- *Corresponding Author:
- Kun Niu
Department of Anesthesiology, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Haidian, Beijing 100142, China
E-mail: niukun100032@163.com
| Date of Received | 26 July 2021 |
| Date of Revision | 11 May 2022 |
| Date of Acceptance | 05 December 2022 |
| Indian J Pharm Sci 2022;84(6):1582-1587 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the effects of propofol and dexmedetomidine on the expression of cytochrome c oxidase in primary hippocampal neurons isolated from neonatal mice. C57 neonatal mice were sacrificed within 24 h of birth and hippocampal tissue was collected. Primary hippocampal neurons were cultured in vitro and then randomly divided into a control group (0 μM propofol and 0 μM dexmedetomidine), propofol group (20 μM propofol and 0 μM dexmedetomidine) and propofol+dexmedetomidine group (20 μM propofol and 10 μM dexmedetomidine). The neurons were incubated for 1 h. Neuron morphology was observed under a light microscope. The expression of cytochrome c protein was detected semiquantitatively by Western blot and immunofluorescence staining. Compared with those in the control group, the primary hippocampal neurons in the propofol group were morphologically degenerated, with sparse axons and dendrites; the expression of cytochrome c protein was significantly higher in the propofol group (p<0.0001). The morphology of primary hippocampal neurons in the propofol+dexmedetomidine group was similar to that of primary hippocampal neurons in the control group, with plump and bright cell bodies and thickened neurites intertwined in a neuron network; additionally, cytochrome c protein expression was significantly lower in the hippocampal neurons in the propofol+dexmedetomidine group than in the hippocampal neurons in propofol group (p<0.0001). Dexmedetomidine reversed propofol induced injury and morphological abnormalities of primary hippocampal neurons. The mechanism may be related to the downregulation of cytochrome c protein expression.
Keywords
Dexmedetomidine, propofol, primary hippocampal neurons, cytochrome C
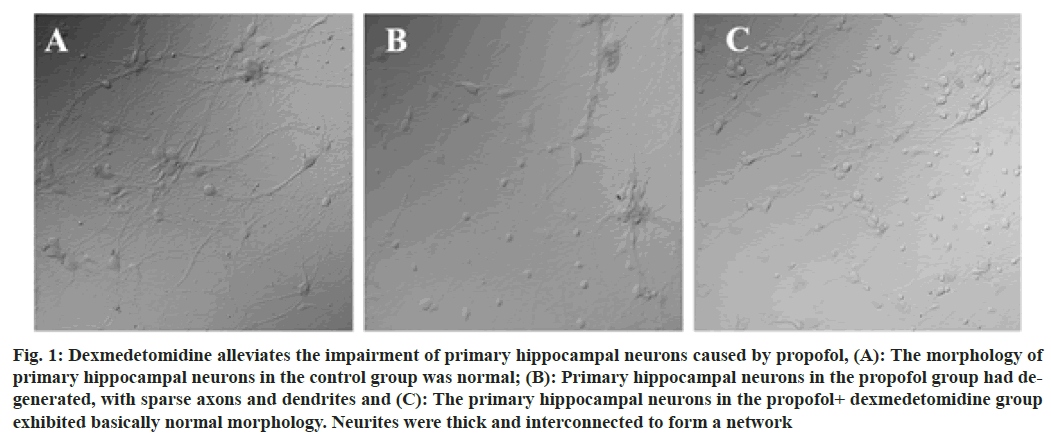
Propofol is the most commonly used intravenous anesthesia drug in clinical practice and is widely used for the induction and maintenance of anesthesia in children and adults. Studies have shown that exposure of the developing brain to general anesthesia with propofol can cause damage to the central nervous system and inhibit the neurogenesis of the hippocampus, thereby affecting the learning and memory functions of infants and young children[1,2]. Dexmedetomidine is an alpha (α)-adrenergic receptor agonist that acts on the presynaptic α2 receptors of noradrenergic nerves, reducing the release of norepinephrine when the receptors are excited, resulting in sedation and analgesic effects[3]. Our preliminary animal studies[4] indicated that dexmedetomidine was able to prevent postoperative spatial memory deficits induced by surgery under propofol anesthesia through neuronal protection and anti-inflammatory effects. The mechanism may be related to the protection of mitochondrial electron transport chain function. Cytochrome c is an important water-soluble mitochondrial protein that exists in the inner mitochondrial membrane and