂0.05 compared with control, # P˂0.05 compared with cerulein group. □ Control; ■ cerulein;  cerulein+2-APB;
cerulein+2-APB;  cerulein+dantrolene;
cerulein+dantrolene;  cerulein+verapamil
cerulein+verapamil
Acute pancreatitis is an inflammatory disease of the pancreas. The incidence of acute pancreatitis is increasing and causes significant morbidity and mortality. There are many experimental and clinical researches, but there is still no specific pharmacological therapy for acute pancreatitis [23]. Although premature intrapancreatic protease activation is a very early event in the development of acute pancreatitis [24], the pathophysiological mechanisms of this process have not yet been completely elucidated. A number of experimental data have suggested that pathological intrapancreatic protease activation is associated with an abnormal rise in cytosolic Ca2+ [7,8]. Intracellular Ca2+, a key physiological signalling element in cell function, plays a central role in controlling normal pancreatic enzyme secretion. It is also a crucial pathological intracellular messenger in cell injury, which is included in the initiation and development of acute pancreatitis [25]. Ward et al. have shown that cytosolic excessive Ca2+ concentration by hyperstimulation of cells caused premature activation of digestive enzymes and pancreatic acinar cell injury [26]. Additionally, it has been reported that there were alterations in intracellular calcium signalling in an experimental animal model of acute pancreatitis induced via supramaximal secretagogue stimulation [27]. Elevated intracellular Ca2+ concentrations cause abnormal intracellular enzyme activation, vacuolization and necrosis, processes that are pivotal in the initiation of acute pancreatitis [7,8]. Several calcium channel blockers that interfere with either the uptake of calcium or the rapid release of calcium from intracellular stores can completely prevent intracellular activation of trypsinogen [8,28].
In this study, it was found that calcium channel blockers reduced intra-acinar zymogen activation. Verapamil is a calcium antagonist, which is widely used as an antiarrhythmic agent to control supraventricular tachyarrhythmias as well as for hypertension [29]. It has been reported that verapamil administration protects against the course of experimental pancreatitis in rats and is related to the effect of verapamil in pancreatic blood flow and plasma prostanoids [30]. Zhou et al. have shown that verapamil administration was associated with a significant protection against inflammatory process in acute pancreatitis induced by high doses of cerulein [31]. Our present work evaluated the effect of verapamil administration on the changes of protease activation and acinar injury. The results obtained indicated that verapamil ameliorated the severity of acute pancreatitis caused by cerulein hyperstimulation in rats. Lake- Bakaar et al. have pointed out that increasing doses of verapamil protect against diet-induced pancreatitis in mice by reduction in zymogen granule membrane dissolution [14]. It has been demonstrated that verapamil can exert a therapeutic effect in pancreatic injury associated with cerulein-induced acute pancreatitis. Although verapamil was strongly suggested against ischemic lesions of pancreatitis in an animal study of experimental pancreatitis [32], Prat et al. selected nifedipine because the haemodynamic effects of verapamil are more difficult to control, especially during and shortly after anaesthesia [33].
Increases in cytosolic Ca2+ occur by release of Ca2+ from intracellular stores located in the endoplasmic reticulum. There are two mostly known types of Ca2+ channel that regulate release from Ca2+ stores: IP3R and RyR [34]. IP3 opens the IP3R and is used as a second messenger in many cells to release Ca2+. IP3R is concentrated in the apical membrane. All Ca2+ signals start in the apical pole and the apical Ca2+ signals are sufficient for stimulation of both enzyme and fluid secretion [35]. In the current study, it has been found that pre-treatment with 2-APB reduced the early protease activation and histological severity of the disease. Maruyama et al. have reported that 2-APB inhibits IP3-induced Ca2+ release [36]. Later, researches have showed that 2-APB inhibited the sarco-endoplasmic reticulum Ca2+-ATPase [37] and blocked the storeoperated Ca2+ pathway [38]. Its mechanism of action is more complicated than thought at first. 2-APB may be useful in investigating the details of Ca2+ signalling mechanism.
