- *Corresponding Author:
- Caijie Li
Department of Cardiovascular Medicine, Second Affiliated Hospital of Qiqihar Medical College, Qiqihar, Heilongjiang Province 161006, China
E-mail: licaijie6789@163.com
| Date of Received | 03 February 2023 |
| Date of Revision | 05 September 2023 |
| Date of Acceptance | 24 January 2024 |
| Indian J Pharm Sci 2024;86(1):193-201 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To study the effects of paeoniflorin on the behavioral manifestations, levels of neurotransmitters in the hippocampus, and axial response in an anxiety model of acute myocardial infarction. 21 rats were randomly divided into 3 groups; control group, acute myocardial infarction+anxiety group, and acute myocardial infarction+anxiety+paeoniflorin group, with 7 rats in each group. An acute myocardial infarction model was constructed by ligating the left anterior descending coronary artery in rats, and mild stress was used to induce anxiety in rats. In the open field test, compared with the horizontal and vertical exercise of the control group, the acute myocardial infarction+anxiety group decreased, and the acute myocardial infarction+anxiety+paeoniflorin group increased compared with the horizontal and vertical exercise of acute myocardial infarction+anxiety group. In the elevated test, compared with the control group, the time of staying at the open arm and entering the open arm was decreased in the acute myocardial infarction+anxiety group, and increased in the acute myocardial infarction+anxiety+paeoniflorin group compared with the acute myocardial infarction+anxiety group. Compared with the control group, chemokine receptor type 4, interleukin-1 beta, interleukin-18 and norepinephrine increased and C-X-C motif chemokine ligand 12 decreased in acute myocardial infarction+anxiety group. Compared with acute myocardial infarction+anxiety group, chemokine receptor type 4, interleukin-1 beta, interleukin-18 and norepinephrine increased and C-X-C motif chemokine ligand 12 decreased in acute myocardial infarction+anxiety+paeoniflorin group. Compared with the control group, 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in acute myocardial infarction+anxiety group were higher than those in control group, acute myocardial infarction+anxiety+paeoniflorin 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in acute myocardial infarction+anxiety group were significantly higher than those in acute myocardial infarction+anxiety group, C-X-C motif chemokine ligand 12 and chemokine receptor type 4 protein expression in acute myocardial infarction+anxiety group was higher than that in control group, while acute myocardial infarction+anxiety+paeoniflorin C-X-C motif chemokine ligand 12 and chemokine receptor type 4 protein expression was lower than that in acute myocardial infarction+anxiety group. Paeoniflorin has anti-anxiety effect, can restore the balance of neurotransmitters in hippocampus, and can improve the inflammatory response of acute myocardial infarction with anxiety model.
Keywords
Acute myocardial infarction, anxiety model, paeoniflorin, neurotransmitter, hippocampus
Acute Myocardial Infarction (AMI) is a life-threatening disease characterized by obstruction of myocardial blood flow[1]. Its clinical manifestations include not only cardiac dysfunction, but also anxiety and other complications[2]. In patients with AMI, anxiety model behavior can reduce the level of anxiety through cognitive behavioral therapy, psychological education and social support. Hippocampal neurotransmitters also have an important effect on anxiety in patients with AMI[3]. The hippocampus is a part of the brain that participates in functions such as memory and mood regulation. Some studies have shown that AMI may lead to changes in neurotransmitters in the hippocampus, leading to anxiety and other psychological symptoms[4]. Glutamate, glycine and serotonin may show abnormal levels in patients with AMI[5]. Changes in these neurotransmitters may be associated with anxiety. C-X-C motif Chemokine Ligand 12 (CXCL12) is a chemokine, and Chemokine Receptor Type 4 (CXCR4) is its receptor. CXCL12/CXCR4 axis plays an important role in various physiological and pathological processes such as neurodevelopment, neuroinflammation and nervous system diseases[6]. In the early days of AMI, CXCL12 was usually added through a variety of mechanisms. Myocardial ischemia causes the release of cytokines and chemokines, and then activates the CXCL12/ CXCR4 signal pathway. This process may play a role in inflammatory cells and other related cell types, leading to inflammatory response and reperfusion injury. Some studies have found that paeoniflorin has anti-inflammatory, neuroprotective and anti- anxiety effects[3-5]. However, the mechanism of its effect on AMI with anxiety model is not clear. Based on CXCL12/CXCR4 axis, this study will explore the effects of paeoniflorin on behavior, hippocampal neurotransmitters and axial response in anxiety model of AMI.
Materials and Methods
General information:
A total of 21 male Sprague Dawley (SD) rats of Specific-Pathogen Free (SPF) grade, weighing 440~500 g, were selected and examined by animal ethics in the experimental animal center. Echocardiography, elevated maze test equipment, Enzyme-Linked Immunosorbent Assay (ELISA) instrument, high performance liquid chromatography, electrochemical detection equipment, neurotransmitter 5-Hydroxytryptamine (5-HT) and 5-Hydroxyindoleacetic Acid (5-HIAA) detection reagent.