its main function is to transport electrons to the mitochondrial oxidative respiratory chain complex[5]. The aim of this study was to observe the effect of dexmedetomidine on mitochondrial protein cytochrome c in neonatal mouse primary hippocampal neurons incubated with propofol. The results of this study provide an experimental basis for the clinical application of dexmedetomidine for the prevention of the developmental neurotoxicity of propofol. C57 neonatal mice within 24 h of birth were used. The animals were clean grade, weighed 4.0 to 6.0 g and were provided by the Institute of Biophysics, Chinese Academy of Sciences. Propofol (500 mg/50 ml) was purchased from Xi'an Libang Pharmaceutical Co., Ltd. Dexmedetomidine (100 μg/1 ml) was purchased from Yangtze River Pharmaceutical Group Co., Ltd. Dulbecco's Modified Eagle's Medium (DMEM), Opti-Minimal Essential Medium (MEM), neurobasal medium and GlutaMAX medium additives were purchased from Gibco, USA. Fetal Bovine Serum (FBS) and anti-fluorescence quenching mounting medium were purchased from Invitrogen, Canada. Trypsin (0.125 %) and poly-L-lysine were purchased from Sigma Aldrich, USA. Anti-cytochrome c antibody and Alexa Fluor® 488 goat anti-rabbit reagent were purchased from Abcam Company. Normal goat serum (5 %) was purchased from Jackson Immuno Research Labs. The day before the material was collected; culture dishes or culture flasks were coated with 100 μg/ml poly-D-ornithine and placed in a 37° incubator overnight. After cleaning the dissection instruments, they were sterilized. H buffer was prepared. 24 h after birth, the external surface of the mouse was sterilized with 70 % ethanol and brain tissue was quickly harvested after decapitation with ophthalmic scissors. Hippocampal tissue was isolated under a stereo dissecting microscope, soaked in fresh H buffer, chopped with Venus scissors and then transferred to a petri dish. During digestion, the solution was gently shaken 3 times. After 30 min of digestion, 1 ml of precooled DMEM containing 10 % FBS was added to stop the digestion; after 2 min, the medium was removed and the process was repeated. After the digestion was terminated, 1 ml of prechilled DMEM containing 10 % FBS and 10 μl of 10 mg/ml DNase I (Roche) were added. The cells were subjected to repeat pipetting at a constant speed with a 1 ml pipette and then passed through a 200-mesh cell sieve; 0.9 ml of the cell suspension was collected and the cells were counted. Prewarmed DMEM containing 10 % FBS (1 ml) was used to resuspend and dilute the cells; the cells were seeded in a coated petri dish and then incubated for 1 h in an incubator at a constant temperature of 37° and 5 % CO2. The medium was replaced with prewarmed neurobasal medium containing B27 and half the medium was changed every 3 d. The growth of neurons was observed under a microscope. Cultured primary hippocampal neurons were treated with different concentrations of propofol and dexmedetomidine based on the experimental groupings[6,7]; control group (0 μM propofol and 0 μM dexmedetomidine); propofol group (20 μM propofol and 0 μM dexmedetomidine) and propofol+dexmedetomidine group (20 μM propofol and 10 μM dexmedetomidine). All cells were incubated for 1 h. After centrifuging the cell