- *Corresponding Author:
- Junjie Lin
Department of Anesthesiology, Ningbo Yinzhou Hospital, Ningbo, Zhejiang Province 315192, China
E-mail: 165607507@qq.com
| Date of Received | 30 October 2021 |
| Date of Revision | 24 September 2022 |
| Date of Acceptance | 03 March 2023 |
| Indian J Pharm Sci 2023;85(2):338-343 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect and related mechanism of propofol anesthesia on pyroptosis in neonatal rat hippocampus. Neonatal rats were divided into a control group (injected intraperitoneally with saline), a propofol group (injected intraperitoneally with 80 mg/kg propofol) and a propofol+MCC950 group (injected intraperitoneally with 80 mg/kg propofol and 10 mg/kg MCC950) and administered for 5 d. The day after the end of drug administration, hippocampi were analyzed for pyroptosis by transferase dUTP nick end labeling assay and for interleukin beta-1, interleukin-18 levels, nod-like receptor protein 3, ASC and caspase-1 protein expression in the hippocampus were determined by Western blotting; at the end of drug administration on the 19th d, the escape latency, the number of crossing the platform and the percentage of time spent in the target quadrant were recorded in the water maze test. Pyroptosis, levels of interleukin-beta and interleukin-18 and the protein levels of nod-like receptor protein 3, ASC and caspase-1, as well as the escape latency of the rats of hippocampal tissues were significantly higher in the propofol group than those in the control group (p<0.05) and the percentage of time crossing the platform and staying in the target quadrant was significantly lower (p<0.05) in the propofol group than those in the control group; pyroptosis, levels of interleukin-beta and interleukin-18 and the protein levels of nod-like receptor protein 3, ASC and caspase-1, as well as the escape latency of the rats of propofol+MCC950 group were significantly lower in the propofol group than those in the control group (p<0.05), whereas the number of crossing the platform and the percentage of time spent in the target quadrant were significantly higher (p<0.05). Propofol anesthesia may trigger inflammatory responses by activating the nod-like receptor protein 3/caspase-1 pathway, which in turn induces pyroptosis in the neonatal rat hippocampus, leading to reduced learning and memory performance.
Abstract
To investigate the effect and related mechanism of propofol anesthesia on pyroptosis in neonatal rat hippocampus. Neonatal rats were divided into a control group (injected intraperitoneally with saline), a propofol group (injected intraperitoneally with 80 mg/kg propofol) and a propofol+MCC950 group (injected intraperitoneally with 80 mg/kg propofol and 10 mg/kg MCC950) and administered for 5 d. The day after the end of drug administration, hippocampi were analyzed for pyroptosis by transferase dUTP nick end labeling assay and for interleukin beta-1, interleukin-18 levels, nod-like receptor protein 3, ASC and caspase-1 protein expression in the hippocampus were determined by Western blotting; at the end of drug administration on the 19th d, the escape latency, the number of crossing the platform and the percentage of time spent in the target quadrant were recorded in the water maze test. Pyroptosis, levels of interleukin-beta and interleukin-18 and the protein levels of nod-like receptor protein 3, ASC and caspase-1, as well as the escape latency of the rats of hippocampal tissues were significantly higher in the propofol group than those in the control group (p<0.05) and the percentage of time crossing the platform and staying in the target quadrant was significantly lower (p<0.05) in the propofol group than those in the control group; pyroptosis, levels of interleukin-beta and interleukin-18 and the protein levels of nod-like receptor protein 3, ASC and caspase-1, as well as the escape latency of the rats of propofol+MCC950 group were significantly lower in the propofol group than those in the control group (p<0.05), whereas the number of crossing the platform and the percentage of time spent in the target quadrant were significantly higher (p<0.05). Propofol anesthesia may trigger inflammatory responses by activating the nod-like receptor protein 3/caspase-1 pathway, which in turn induces pyroptosis in the neonatal rat hippocampus, leading to reduced learning and memory performance.
Keywords
Propofol, neonatal rats, hippocampus, pyroptosis, nod-like receptor protein 3/caspase-1
Propofol, a new type of rapid and effective intravenous anesthetics with the advantages of few adverse effects and fast ghostwriting clearance, is currently one of the most widely used intravenous anesthetics in the clinic[1]. Pediatric patients are unable to cooperate actively with anesthesia for their own reasons, resulting in pediatric surgery often requiring general anesthesia for surgical treatment[2].