Model preparation:
AMI model: Myocardial infarction was simulated by ligating the left anterior descending coronary artery in rats. Using 2.5 % isoflurane anesthesia (1 l/min), the rats were placed in a 20°-26° incubator with a relative humidity of 50 %-60 % to maintain a stable body temperature. Disinfect the skin on the left 3 and 4 intercostal areas of the sternum, open the chest and prepare for surgery. The left anterior descending coronary artery is exposed through a small incision and the coronary artery is ligated with sutures in place. Make sure the coronary artery is completely occluded and postoperative intraperitoneal injection of 0.2 ml lidocaine to prevent arrhythmia, 400 000 U of penicillin to prevent postoperative infection, and effective measures to keep warm after operation. The electrocardiogram examination showed that the Q wave modeling was successful on the 2nd d after operation.
Anxiety model:
22 d after the establishment of the model, anxiety in rats was induced by mild stress methods such as ordinary noise, hot and cold stimulation and light changes, and simulated situations that might lead to anxiety in real life.
Compound model:
Myocardial infarction model was established for 15 d after the establishment of anxiety model, mild stress such as common noise, hot and cold stimulation and light changes continued to be given, and behavior was evaluated for 23 d.
Mode of administration and grouping:
21 rats were randomly divided into three groups; control group, AMI+anxiety group and AMI+anxiety+paeoniflorin group with an average of 7 rats in each group. Paeoniflorin (production batch number: 110736-202145, Chengdu Pusi Biotechnology Co., Ltd.) 1 mg/ml was administered orally for 4 w, while the control group was treated with normal saline.
Observation index and method:
Echocardiographic examination: Ensure the normal operation of the echocardiography machine, including calibration and debugging. The rats were fixed on the examination table to make the position of the heart exposed and easy to observe. Anesthetics or sedatives are appropriately recommended to reduce exercise or discomfort in rats. The ultrasonic probe was placed on the left side of the sternum of the rat, and the structure of the heart was observed by properly adjusting the position and angle of the probe. The structures of ventricular wall, ventricular cavity, valve and pericardium were observed by two-dimensional model, and the quantitative and qualitative analysis was carried out. The motion mode was used to observe the amplitude and velocity of cardiac wall motion. Left Ventricular Ejection Fraction (LVEF), Left Ventricular Fraction Shortening (LVFS), Left Ventricular End-Diastolic Diameter (LVID; d) and LVID; s were measured to evaluate cardiac pumping function. LVEF indicates the percentage of blood ejected from the left ventricle as a percentage of the total filling volume during each contraction of the heart, and LVFS indicates the contraction amplitude of the left ventricular wall during each contraction.
Using 100 cm×100 cm×40 cm wooden black box, the bottom plate is 25 squares, first adapt to the mobile 8 min in the wooden black box, in the bottom box, record the vertical movement and movement scores, and observe the 5 min activity.
Elevated Maze (EMP) experiment:
Two relative open arms (50 cm×10 cm) and relative closed arms (50 cm×10 cm×40 cm) are composed. There is an open area (10 cm×10 cm) in the center, and the cross maze is 50 cm from the ground. Set up video recording equipment to record the behavior of rats in the maze. Before the start of the experiment, the rats were placed in an environment similar to an elevated maze. Make sure that the light in the laboratory is moderate, usually weak, so as not to cause unnecessary pressure or interference. The rats were placed separately at the central starting point of the maze, usually on a closed arm. The behavior of rats in the maze was observed, including the number of times to enter the open arm (OE), the stay time (OT) and the number of closed arm (CE) OT. Continuous observation and recording of behavior was for 5-10 min. The ratio of OE/(OE+CE) to OT/(OT+CT) was calculated.
Observation of pathological changes of hippocampus in rats by Nissl-staining: Prepare samples containing rat hippocampal tissue. Thin sections of 30-40 microns were prepared from rat brain tissue. Take out the required number of slices and place them on glass slides. The slices in the slide were immersed in methanol and soaked for 5 min to remove fat. Remove the slices from methanol and wash them gently in distilled water. Put the slices in Nissl-staining solution and soak in the staining solution for 10-15 min. Transfer the staining section to 75 % ethanol and remove the excess staining solution. Wash the decolorized slices gently with distilled water. The slices were gradually dehydrated by soaking in 95 % ethanol and 100 % ethanol. Soak the slices in Kodak trimming glue, cover them with glass slides, and finally dry the slides. Cover the slice on the slide with a cover slide and seal it with sealing glue.