suspension, the supernatant was discarded and the cells were washed 2-3 times with Phosphate- Buffered Saline (PBS). Then, 4 % paraformaldehyde was used to fix the cells for 10-15 min at room temperature. The cells were washed 3 times (5 min each) with PBS, after which 0.1 % Triton X-100 was added to permeabilize the cell membrane for 2 min at room temperature. Nonspecific binding sites were blocked with 5 % goat serum in PBS for 30 min at room temperature. After removing the blocking solution, the primary antibody (rabbit anti-cytochrome c) was added and the cells were incubated overnight at 4°. The cells were then washed 3 times, followed by the dropwise addition of the secondary antibody (Alexa Fluor® 488 goat anti-rabbit Immunoglobulin G (IgG)) and incubation at room temperature for 1 h. Then, the cells were washed and mounted with antifluorescence quenching mounting medium. The cells were excited with DyLight 488 at different excitation wavelengths and observed and photographed under an Olympus FluoViewTM FV3000 confocal laser scanning microscope equipped with an oil lens. Hippocampal tissue was lysed in 10 times the volume of Radioimmunoprecipitation Assay (RIPA) lysis buffer, Phenylmethylsulfonyl Fluoride (PMSF), cytoplasmic protein extraction reagent A and cytoplasmic protein extraction reagent B mixture and ground with a grinder for 60 s. The tissue homogenate was centrifuged at low temperature (4°) at 13 000 rpm/min for 20 min. The supernatant was aspirated with a pipette and the protein concentration was measured using a Bicinchoninic acid (BCA) protein concentration assay kit. The 3 groups were subjected to Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS‒PAGE), and the membranes were transferred to a Polyvinylidene Difluoride (PVDF) membrane using a wet transfer method. Then, the PVDF membrane was immersed in 5 % nonfat milk blocking solution and slowly stirred on a shaker at room temperature for 1 h. The membrane was incubated with primary antibody, i.e., rabbit anticytochrome c antibody (1:5000, ab133504, Abcam, Cambridge, UK) and anti-beta actin rabbit pAb (1:1000, GB11001, Servicebio, Wuhan, China) overnight at 4°. After washing off the primary antibody with Tris Buffered Saline with Tween (TBST), the secondary antibody, i.e., goat antirabbit IgG (1:3000, GB23303, Servicebio, Wuhan, China) was added and the membrane was incubated at room temperature for 1 h. Then, the secondary antibody was recovered, and the membrane was washed with TBST 3 times on a shaker. Electrochemiluminescence (ECL) was used to visualize the bands. The content of the target protein was calculated as the ratio of the gray value of the target protein band to that of the internal reference band. Statistical Package for the Social Sciences (SPSS) 19.0 software was used for the statistical analyses. All data are expressed as the mean±Standard Deviation (mean±SD) and graphing was performed using GraphPad Prism 5.0. Analysis of Variance (ANOVA) or t test statistics are described in the legends. p<0.05 indicated that difference was statistically significant. In the control group, the primary hippocampal neurons of the neonatal mice were mature and the neurites were intertwined into a relatively dense neural network, with full cell bodies