However, the child’s neurological function is in its developmental stage and inappropriate anesthesia will lead to neurological impairment, which will eventually affect long-term learning and memory function[3]. Studies have found that propofol causes apoptosis of neurons in the brain of animals during development, triggering cognitive dysfunction, but the specific mechanism of action is currently unknown[4]. Studies have argued that propofol caused apoptosis of hippocampal neurons in rats during development is associated with inflammatory responses[5,6]. Nod-Like Receptor Protein 3 (NLRP3)/ caspase-1 pathway is an important inflammatory response pathway that mediates pyroptosis and induces inflammatory cascades[7,8]. Studies have shown that the NLRP3/caspase-1 pathway plays an important role during brain injury in rats with orthotropic liver transplantation[9]. This study will investigate the effects and related mechanisms of propofol anesthesia on pyroptosis in the neonatal rat hippocampus.
Materials and Methods
Materials:
Experimental animals: Specific-Pathogen Free (SPF) grade Sprague Dawley (SD) male rats (7 d old, weighing 9-13 g) were purchased from Liaoning Changsheng Biotechnology Co., Ltd., license No.: SCXK (L) 2018-0015, housed in an incubator.
Main reagents: Propofol (Cat No.: T1300) was purchased from target molecule Corp, United States of America (USA); the NLRP3 inhibitor MCC950 (Cat No: T6887) was purchased from TargetMol, USA; Transferase dUTP Nick End Labeling (TUNEL) kit (Cat No.: AT2190-50T) was purchased from Shanghai ACMEC Biochemical Technology Co., Ltd.; Interleukin-1 beta (IL-1β), Interleukin-18 (IL-18) detection kit (Cat No.: YAD1545 and YAD1758) was purchased from Beijing Jiehui Bogao Biotechnology Co., Ltd.; Bicinchoninic Acid (BCA) protein assay kit (Cat No.: 701780-480) was purchased from Cayman, USA; primary antibodies NLRP3 antibody, ASC antibody, caspase-1 antibody, Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) antibody and secondary antibodies Immunoglobulin G (IgG) antibody (Cat No.: ab263899, ab175449, ab138483, ab59164 and ab6721) were purchased from Abcam.
Main instruments: Microscope (Model: CKX41SF) was purchased from Olympus corporation, Japan; Enzyme-Linked Immunosorbent Assay (ELIASA) (Model: SPECTRAmax®M2e) was purchased from molecular devices, USA; the gel scanning imaging system (Model No: TH-690B) was purchased from Beijing Tianheng.
Methods:
Animal grouping and administration: The neonatal rats were randomly divided into a control group, propofol group and propofol+MCC950 group with 26 rats in each group. Rats in the propofol group were administered 80 mg/kg propofol intraperitoneally[10], rats in the propofol+MCC950 group were administered 80 mg/kg propofol and 10 mg/kg MCC950 intraperitoneally[11] and rats in the control group were administered normal saline intraperitoneally at 150 μl, once daily for 5 d, after each injection and put it back into the incubator for feeding.
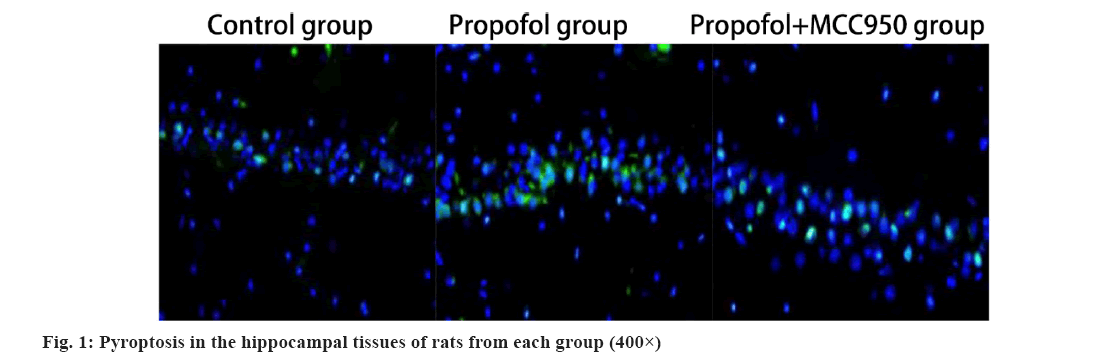
TUNEL assay for detection of pyroptosis in hippocampal tissues: The day after the last administration, 18 rats from each group were sacrificed, hippocampal tissues were dissected and separated on ice and hippocampal tissues from 12 rats were frozen with liquid nitrogen and stored in a −80° freezer; hippocampal tissues from another six rats were fixed with 4 % paraformaldehyde to make paraffin sections, which were incubated and then blocked using TUNEL detection solution and excited under excitation light of 450-500 nm wavelength. Six high-power fields were selected under a fluorescence microscope (400×) for observation to analyze pyroptosis in the hippocampus.