ELISA:
After anesthesia, the abdominal cavity of the rats was fixed and exposed, and the blood was collected from the abdominal aorta for 10 min. The serum was collected after centrifugation and operated according to the instructions of the ELISA detection kit.
Detection of 5-HT and 5-HIAA in hippocampus of mice:
The brain tissue samples of mice stored under frozen conditions were taken. Use a mechanical tissue breaker to break the sample evenly. Adding proper amount of extraction buffer, 5-HT and 5-HIAA were extracted by Hydrochloric acid (HCl). The samples were incubated at 4° for a period of time, and 5-HT and 5-HIAA were extracted. The extracted samples are centrifuged using a high-speed centrifuge to separate cell fragments and other impurities. Transfer the supernatant containing 5-HT and 5-HIAA to a new tube. 5-HT and 5-HIAA usually need to be derivatized with hexafluoroacetone to increase the sensitivity and stability of their detection. Efficient Liquid Chromatography–Mass Spectrometry (LC– MS)/MS technology is used to analyze the derived 5-HT and 5-HIAA.
According to the output data of the instrument, the concentrations of 5-HT and 5-HIAA (mol/l) were calculated.
Western blot:
Protein samples were extracted and dissolved with Radioimmunoprecipitation Assay (RIPA) cell lysate, and protease inhibitors and phosphatase inhibitors were added. Shake the sample with an ultrasonic oscillator for 10 min, then centrifuge at 4° for 10 min collect the supernatant. The extracted protein samples were mixed with appropriate concentration of Laemmli buffer and heated in a hot water bath for 5 min. The sample was loaded into the Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel pore and the protein sample was electrophoretic at appropriate voltage using an electrophoretic apparatus. The separated protein was transferred to Polyacrylamide Membrane (PVDF) by wet transfer method. Before transferring the membrane, the PVDF membrane was pretreated for 15 min, soaked in methanol, and wetted with standard transfer buffer. The PVDF membrane was pretreated with Tris-Buffered Saline with 0.1 % Tween® 20 (TBST) buffer, and then the non-specific binding was blocked in 5 % skimmed milk powder. The specific antibody was diluted to an appropriate concentration, and the TBST buffer containing 0.1 % skimmed milk powder was used to incubate the membrane overnight. Wash the membrane to remove unbound primary antibodies and incubate the membrane with properly diluted Horseradish Peroxidase (HRP)-labeled secondary antibodies for 1 h to detect the binding of primary antibodies. Enhanced Chemiluminescence Reagent (ECL) was used for development. The ECL reagent was sprayed or dripped evenly on the membrane and exposed in the darkroom for 3-4 min. Capture and record images of protein bands using chemiluminescence imaging system.
Statistical analysis:
Statistical analysis is carried out by using Statistical Package for the Social Sciences (SPSS) 17.0 software package, and the measurement data are expressed by (x͞ ±s); the counting data rate is expressed, and the comparison is done by Chi-square (χ2) test. The single factor between groups was tested by Least Significant Difference (LSD)-t test, and the difference was statistically significant. Compared with control group, ap<0.05 and compared with AMI+anxiety group bp<0.05.
Results and Discussion
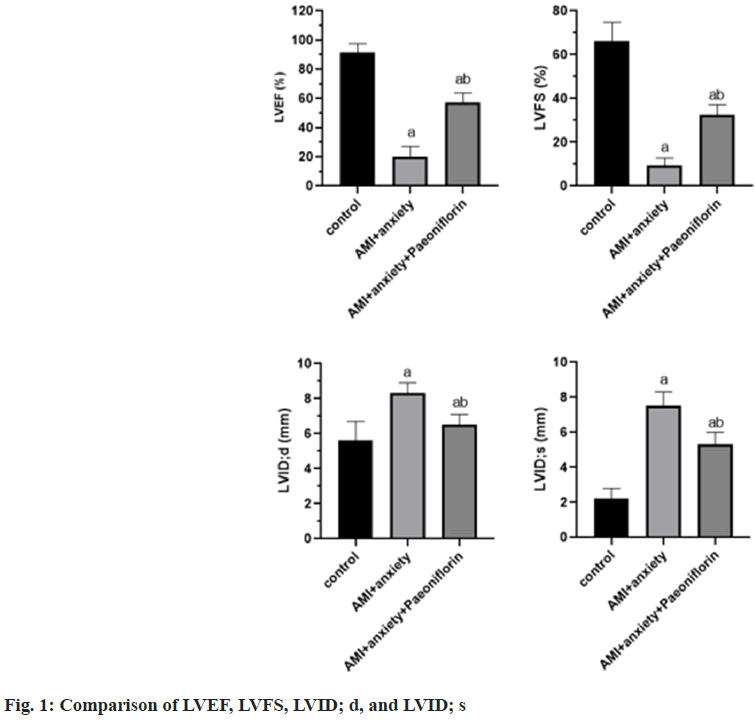
Compared with the control group, LVEF and LVFS were significantly decreased and LVID was significantly increased in AMI+anxiety group. LVID; d, compared with AMI+anxiety group, LVEF and LVFSS in AMI+anxiety+paeoniflorin group were higher, LVID; d, LVID; s were significantly decreased (Table 1 and fig. 1).