and rich cytoplasm. Neurons in the propofol group showed obvious degeneration, manifesting as shortened neurites, sparse axons and dendrites, and blank space in some areas. The morphology of neurons in the propofol+dexmedetomidine group was close to that of neurons in the control group. The cell body was full and translucent, and the neurites were thick and interconnected and formed a network, with little degeneration and sparse protrusions as shown in fig. 1. Clinical studies have shown that general anesthesia in infants and young children may lead to abnormal development of the central nervous system, manifesting as changes in neuronal toxicity, impaired synaptic plasticity and indirect effects on learning and memory functions[8]. Hippocampal neuronal synapse formation is the basis of learning and memory function[9]. Because the peak period of rodent neurodevelopment is from 2 d before birth to 1 w after birth, primary hippocampal neurons were obtained from the hippocampal tissue of neonatal mice. When prepared as a single cell suspension for in vitro culture, the biological traits are closer to those in the in vivo state. Therefore, this study selected the hippocampal neurons of neonatal mice within 1 d of birth as the research object. Referring to the methods described by Ge et al.[6] and Lv et al.[7], in this study hippocampal neurons were incubated with 20 μM propofol to induce neuronal damage and the final concentration of dexmedetomidine was set to 10 μM. The results of this study suggest that compared with those in the control group, the primary hippocampal neurons in the propofol group had an abnormal morphology, with shortened neurites and sparse axons and dendrites. Compared with that in the propofol group, the proportion of neuronal degeneration and sparse neurites in the propofol+dexmedetomidine group was significantly lower. These results suggest that dexmedetomidine can reverse propofol induced damage and morphological abnormalities in hippocampal neurons as shown in fig. 2 and fig. 3. The results of previous animal studies[4] showed that postoperative spatial memory deficits occurred in rats after propofol anesthesia and that dexmedetomidine ameliorated this cognitive impairment. The results of an investigation of the molecular mechanism by hippocampal transcriptome sequencing and differential gene enrichment analysis suggested that the differential expression of mitochondrial function-related genes may be involved in the process of dexmedetomidine improving spatial memory impairment after propofol anesthesia. Anesthesia and surgery can induce a systemic stress response, followed by the massive release of neuroendocrine hormones and inflammatory mediators. However, the ability of the central nervous system to secrete antioxidant substances is relatively weak, the overall level of antioxidative stress is low and neurons are vulnerable to free radicals and other substances[10]. Hippocampal neurons are rich in unsaturated fatty acids, making these neurons particularly sensitive to external oxidative stress stimuli[11]. Mitochondria are the energy metabolism core of neurons and generate Adenosine Triphosphate (ATP) to maintain cell life activities. Oxidative stress can cause mitochondrial dysfunction by