The RyR is responsible for calcium-induced calcium release [39] and is diffusely distributed in the basolateral region of the acinar cell [40]. It has been shown that the RyR modulates pathological protease activation in isolated acinar cells [41]. RyR inhibitors reduced intra-acinar protease activation due to secretagogue hyperstimulation without affecting physiological enzyme secretion from the acinar cell [42]. In the present study, RyR was inhibited with dantrolene, a drug that is clinically used to treat malignant hyperthermia [43]. Nathanson et al. showed that dantrolene appeared to selectively reduce basal Ca2+ signals without affected enzyme secretion [44]. Lewarchik et al. found that the RyR is expressed in human acinar cells and it modulates acinar Ca2+ signals and cell injury as well as RyR inhibition attenuates acinar cell injury [12]. Orabi et al. suggested that RYR is a potential therapeutic target for pancreatitis [45]. According to these findings, dantrolene reduced histological pancreatitis severity and zymogen activation. Also these results demonstrated that dantrolene is not effective in reducing the activity of trypsin and its effect on lowering TAP levels is less than that of other calcium channel blockers. In addition, dantrolene modestly increased PSTI that is believed to protect against intrapancreatic trypsin activity. While trypsinogen auto-activation requires an acidic pH and is enhanced in the presence of Ca2+ [38], the affinity of a pancreatic trypsin inhibitor is greatest at a neutral pH and is reduced at an acidic pH+ [3]. According to the results obtained, it is possible to suggest that dantrolene can be used with therapeutic effect on pancreatic damage, but it has little benefit on preventing early protease activation. Future works need to enlighten the molecular mechanism of these agents and to clarify the specific affinity of candidate agents.
In conclusion, the present study demonstrated that calcium channel blockers can mitigate early protease activation and acinar cell injury due to ceruleininduced acute pancreatitis. In this sense, it is possible to speculate that calcium channel blockers may exert a preventive effect on the first and progressive steps of the disease. Calcium channel inhibition might help to protect against acute pancreatitis. In future investigations, the sophisticated molecular approaches related with Ca2+ homeostasis including ion exchange mechanisms, gene expressions of ion channels, and granular membrane permeability in zymogen granules should be examined in other experimental models of pancreatitis.
Financial support and sponsorship
IUBAP.
Conflict of interest
The authors declare that this paper content has no conflict of interests
References
- Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008;371:43-52.
- Schäfer C, Tietz AB, Göke B. Pathophysiology of acute experimental pancreatitis: lessons from genetically engineered animal models and new molecular approaches. Digestion 2005;71:162-72.
- Gorelick FS, Otani T. Mechanisms of intracellular zymogen activation. Baillieres Best Pract Res Clin Gastroenterol 1999;13:227-40.
- Mitchell RMS, Byrne MF, Baillie J. Pancreatitis. Lancet 2003;361:1447-55.
- Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology 2009;136:2040-44.
- Voronina S, Longbottom R, Sutton R, Petersen OH, Tepikin A. Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J Physiol 2002;540:49-55.
- Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signalling in premature protease activation and the onset of pancreatitis. Am J Pathol 2000;157:43-50.
- Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA 2000;97:13126-31.
- Tsien RW, Tsien RY. Calcium channels, stores and oscillations. Annu Rev Cell Biol 1990;6:715-60.
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 1983;306(5938):67-9.
- Petersen OH. Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium 2005;38:171-200.
- Lewarchik CM, Orabi AI, Jin S, Wang D, Muili KA, Shah AU et al. The ryanodine receptor is expressed in human pancreatic acinar cells and contributes to acinar cell injury. Am J Physiol Gastrointest Liver Physiol 2014;307:574-81.
- Gerasimenko JV, Gerasimenko OV, Petersen OH. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol 2014;592(2):269-80.
- Lake-Bakaar G, Lyubsky S. Dose-dependent effect of continuous subcutaneous verapamil infusion on experimental acute pancreatitis in mice. Dig Dis Sci 1995;40:2349-55.
- Gerasimenko JV, Gryshchenko O, Ferdek PE, Stapleton E, Hébert TOG, Bychkova S, et al. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc Natl Acad Sci USA 2013;110:13186-91.
- Lowry OH, Rosebrough NJ, Farr AL, Ronndall, RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951;193:265-71.
- http://www.worthington-biochem.com/TRY/assay.html.
- http://www.worthington-biochem.com/TI/assay.html.