Enzyme-Linked Immunosorbent Assay (ELISA) detection: Hippocampal tissues from six rats were obtained and homogenized by grinding after being sheared by ophthalmic scissor and the supernatants were centrifuged to detect the expression of inflammatory factors using ELISA.
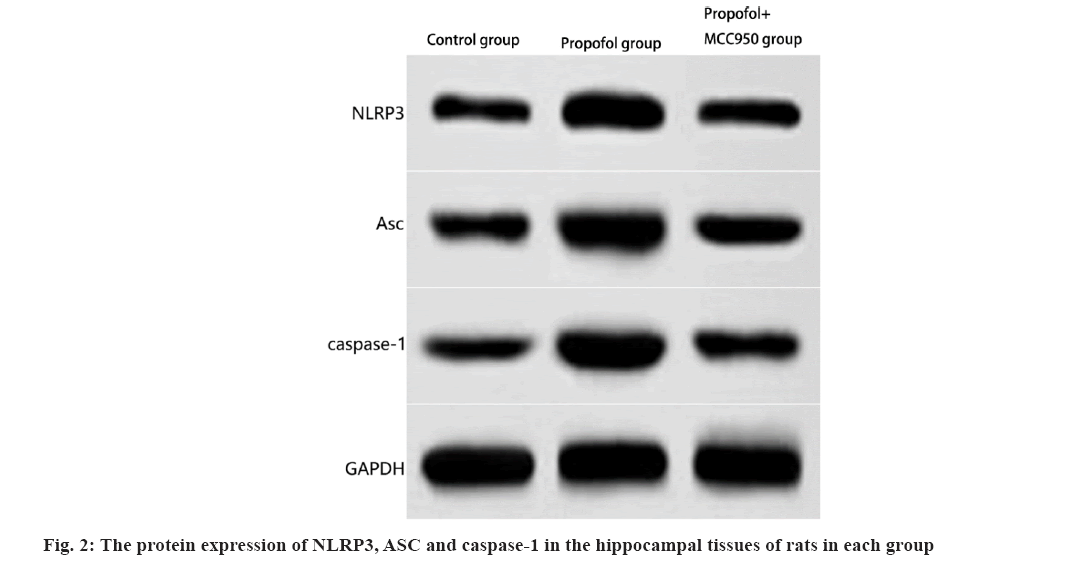
Western blotting: This was performed to detect the expression of NLRP3/caspase-1 pathway related proteins in the hippocampal tissues. The hippocampal tissues of the remaining six rats were frozen, the homogenates were ground after ophthalmic clipping, the supernatants were centrifuged, the protein expression was detected after extraction of total protein and the gray scale values of protein bands in protein band charts were analyzed by Image J software to calculate the protein levels of NLRP3, ASC and caspase-1.
Water maze test: This test was used to examine the learning and memory ability of rats. On d 19 after the last administration (30 d after the rats were born), the water maze test was performed on the remaining eight rats in each group. A pool of 90 cm in diameter and 21 cm in water depth was divided into four quadrants and the cylindrical platform with a diameter of 8 cm and a height of 20 cm was placed in the fourth quadrant, where ink was added to the water to black and the water temperature ranged from 19° to 21°. The time taken from entering the water to climbing on the platform was recorded as the escape latency. If the platform was not found in 90 s, it was guided to the platform using a guide bar. The escape latency was recorded as 90 s and the rats were trained once daily in each quadrant by using the guide bar for 10 min per training interval. The escape latency was recorded for 5 d and the rat was withdrawn on the 6th d. The rats were placed into the water from the second quadrant and the time it took to go through the original platform position (escape latency), the number of crossing the original platform location in 90 s (the number of crossing the platform) and the percentage of residence time in the second quadrant in the total time within 90 s (percentage of time spent in the target quadrant) were recorded.