| Group | LVEF (%) | LVFS (%) | LVID; d (mm) | LVID; s (mm) |
|---|---|---|---|---|
| Control | 91.8±5.8 | 66.3±8.4 | 5.6±1.1 | 2.2±0.6 |
| AMI+anxiety | 20.1±7.1a | 9.2±3.5a | 8.3±0.6a | 7.5±0.8a |
| AMI+anxiety+paeoniflorin | 57.3±6.5ab | 32.5±4.6ab | 6.5±0.6ab | 5.3±0.7ab |
Note: ap<0.05, compared with AMI+anxiety and bp<0.05, compared with AMI+anxiety+paeoniflorin group
Table 1: Echocardiographic Indexes of Rats in Each Group (x͞ ±s)
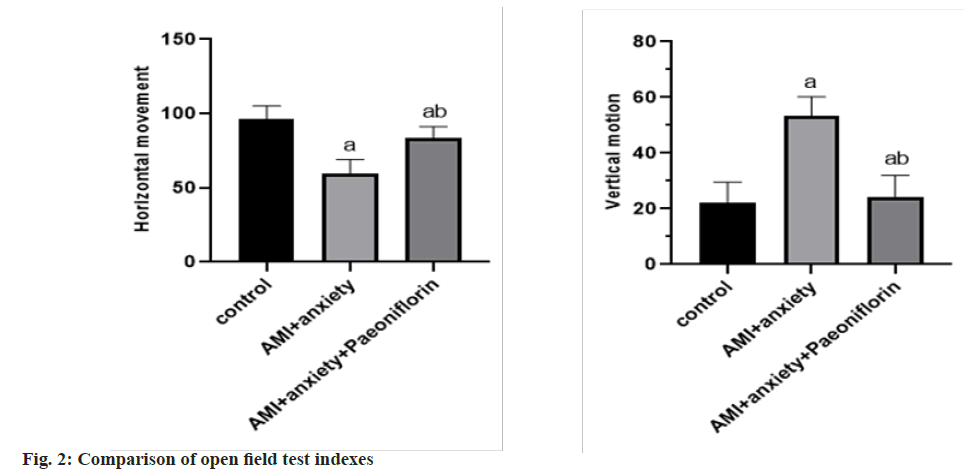
In the open field test, compared with the horizontal exercise and vertical exercise of the control group, the AMI+anxiety group decreased and the AMI+anxiety+paeoniflorin group increased compared with the horizontal and vertical exercise of AMI+anxiety group as shown in Table 2 and fig. 2.
| Group | Horizontal movement | Vertical motion |
|---|---|---|
| Control | 96.5±8.7 | 22.3±7.2 |
| AMI+anxiety | 59.5±9.6a | 53.3±6.9a |
| AMI+anxiety+paeoniflorin | 83.6±7.6ab | 24.2±7.8ab |
Note: ap<0.05, compared with AMI+anxiety and bp<0.05, compared with AMI+anxiety+paeoniflorin group
Table 2: Comparison of Behavioral Indexes of Rats in Different Groups
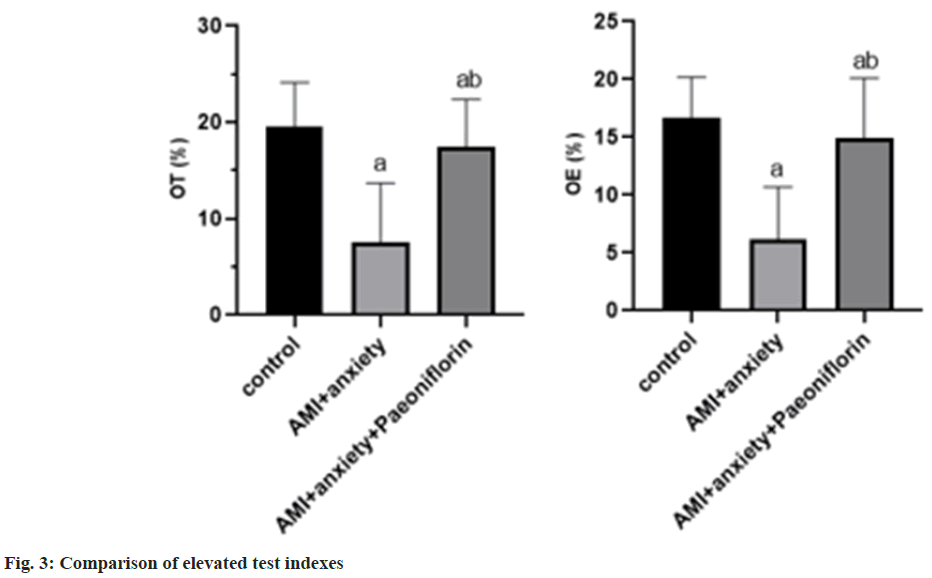
In the elevated test, compared with the control group, the time of staying at the open arm and entering the open arm was creased in the AMI+anxiety group, and increased in the AMI+anxiety+paeoniflorin group compared with the AMI+anxiety group as shown in Table 3 and fig. 3.