disrupting the mitochondrial membrane potential, eventually leading to neuronal damage and even apoptosis[12]. Cytochrome c oxidase (molecular weight, 12 kDa) is an important water-soluble mitochondrial protein encoded by nuclear genes. Cytochrome c is mainly involved in the mitochondrial electron transport chain, utilizing heme groups as redox intermediates to transport electrons to mitochondrial complex IV[5]. Cytochrome c is released from mitochondria to the cytoplasm when neurons are strongly stimulated (e.g., DNA damage, metabolic stress or the presence of unfolded proteins), acting as a key signaling molecule in neuroinflammation[13]. Studies have reported increased levels of cytochrome c during traumatic brain injury, cancer treatment and ischemic encephalopathy[14]. Cytochrome c can mediate glial cell activation by participating in Toll-like receptor interactions, causing neurotoxic inflammatory responses[15]. Zheng et al.[16] established a rat Postnatal Day (PND) model by performing heart bypass surgery on rats; the expression of cytochrome c in the hippocampus of the postoperative neurocognitive disorder rat model was abnormally increased compared with that in the control group. In this study, immunofluorescence staining and Western Blot (WB) were used to assess cytochrome c content and similar results were obtained; i.e., primary hippocampal neurons incubated with propofol expressed cytochrome c at high levels, but expression decreased to a level similar to that in the control group in cells coincubated with dexmedetomidine and propofol. The results suggest that dexmedetomidine significantly inhibits high cytochrome c expression in hippocampal neurons induced by propofol.
Fig. 1: Dexmedetomidine alleviates the impairment of primary hippocampal neurons caused by propofol, (A): The morphology of primary hippocampal neurons in the control group was normal; (B): Primary hippocampal neurons in the propofol group had degenerated, with sparse axons and dendrites and (C): The primary hippocampal neurons in the propofol+ dexmedetomidine group exhibited basically normal morphology. Neurites were thick and interconnected to form a network
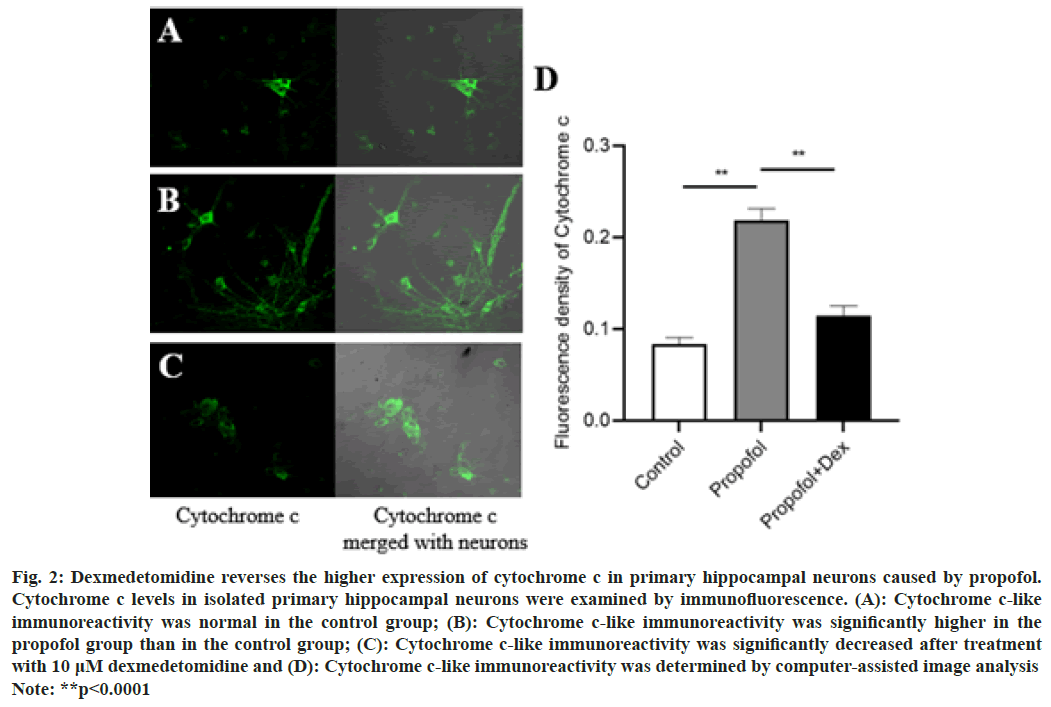
Fig. 2: Dexmedetomidine reverses the higher expression of cytochrome c in primary hippocampal neurons caused by propofol.
Cytochrome c levels in isolated primary hippocampal neurons were examined by immunofluorescence. (A): Cytochrome c-like
immunoreactivity was normal in the control group; (B): Cytochrome c-like immunoreactivity was significantly higher in the
propofol group than in the control group; (C): Cytochrome c-like immunoreactivity was significantly decreased after treatment
with 10 μM dexmedetomidine and (D): Cytochrome c-like immunoreactivity was determined by computer-assisted image analysis
Note: **p<0.0001
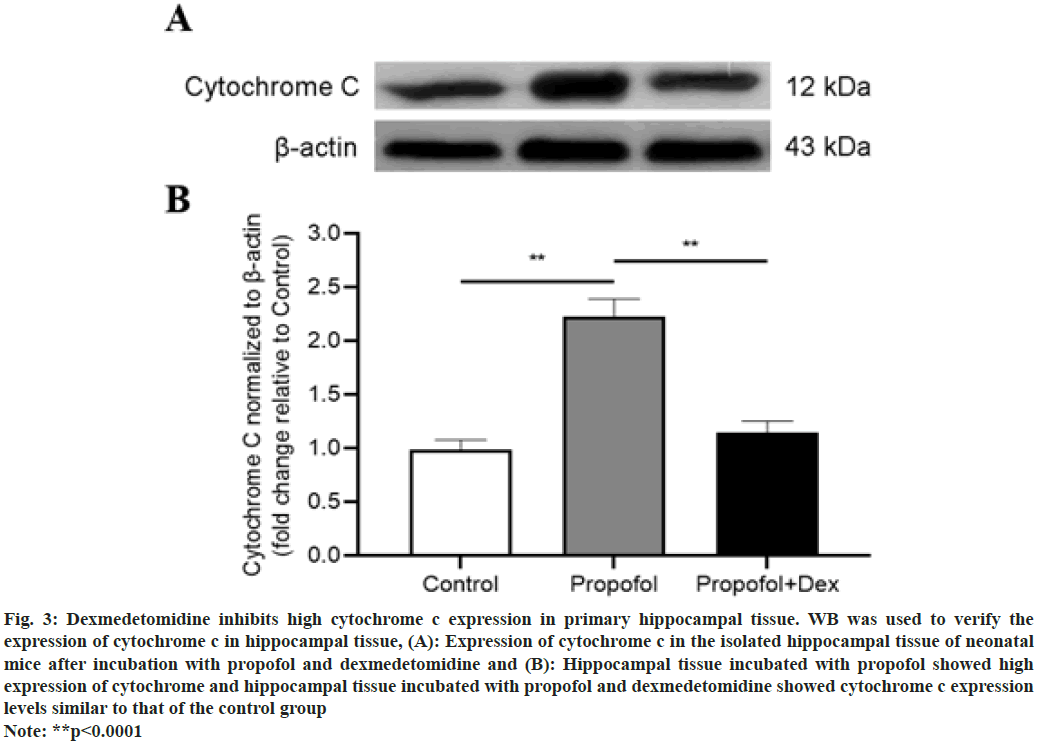
Fig. 3: Dexmedetomidine inhibits high cytochrome c expression in primary hippocampal tissue. WB was used to verify the
expression of cytochrome c in hippocampal tissue, (A): Expression of cytochrome c in the isolated hippocampal tissue of neonatal
mice after incubation with propofol and dexmedetomidine and (B): Hippocampal tissue incubated with propofol showed high