Statistical analysis:
Sigma plot 12.0 software was used to analyze the data. Mean±Standard Deviation (SD) (x?±s) of metrology data was expressed and comparisons among multiple groups were performed using one-way Analysis of Variance (ANOVA) (Student–Newman–Keuls (SNK) test was used for pairwise comparisons between groups). p<0.05 was considered statistically significant.
Results and Discussion
A significant increase in pyroptosis was observed in the hippocampus of propofol group treated rats compared with that of control group (p<0.05); a significant decrease (p<0.05) in pyroptosis was observed in the hippocampal tissues of propofol+MCC950 group rats compared with that of control group as shown in fig. 1 and Table 1.
| Group | Pyroptosis (%) |
|---|---|
| Control group | 12.64±0.85 |
| Propofol group | 26.85±1.13a |
| Propofol+MCC950 group | 17.44±1.12ab |
| F | 289.058 |
| p | 0.000 |
Notes: Compared with the control group, ap<0.05 and compared with the propofol group, bp<0.05
Table 1: Comparison of Pyroptosis in Hippocampal Tissues of RATS in each group (x?±s, n=6)
IL-1β and IL-18 expression levels in the hippocampus of propofol group treated rats were significantly higher (p<0.05) than those in the control group; IL-1β and IL-18 expression levels in the hippocampal tissues of propofol+MCC950 group were significantly (p<0.05) lower than those in the propofol group as shown in Table 2.
| Group | IL-1β (ng/mg) | IL-18 (ng/mg) |
|---|---|---|
| Control group | 14.62±2.85 | 37.88±4.65 |
| Propofol group | 31.05±3.63a | 94.22±5.76a |
| Propofol+MCC950 group | 20.14±2.50ab | 48.53±4.52ab |
| F | 45.675 | 214.349 |
| p | 0.000 | 0.000 |
Notes: Compared with the control group, ap<0.05 and compared with the propofol group, bp<0.05
Table 2: IL-1Β and IL-18 Expression Levels in the HIPPOCAMPAL Tissues from each group (x?±s, n=6)
The protein expression levels of NLRP3, ASC and caspase-1 in the hippocampus of propofol group treated rats were significantly higher than those in the control group (p<0.05); the protein expression levels of NLRP3, ASC and caspase-1 in the hippocampal tissues of propofol+MCC950 group rats were significantly lower than those in the propofol group (p<0.05, as shown in fig. 2 and Table 3.
| Group | NLRP3/GAPDH | ASC/GAPDH | Caspase-1/GAPDH |
|---|---|---|---|
| Control group | 0.40±0.05 | 0.42±0.06 | 0.32±0.06 |
| Propofol group | 0.82±0.06a | 0.89±0.06a | 0.88±0.07a |
| Propofol+MCC950 group | 0.48±0.05b | 0.55±0.05ab | 0.42±0.07b |
| F | 104.093 | 109.299 | 119.821 |
| p | 0.000 | 0.000 | 0.000 |
Notes: Compared with the control group, ap<0.05 and compared with the propofol group, bp<0.05
Table 3: Comparison of the Protein Expression Levels of NLRP3, ASC and CASPASE-1 in the HIPPOCAMPAL tissues of RATS in each group (x?±s, n=6)
The escape latency of the rats in the propofol group was significantly higher (p<0.05) than that of the control group, as was the number of crossing the platform and the percentage of time spent in the target quadrant (p<0.05); the escape latency of the rats in the propofol+MCC950 group was significantly lower than that in the propofol group (p<0.05), as was the number of crossing the platform and the percentage of time spent in the target quadrant (p<0.05), as shown in Table 4.