| Group | OT (%) | OE (%) |
|---|---|---|
| Control | 19.5±4.6 | 16.7±3.5 |
| AMI+anxiety | 7.5±6.2a | 6.2±4.5a |
| AMI+anxiety+paeoniflorin | 17.5±4.9ab | 14.9±5.2ab |
Note: ap<0.05, compared with AMI+anxiety and bp<0.05, compared with AMI+anxiety+paeoniflorin group
Table 3: Comparison of Elevated Test Indexes of Rats in Each Group
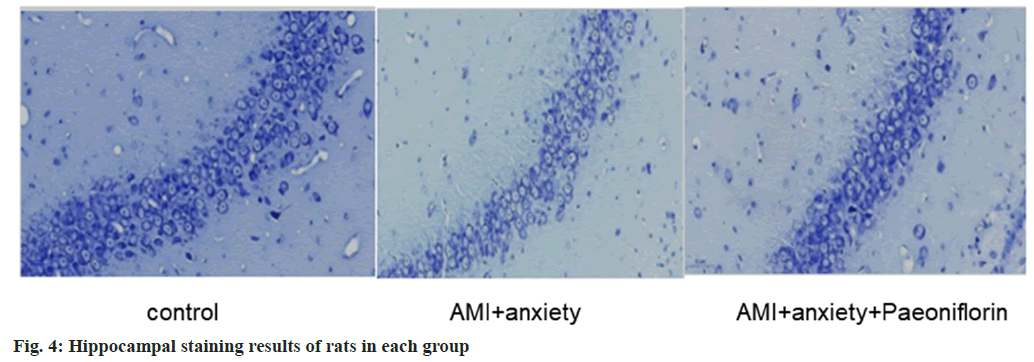
AMI and anxiety state lead to structural damage in the hippocampus, including neuronal loss and morphological changes. AMI+anxiety+paeoniflorin group alleviates the structure of hippocampal region, as shown in fig. 4.
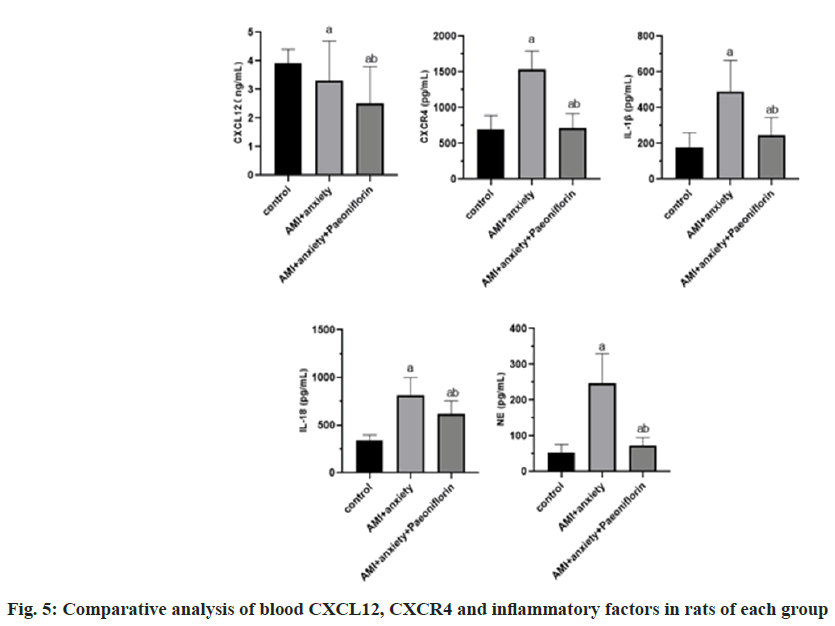
Compared with the control group, CXCR4, Interleukin-1 Beta (IL-1β), IL-18 and Norepinephrine (NE) in AMI+anxiety group were significantly increased, while CXCL12 was decreased, compared with AMI+anxiety group, CXCR4, IL-1β, IL-18 and NE in AMI+anxiety+paeoniflorin group were increased, while CXCL12 was significantly decreased (Table 4 and fig. 5).