expression of cytochrome and hippocampal tissue incubated with propofol and dexmedetomidine showed cytochrome c expression
levels similar to that of the control group
Note: **p<0.0001
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist widely used in clinical anesthesia. Dexmedetomidine can reduce the stress response, maintain hemodynamic stability, and reduce or prevent the transmission of noxious stimuli and is also recognized to have antiinflammatory and neuroprotective effects[17]. Scholars have created a hypoxic brain injury rat model and found that the learning and memory abilities of the rats significantly improved after dexmedetomidine treatment, an effect that may be due to the downregulation of cytochrome c, Apoptotic protease activating factor-1 (Apaf-1) and caspase-3 protein expression by dexmedetomidine, thus exerting neuroprotective effects[18]. Scholars have also reported that dexmedetomidine can prevent synaptic loss and cognitive dysfunction in neonatal rats with hypoxic-ischemic brain injury by reducing oxidative stress, neuroinflammation and excessive autophagy in the central nervous system[19,20]. However, there are also researchers who hold the opposite view, i.e., dexmedetomidine, when used in combination with other drugs, does not reduce the neurotoxicity to the neonatal brain caused by general anesthesia, a finding that may be related to the use of inhaled anesthetic rather than intravenous propofol in that study[21]. In conclusion, dexmedetomidine can reverse propofol-induced damage and morphological abnormalities in primary hippocampal neurons and the molecular mechanism may be related to inhibiting the overexpression of the mitochondrial protein cytochrome c in the hippocampus.
Funding:
This work was supported by Science Foundation of Peking University Cancer Hospital (No.2022-13).
Author’s contributions:
Xichun Yang and Lei Yang have contributed same to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- McCann ME, Soriano SG. Does general anesthesia affect neurodevelopment in infants and children? BMJ 2019;367:16459.
[Crossref] [Google Scholar] [PubMed]
- Aksenov DP, Miller MJ, Dixon CJ, Drobyshevsky A. Impact of anesthesia exposure in early development on learning and sensory functions. Dev Psychobiol 2020;62(5):559-72.
[Crossref] [Google Scholar] [PubMed]
- Chen R, Sun Y, Lv J, Dou X, Dai M, Sun S, et al. Effects of dexmedetomidine on immune cells: A narrative review. Front Pharmacol 2022;13.
[Crossref] [Google Scholar] [PubMed]
- Niu K, Qin JL, Lu GF, Guo J, Williams JP, An JX. Dexmedetomidine reverses postoperative spatial memory deficit by targeting surf1 and cytochrome c. Neuroscience 2021;466:148-61.
[Crossref] [Google Scholar] [PubMed]
- Ow YL, Green DR, Hao Z, Mak TW. Cytochrome c: Functions beyond respiration. Nat Rev Mol Cell Biol 2008;9(7):532-42.
[Crossref] [Google Scholar] [PubMed]
- Ge J, Huang Y, Zhang Y, Liu L, Gu T, Liu X, et al. Metformin inhibits propofol-induced apoptosis of mouse hippocampal neurons HT-22 through down regulating Cav-1. Drug Des Dev Ther 2020;14:1561-9.
[Crossref] [Google Scholar] [PubMed]
- Lv J, Ou W, Zou XH, Yao Y, Wu JL. Effect of dexmedetomidine on hippocampal neuron development and BDNF-TrkB signal expression in neonatal rats. Neuropsychiatr Dis Treat 2016;12:3153-9.