| Group | Escape latency (s) | The number of crossing the platform (times) | Percentage of time spent in the target quadrant (%) |
|---|---|---|---|
| Control group | 6.03±0.48 | 7.28±0.54 | 38.06±2.24 |
| Propofol group | 6.88±0.42a | 5.46±0.55a | 31.27±2.13a |
| Propofol+MCC950 group | 6.29±0.45b | 6.20±0.59ab | 34.14±2.39ab |
| F | 7.472 | 21.339 | 18.264 |
| p | 0.015 | 0.000 | 0.000 |
Notes: Compared with the control group, ap<0.05 and compared with the propofol group, bp<0.05
Table 4: Comparison of Escape Latency, The Number of Crossing the Platform and time spent in the Target Quadrant among RATS in each group (x?±s, n=8)
It was found that children who had been exposed to general anesthesia in the neonatal period had a greatly increased risk of attention deficit and learning disabilities[12]. Recent studies have shown that the neonatal period is a high-speed developmental period of the nervous system and propofol causes neuronal apoptosis during development, which can affect learning and memory function in severe cases[13]. In order to make a safer use of propofol, this study will use propofol anesthesia to explore its effect on pyroptosis and related mechanisms of action in the neonatal rat hippocampus. The rat nervous system is in a period of rapid development from 7-14 d after birth and the level of development is equivalent to that of a full-term neonate, which is highly vulnerable to external environmental factors[14]. 7 d old rats were selected for this study. Pyroptosis is a new programmed cell death modality and compared with caspase-3 mediated apoptosis, pyroptosis mainly depends on caspase-1 activation[15]. Our results showed that caspase-1 protein levels and pyroptosis were significantly increased in the hippocampus of rats at the end of propofol administration, suggesting that propofol causes pyroptosis in the hippocampus during development, leading to hippocampal tissue damage.
The NLRP3 inflammasome, an innate immune pattern recognition receptor, is composed of NLRP3, ASC and caspase-1[16]. When the organism is subjected to foreign stimuli, NLRP bind the ASC machinery caspase-1 precursor to form the NLRP3 inflammasome, which activates caspase-1 with hydrolase activity to mature cytosolic inactive IL-18 and IL-1β precursors, producing an inflammatory cascade and leading to pyroptosis[17,18]. In this study, we found that the administration of propofol increased the levels of IL-1β, IL-18 as well as the protein levels of NLRP3, ASC and caspase-1, suggesting that propofol may activate the NLRP3/caspase-1 pathway, leading to NLRP3 inflammasome formation and subsequent inflammatory cascade effects that cause pyroptosis in the hippocampus.
The water maze test is a behavioral means of studying hippocampal dependent spatial learning and memory abilities in rodents[19]. The age of 28-30 d in rats is a period of high acquisition of learning and memory ability and the level of development is equivalent to children aged 6-7 y[20]. Therefore, the water maze test was performed in this study when rats were 30 d old. The results showed that the escape latency of the rats in the propofol group was significantly higher than that of the control group, whereas the number of crossing the platform and the percentage of time spent in the target quadrant were significantly lower, suggesting that the activation of the NLRP3/caspase-1 pathway and pyroptosis in the hippocampus caused by propofol would decrease the learning and memory performance of the rats. MCC950, an NLRP3 inhibitor, was found to reduce NLRP3, ASC and caspase-1 protein levels and IL-1β and IL-18 levels in the hippocampal tissues, reduce hippocampal pyroptosis and the escape latency of the rats, as well as increase the number of crossing the platform and the percentage of time spent in the target quadrant when administered concomitantly with propofol to rats compared to propofol alone, further suggested that the pyroptosis in the hippocampus and the reduced learning and memory ability of the rats caused by propofol may act through the activation of the NLRP3/caspase-1 pathway. In conclusion, propofol anesthesia causes pyroptosis and, reduces learning and memory performance in neonatal rat hippocampus and the mechanism may be related to the inflammatory response triggered by activation of the NLRP3/caspase-1 pathway.
Funding:
This work was supported by Natural Science Foundation of China (No.81673909) and Tianjin science and technology project (No.15ZXLCSY00020).
Author contributions:
Fangfang Luo and Junjie Lin designed experiments; Fangfang Luo carried out experiments; Junjie Lin analyzed experimental results and analyzed sequencing data and developed analysis tools; Fangfang Luo assisted with Junjie Lin sequencing; Fangfang Luo and Junjie Lin wrote the manuscript. Fangfang Luo and Junjie Lin have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Li J, Zhang K, Liu Y, LI Y. Effect of RhoA inhibitor Y27632 on propofol-induced apoptosis of hippocampal neurons in neonatal rats. Chin J Behav Med Brain Sci 2018;12:139-44.
- Wang Q, Li Y, Yin S. Repeated propofol anesthesia induces apoptosis and autophagy in neonatal rat hippocampus via mTOR pathway. J Clin Exp Med 2020;19(20):2139-42.