| Group | CXCL12 (ng/ml) | CXCR4 (pg/ml) | IL-1β (pg/ml) | IL-18 (pg/ml) | NE (pg/ml) |
|---|---|---|---|---|---|
| Control | 3.9±0.5 | 693.5±196.8 | 178.3±81.2 | 337.5±61.1 | 52.6±22.9 |
| AMI+anxiety | 3.3±1.4a | 1536.5±256.8a | 489.3±175.2a | 815.1±184.3a | 246.3±83.2a |
| AMI+anxiety+paeoniflorin | 2.5±1.3ab | 717.6±198.5ab | 244.5±99.7ab | 621.5±134.2ab | 72.1±22.9ab |
Note: ap<0.05, compared with AMI+anxiety and bp<0.05, compared with AMI+anxiety+paeoniflorin group
Table 4: Comparative Analysis of CXCL12, CXCR4 and Inflammatory Factors in Blood of Rats in Each Group
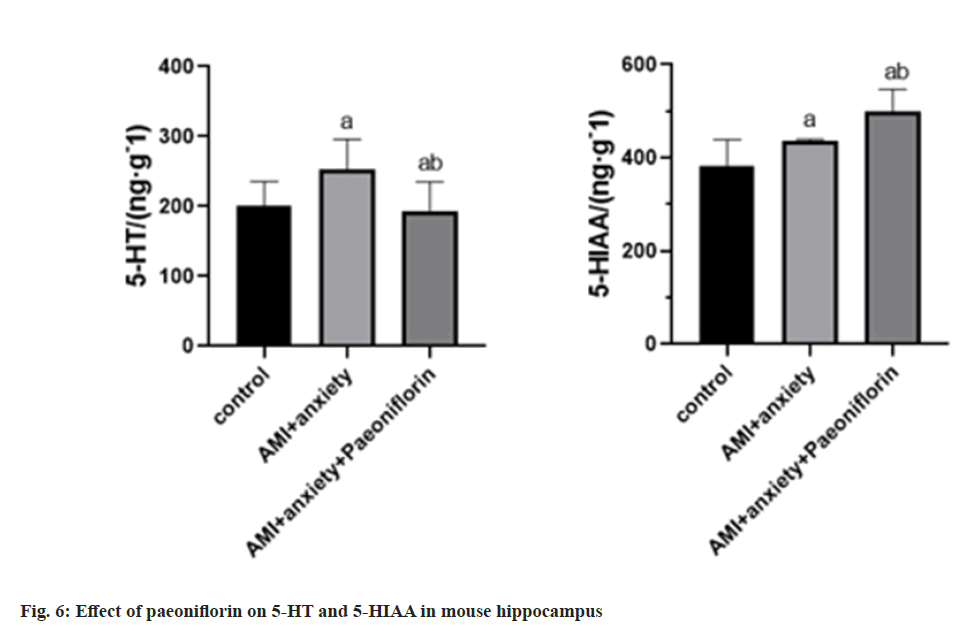
Compared with the control group, 5-HT and 5-HIAA were higher in AMI+anxiety group compared with AMI+anxiety group, AMI+anxiety+paeoniflorin 5-HT and 5-HIAA were higher than those in AMI+anxiety group (Table 5 and fig. 6).
| Group | 5-HT/(ng·g-1) | 5-HIAA/(ng·g-1) |
|---|---|---|
| Control | 200.4±35.27 | 381.1±58.2 |
| AMI+anxiety | 253.6±42.7a | 435.4±41.3a |
| AMI+anxiety+paeoniflorin | 293.7±41.5ab | 498.5±48.4ab |
Note: ap<0.05, compared with AMI+anxiety and bp<0.05, compared with AMI+anxiety+paeoniflorin group
Table 5: Effects of Paeoniflorin on 5-HT and 5-HIAA in Hippocampus of Mice
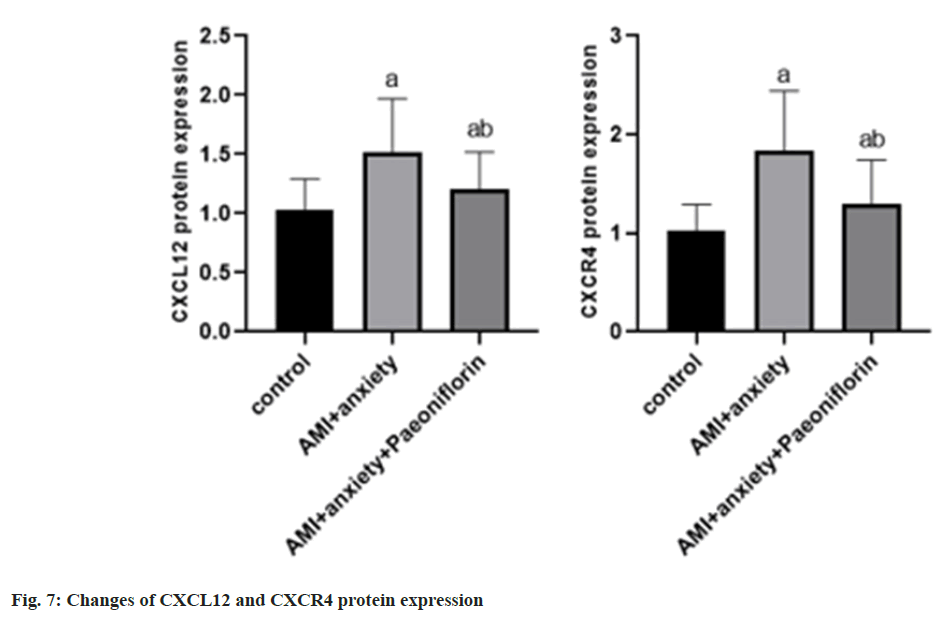
Compared with the control group, the expressions of CXCL12 and CXCR4 proteins in AMI+anxiety group were increased compared with AMI+anxiety group, CXCL12 and CXCR4 protein expressions of paeoniflorin in AMI+anxiety+paeoniflorin were decreased (Table 6 and fig. 7).