[Crossref] [Google Scholar] [PubMed]
- Gascoigne DA, Serdyukova NA, Aksenov DP. Early development of the GABAergic system and the associated risks of neonatal anesthesia. Int J Mol Sci 2021;22(23):12951.
[Crossref] [Google Scholar] [PubMed]
- Benear SL, Ngo CT, Olson IR. Dissecting the fornix in basic memory processes and neuropsychiatric disease: A review. Brain Connect 2020;10(7):331-54.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Shen N, Duan X, Guo Y. An investigation of the mechanism of dexmedetomidine in improving postoperative cognitive dysfunction from the perspectives of alleviating neuronal mitochondrial membrane oxidative stress and electrophysiological dysfunction. Exp Ther Med 2018;15(2):2037-43.
[Crossref] [Google Scholar] [PubMed]
- Bai X, Song Z, Zhou Y, Pan S, Wang F, Guo Z, et al. The apoptosis of peripheral blood lymphocytes promoted by hyperbaric oxygen treatment contributes to attenuate the severity of early stage acute pancreatitis in rats. Apoptosis 2014;19(1):58-75.
[Crossref] [Google Scholar] [PubMed]
- Ning Q, Liu Z, Wang X, Zhang R, Zhang J, Yang M, et al. Neurodegenerative changes and neuroapoptosis induced by systemic lipopolysaccharide administration are reversed by dexmedetomidine treatment in mice. Neurol Res 2017;39(4):357-66.
[Crossref] [Google Scholar] [PubMed]
- Giridharan VV, Collodel A, Generoso JS, Scaini G, Wassather R, Selvaraj S, et al. Neuroinflammation trajectories precede cognitive impairment after experimental meningitis-evidence from an in vivo PET study. J Neuroinflamm 2020;17(1):1-20.
[Crossref] [Google Scholar] [PubMed]
- Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: A marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol 2007;292(2):H767-75.
[Crossref] [Google Scholar] [PubMed]
- Gouveia A, Bajwa E, Klegeris A. Extracellular cytochrome c as an intercellular signaling molecule regulating microglial functions. Biochim Biophys Acta Gen Subj 2017;1861(9):2274-81.
[Crossref] [Google Scholar] [PubMed]
- Zheng J, Min S, Hu B, Liu Q, Wan Y. Nrdp1 is involved in hippocampus apoptosis in cardiopulmonary bypass-induced cognitive dysfunction via the regulation of ErbB3 protein levels. Int J Mol Med 2019;43(4):1747-57.
[Crossref] [Google Scholar] [PubMed]
- Gao Z, Li Z, Deng R, Liu Q, Xiao Q, Han J, et al. Dexmedetomidine improves postoperative neurocognitive disorder after cardiopulmonary bypass in rats. Neurol Res 2021;43(2):164-72.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Zhang Y, Dong Y, Wu X, Liu H. Dexmedetomidine mediates neuroglobin up-regulation and alleviates the hypoxia/reoxygenation injury by inhibiting neuronal apoptosis in developing rats. Front Pharmacol 2020;11:555532.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Chen D, Li Q, Wu S, Pan J, Liao Y, et al. Dexmedetomidine alleviates hypoxia-induced synaptic loss and cognitive impairment via inhibition of microglial NOX2 activation in the hippocampus of neonatal rats. Oxid Med Cell Longev 2021;2021:6643171.
[Crossref] [Google Scholar] [PubMed]
- Xue H, Wu Z, Xu Y, Gao Q, Zhang Y, Li C, et al. Dexmedetomidine post-conditioning ameliorates long-term neurological outcomes after neonatal hypoxic ischemia: The role of autophagy. Life Sci 2021;270:118980.
[Crossref] [Google Scholar] [PubMed]
- Lee JR, Joseph B, Hofacer RD, Upton B, Lee SY, Ewing L, et al. Effect of dexmedetomidine on sevoflurane-induced neurodegeneration in neonatal rats. Br J Anaesth 2021;126(5):1009-21.
[Crossref] [Google Scholar] [PubMed]