- Strøm C, Lundstrøm LH, Afshari A, Lohse N. Characteristics of children aged 2–17 y undergoing anaesthesia in Danish hospitals 2005–2015: A national observational study. Anaesthesia 2018;73(11):1321-36.
[Crossref] [Google Scholar] [PubMed]
- Liang C, Du F, Cang J, Xue Z. Pink1 attenuates propofol-induced apoptosis and oxidative stress in developing neurons. J Anesth 2018;32(1):62-9.
[Crossref] [Google Scholar] [PubMed]
- Li J, Wu X, Zhang Y, Rong J. Effect of 17β estradiol pretreatment on inflammatory responses during propofol-induced apoptosis in hippocampal nerve cells of developing rats. Chin J Anesthesiol 2017;31(2):821-4.
- Zhang C, Wang Y, Jin J, Li K. Erythropoietin protects propofol induced neuronal injury in developing rats by regulating TLR4/NF-κB signaling pathway abstract. Neurosci Lett 2019;712(1):134517.
- Fu Q, Wu J, Zhou XY, Ji MH, Mao QH, Li Q, et al. NLRP3/caspase-1 pathway-induced pyroptosis mediated cognitive deficits in a mouse model of sepsis-associated encephalopathy. Inflammation 2019;42:306-18.
[Crossref] [Google Scholar] [PubMed]
- Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, et al. NOD-like receptor protein 3 inflammasome-dependent IL-1β accelerated ConA-induced hepatitis. Front Immunol 2018;9:758.
[Crossref] [Google Scholar] [PubMed]
- Sun S, Liu Y, Yang G, Li H. Effect of leptin on pyroptosis in hippocampal cells during brain injury in a rat model of orthotopic liver transplantation. Chin J Anesthesiol 2020;40(3):312-5.
- Chang C, Peng L, Luo J, Li J, Liu J, Wang T, et al. Effect of propofol anesthesia on autophagy in hippocampal neurons of newborn rats. Chin J Anesthesiol 2020;40(6):669-71.
- Zhang J, Fan Y, Fu Q, Wu J, Zhou Z, Li G. Effects of MCC950 on cognitive function in a mouse model of sepsis-associated encephalopathy. Chin J Emerg Med 2019;28(7):851-4.
- Ing C, Sun M, Olfson M, di Maggio CJ, Sun LS, Wall MM, et al. Age at exposure to surgery and anesthesia in children and association with mental disorder diagnosis. Anesth Analg 2017;125(6):1988-98.
[Crossref] [Google Scholar] [PubMed]
- Guo X, Cheng M, Ke W, Wang Y, Ji X. MicroRNA-214 suppresses propofol-induced neuroapoptosis through activation of phosphoinositide 3-kinase/protein kinase B signaling by targeting phosphatase and tensin homolog expression. Int J Mol Med 2018;42(5):2527-37.
[Crossref] [Google Scholar] [PubMed]
- Hu X, Luan L, Guan W, Zhang S, Li B, Ji W, et al. Hydrogen sulfide attenuates isoflurane-induced neuroapoptosis and cognitive impairment in the developing rat brain. BMC Anesthesiol 2017;17(1):123-31.
[Crossref] [Google Scholar] [PubMed]
- She Y, Hu Y, Zhang J. Involvement of pyroptosis in cerebral ischemia-reperfusion injury: A preliminary study. Chin J Pathophysiol 2019;35(8):1379-86.
- Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis 2019;10(8):542-56.
[Crossref] [Google Scholar] [PubMed]
- Chen F, Wei G, Xu J, Ma X, Wang Q. Naringin ameliorates the high glucose-induced rat mesangial cell inflammatory reaction by modulating the NLRP3 Inflammasome. BMC Complement Altern Med 2018;18(1):192-202.
- Sun Li, Lu We, Song X. Exploration on the mechanism of total saponin from Araliaceae longa against myocardial ischemia-reperfusion injury from the NLRP3 inflammasome pathway. Chin J Tradit Chin Med Pharm 2020;35(3):1441-3.
- Tian H, Ding N, Guo M, Wang S, Wang Z, Liu H, et al. Analysis of learning and memory ability in an Alzheimer's disease mouse model using the Morris water maze. J Vis Exp 2019;152(1):e60055.
[Crossref] [Google Scholar] [PubMed]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 2013;106:1-6.
[Crossref] [Google Scholar] [PubMed]