| Group | CXCL12 | CXCR4 |
|---|---|---|
| Control | 1.03±0.26 | 1.03±0.26 |
| AMI+anxiety | 1.52±0.45a | 1.83±0.61a |
| AMI+anxiety+paeoniflorin | 1.21±0.31ab | 1.29±0.45ab |
Note: ap<0.05, compared with AMI+anxiety and bp<0.05, compared with AMI+anxiety+paeoniflorin group
Table 6: Changes of CXCL12 and CXCR4 Protein Expression
AMI is caused by myocardial ischemia, necrosis and inflammation caused by coronary artery occlusion. Anxiety is a common mental symptom in patients with myocardial infarction[7]. The behavioral study of anxiety model is a method to evaluate the behavior changes of animals in the state of anxiety. In the behavioral study of anxiety model of AMI, the degree of anxiety can be evaluated by analyzing the behavior of animals. AMI may cause changes in anxiety behavior. Some studies have found that[8], rats after AMI showed obvious anxiety behavior in open field and light conditions, such as reducing exploratory behavior and increasing hair removal behavior. The changes of neurotransmitters in hippocampus may play a role in anxiety caused by AMI. Hippocampal neurotransmitters include dopamine, Gamma (γ)-aminobutyric acid and 5-HT, which are closely related to the occurrence of anxiety. Research findings[9], AMI can lead to the disorder of hippocampal neurotransmitters, thus aggravating anxiety symptoms. For example, after AMI, the content of 5-HT in hippocampus decreased significantly, while the content of γ-aminobutyric acid increased significantly. CXCL12/CXCR4 axis is a signal transduction pathway between cytokines and receptors, which is involved in the occurrence and development of many diseases[10]. The potential role of CXCL12/ CXCR4 axis in myocardial infarction is mainly related to inflammatory response and myocardial regeneration[11]. CXCL12 can attract CXCR4 positive bone marrow mesenchymal stem cells to migrate to the infarcted myocardium, thus promoting myocardial regeneration and repair[12]. CXCL12/CXCR4 axis also plays an important role in the progression of coronary artery disease. CXCR4 positive endothelial progenitor cells promote angiogenesis through CXCL12 signaling and reduce coronary artery stenosis and poor blood flow[13].
Paeoniflorin has a certain therapeutic effect on cardiovascular diseases[14]. Paeoniflorin has a significant antidepressant effect and can alleviate the depressive symptoms of model animals. Paeoniflorin can regulate the level of neurotransmitters in the brain, such as serotonin, norepinephrine, etc., so as to play an antidepressant effect. In the behavioral test, the anxiety behavior of AMI+anxiety group was significantly higher than that of the control group, while the anxiety behavior of AMI+anxiety+paeoniflorin group was significantly decreased. Biochemical examination showed that the levels of CXCR4, IL-1β, IL-18 and NE in AMI with anxiety group increased significantly, while the level of CXCL12 decreased. However, after paeoniflorin treatment, these indicators were reversed, suggesting that paeoniflorin may reduce anxiety symptoms by regulating the CXCL12/ CXCR4 axis and related inflammatory factors. Paeoniflorin can protect cardiomyocytes from injury by reducing inflammatory reaction and oxidative stress. Paeoniflorin can also prevent thrombosis by inhibiting thrombosis and platelet aggregation[15]. Paeoniflorin can increase the content of 5-HT in the hippocampus of rats. 5-HT is a neurotransmitter that regulates emotional and cognitive function and plays an important role in the hippocampus. This increase of paeoniflorin may be achieved by promoting the release of 5-HT or reducing the metabolism of 5-HT. 5-HIAA is the metabolite of 5-HT, and its level can indirectly reflect the metabolic activity of 5-HT. To sum up, paeoniflorin has anti-anxiety effect, can restore the balance of neurotransmitters in hippocampus, and can improve the inflammatory response of AMI and anxiety model.