- Dembin´ski A, Warzecha Z, Ceranowicz P, Warzecha AM, Pawlik WW, Dembin´ski M, et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of Sensory Nerves. Eur J Pharmacol 2008;591:284-92.
- Thrower EC, Osgood S, Shugrue CA, Kolodecik TR, Chaudri AM, Reeve Jr JR, et al. The novel protein kinase C isoforms – δ and - ε modulate cerulein-induced zymogen activation in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 2008;294:1344-53.
- Mayer J, Rau B, Schoenberg MH, Beger HG. Mechanism and role of trypsinogen activation in acute pancreatitis. Hepatogastroenterology 1999;46:2757-63.
- Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 2000;25:213-16.
- Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA 2004;291:2865-68.
- Steer ML. Early events in acute pancreatitis. Baillieres Best Pract Res Clin Gastroenterol 1999;13:213-25.
- Lum H, Aschner JL. Phillips PG. Time course of thrombin induced increase in endothelial permeability: relationship to Ca2+i and inositol polyphosphates. Am J Physiol 1992;263:219-25.
- Ward JB, Petersen OH, Jenkins SA, Petersen OH. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet 1995;346:1016-19.
- Ward JB, Sutton R, Jenkins SA, Petersen OH. Progressive disruption of acinar cell calcium signalling is an early feature of cerulein-induced pancreatitis in mice. Gastroenterology 1996;111:481-91.
- Grady T, Mah’Moud M, Otani T, Rhee S, Lerch MM, Gorelick FS. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol 1998;275:1010-17.
- Hamann SR, Blouin RA, McAllister RG. Clinical pharmacokinetics of verapamil. Clin Pharmacokinet 1984;9:26-41.
- Shen J, Zhao S, Teng C, Shao H, Wu Z, Wang K, et al. Calcium channel blockade protects against the development of experimental acute pancreatitis in rats: effects of verapamil on pancreatic blood flow and plasma arachidonic acid metabolites. Med Sci Res 1991;19:667-69.
- Zhou W, Shen F, Miller JE, Han Q, Olson MS. Evidence for altered cellular calcium in the pathogenetic mechanism of acute pancreatitis in rats. J Surg Res 1996;60:147-55.
- Leahy AL, Darzi A, Grace P, Qureshi A, Redmond P, Leader M, et al. Verapamil is beneficial in a model of post-ERCP pancreatitis. Eur J Gastroenterol Hepatol 1993;5:467-69.
- Prat F, Amaris J, Ducot B, Bocquentin M, Fritsch J, Choury AD, et al. Nifedipine for prevention of post-ERCP pancreatitis: a prospective, double-blind randomized study. Gastrointest Endosc 2002;56:202-08.
- Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci 2006;27(2):113-20.
- Nathanson MH, Fallon MB, Padfield PJ, Maranto AR. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J Biol Chem 1994;269:4693-96.
- Maruyama T, Kanaji T, Nakada S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membranepenetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 1997;505:498-505.
- Bilmen JG, Wootton LL, Godfrey RE, Smart OS, Michelangeli F. Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenylborate (2-APB). 2APB reduces both Ca2+ binding and phosphoryltransfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites. Eur J Biochem 2002;269:3678-87.
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 2002;16:1145-50.
- Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 1970;228:34-6.
- Fitzsimmons TJ, Gukovsky I, McRoberts JA, Rodriguez E, Lai FA, Pandol SJ. Multiple isoforms of the ryanodine receptor are expressed in rat pancreatic acinar cells. Biochem J 2000;351:265-71.
- Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. PNAS 2005;102:14386-91.
- Husain SZ, Orabi AI, Muili KA, Luo Y, Sarwar S, Mahmood SM, et al. Ryanodine receptors contribute to bile acid induced pathological calcium signalling and pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 2012;302:1423-33.
- Krause T, Gerbershagen MU, Fiege M, Weißhorn R, Wappler F. Dantrolene-a review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004;59:364-73.
- Nathanson MH, Padfield PJ, O'Sullivan AJ, Burgstahler AD, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem 1992;267:18118-21.
- Orabi AI, Shah AU, Ahmad MU, Choo-Wing R, Parness J, Jain D et al. Dantrolene mitigates caerulein-induced pancreatitis in vivo in mice. Am J Physiol Gastrointest Liver Physiol 2009;299:196-204.