Acknowledgement:
Innovation incentive plan of Qiqihar Science and Technology Bureau, (No: CSFGG-2020155).
Conflict of interests:
The authors declared no conflict of interests.
References
- Stepinska J, Lettino M, Ahrens I, Bueno H, Garcia-Castrillo L, Khoury A, et al. Diagnosis and risk stratification of chest pain patients in the emergency department: Focus on acute coronary syndromes. A position paper of the acute cardiovascular care association. Eur Heart J Acute Cardiovasc Care 2020;9(1):76-89.
[Crossref] [Google Scholar] [PubMed]
- Wilhelm AB, Pinsky S, Ahmad S, Cunningham A, Chatila K, Boor PJ, et al. Endomyocardial biopsy facilitates diagnosis of eosinophilic granulomatosis with polyangiitis (EGPA): A case report. Cardiovasc Pathol 2022;58:107407.
[Crossref] [Google Scholar] [PubMed]
- Guo W, Yao X, Cui R, Yang W, Wang L. Mechanisms of paeoniaceae action as an antidepressant. Front Pharmacol 2023;13:934199.
- Li LC, Kan LD. Traditional Chinese medicine for pulmonary fibrosis therapy: Progress and future prospects. J Ethnopharmacol 2017;198:45-63.
[Crossref] [Google Scholar] [PubMed]
- An D, Kochanska G. Theory of mind as a mechanism that accounts for the continuity or discontinuity of behavioral inhibition: A developmentally informed model of risk for social anxiety. Res Child Adolesc Psychopathol 2021;49(10):1333-44.
[Crossref] [Google Scholar] [PubMed]
- Tang H, Xu Y, Zhang Z, Zeng S, Dong W, Jiao W, et al. SDF-1/CXCR4 induces epithelial-mesenchymal transition through activation of the Wnt/β-catenin signaling pathway in rat chronic allograft nephropathy. Mol Med Rep 2019;19(5):3696-706.
[Crossref] [Google Scholar] [PubMed]
- Yamada T, Hattori H, Kikuchi N, Haruki S, Minami Y, Ichihara Y, et al. Acute myocardial infarction and sudden cardiac arrest caused by anomalous left coronary artery arising from the noncoronary cusp. J Cardiol Cases 2022;25(5):312-5.
[Crossref] [Google Scholar] [PubMed]
- Seifi M, Hamedi R, Khavandegar Z. The effect of thyroid hormone, prostaglandin E2, and calcium gluconate on orthodontic tooth movement and root resorption in rats. J Den 2015;16(1):35-42.
[Google Scholar] [PubMed]
- Saito R, Koyama K, Kongoji K, Soejima K. Acute myocardial infarction with simultaneous total occlusion of the left anterior descending artery and right coronary artery successfully treated with percutaneous coronary intervention. BMC Cardiovasc Dis 2022;22(1):1-9.
- Daniel SK, Seo YD, Pillarisetty VG. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin Cancer Biol 2020;65:176-88.
[Crossref] [Google Scholar] [PubMed]
- Han L, Zhang L. CCL21/CCR7 axis as a therapeutic target for autoimmune diseases. Int Immunopharmacol 2023;121:110431.
[Crossref] [Google Scholar] [PubMed]
- He X, Li C, Yin H, Tan X, Yi J, Tian S, et al. Mesenchymal stem cells inhibited the apoptosis of alveolar epithelial cells caused by ARDS through CXCL12/CXCR4 axis. Bioengineered 2022;13(4):9060-70.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Li J, Lei W, Wang H, Ni Y, Liu Y, et al. CXCL12-CXCR4/CXCR7 axis in cancer: From mechanisms to clinical applications. Int J Biol Sci 2023;19(11):3341.
[Crossref] [Google Scholar] [PubMed]
- Zhang XE, Pang YB, Bo Q, Hu SY, Xiang JY, Yang ZR, et al. Protective effect of paeoniflorin in diabetic nephropathy: A preclinical systematic review revealing the mechanism of action. Plos One 2023;18(9):e0282275.
[Crossref] [Google Scholar] [PubMed]
- Zhu Z, Wang L, Guo R, Pang D, Wang W, Wu Y, et al. XJ-8, a natural compound isolated from Sanguis draxonis, inhibits platelet function and thrombosis by targeting MAP3K3. J Thromb Haemos 2022;20(3):605-18.
[Crossref] [Google Scholar] [PubMed]