- *Corresponding Author:
- Yi Zhang
Department of Nursing, Pingdingshan University Medical College, Pingdingshan, Henan Province 467000, China
E-mail: chengwang20180803@163.com
| Date of Received | 04 December 2022 |
| Date of Revision | 29 October 2023 |
| Date of Acceptance | 15 March 2024 |
| Indian J Pharm Sci 2024;86(2):654-659 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the effect of vitamin D deficiency on metabolic diseases induced by high-fat diet in young male rats and its possible mechanism. Twenty four 4 w old male mice were randomly divided into three groups; control group (group A), model group (group B) and experimental group (group C). The mice in the group A were given normal diet, the mice in the group B were given high-fat diet, and the mice in the group C were given high-fat diet plus vitamin D deficiency. The changes of body length, body weight and liver mass of mice in each group were observed; fasting blood samples were collected to measure the changes of blood glucose and blood lipids; hepatic steatosis and lipid droplet content were observed by hematoxylin and eosin staining; serum interleukin-1 beta and interleukin-8 levels were compared by enzyme-linked immunosorbent assay; nucleotide oligomerization domain-like receptor protein 3, ASC, pro-caspase-1, interleukin-1 beta and gasdermin D protein levels in three groups were detected by Western blot. Compared with the group A, the body weight, liver weight, blood glucose and blood lipid increased in the group B, while these in the group C decreased compared with the group B. The liver tissue of the group B and the group C showed steatosis and the content of lipid droplets increased under microscope. Vitamin D deficiency aggravates metabolic diseases in young male rats induced by high-fat diet, which is mainly related to inflammatory response and activation of cell pyrogenesis pathway. Through the supplement of vitamin D deficiency, it can effectively reduce the occurrence of metabolic diseases such as weight, blood glucose, blood lipids and liver steatosis caused by high fat diet.
Keywords
Vitamin D, high-fat diet, triglyceride, autoimmune disease, Western blot
Vitamin D (VD) is a fat-soluble vitamin, which is widely involved in human bone health, immune regulation, nervous system function and cell life cycle and other important physiological processes[1]. In addition to playing critical in the absorption and utilization of calcium and phosphorus, VD, as an important regulator, is closely related to cardiovascular diseases, tumor growth, autoimmune diseases, diabetes and other metabolic diseases[2]. High-Fat Diet (HFD) is a widespread dietary pattern in modern society, and its long-term intake can cause a series of chronic metabolic diseases, such as obesity, diabetes and cardiovascular disease[3]. The pathogenesis of these diseases involves a variety of factors, including chronic inflammation, islet dysfunction, mitochondrial dysfunction and fat deposition. In recent years, clinical and experimental studies have shown that lack of VD may be one of the important factors of metabolic diseases caused by HFD. Some studies have shown that lack of VD is related to the progression of metabolic diseases[4]. Some studies have found that low VD levels are associated with an increased risk of obesity, hypertension and cardiovascular disease[5]. In addition, lack of VD is associated with abnormal lipid metabolism, islet dysfunction, chronic inflammation and bone health problems[6]. However, the specific mechanism of the lack of VD on metabolic diseases induced by HFD is still limited. Therefore, further explore the relationship between VD deficiency and metabolic diseases induced by HFD and its underlying mechanism.
Materials and Methods
Experimental animal:
The 28 d old young mice, Specific-Pathogen-Free (SPF) grade, with a body weight of (30.72±4.56) g, were purchased from Shanghai Slyke Experimental Animal Co., Ltd., license number: SOXK (Shanghai) 2007-0005. The rats were fed freely and fed at 20°-26°, 50 %-60 % humidity and provided quiet, dry and ventilated conditions.
Experimental materials:
Mouse cage, feed trough, drinking bottle, laboratory scale, microscope, centrifuge, refrigerator, over speed freezing centrifuge, body length measuring device, small animal weight meter, blood collection tube, syringe, small animal forceps, tissue slicing knife, micro slicer, staining plate, micropipette, enzyme labeling instrument, oscillator, plate washer, electrophoresis instrument, transmembrane apparatus, developer, protein quantifier, blood glucose kit, blood lipid detection kit, basic staining reagent, dehydration and transparency agent, Interleukin-1 Beta (IL-1β) and IL-8 Enzyme- Linked Immunosorbent Assay (ELISA) kit, protein extraction kit, Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel, Western blot membrane, antibodies NOD-Like Receptor Protein 3 (NLRP3), ASC, pro-caspase-1, IL-1β, Gasdermin D (GSDMD), buffer, reagent grade alcohol, and Dimethyl Sulfoxide (DMSO).
Experimental method:
Animal grouping: Twenty four 4 w old male mice were selected to ensure that the mice were in good health and free from diseases and physiological defects. According to the random number table method, the mice were divided into two groups; control group (group A), model group (group B) and experimental group (group C), with 8 mice in each group. The mice in the group A were given normal diet, the mice in the group B were given HFD, and the mice in the group C were given HFD plus VD3 diet. The ratio of fat to total calories was 10 % in the group A, 60 % in the group B and 60 % in the group C, and lasted for 12 w. 0.6 % calcium and 1000 U/kg VD3 were added to the diet of mice in the group A and group B. No calcium and VD3 were added to the diet of mice in the group C. During the experiment, the body weight of the young mice was measured and recorded every week. The young mice were euthanized with appropriate anesthetic. Collect blood samples and liver tissues of young rats.
Fasting blood samples were collected to measure the changes of blood glucose and blood lipids:
First of all, the mice were adaptively raised to adapt to the experimental diet. At the end of the experimental cycle, fasting blood samples were taken from mice. Put the mouse in the fixator, wipe the tail of the mouse with alcohol cotton ball, and use a disposable aseptic blood collection needle to pierce the tail vein of the mouse after the alcohol volatilized. The blood collection needle takes a certain amount of blood, puts it into the test tube containing anticoagulant, and gently reverses the test tube several times, so that the blood is fully mixed with the anticoagulant. Immediately put the test tube in an ice bath to prevent the blood from clotting. After collecting the blood, gently take out the test tube, label marked the group, blood collection date and other information, stored in the cryogenic refrigerator for follow-up laboratory analysis. The collected blood samples were sent to the laboratory for the detection of blood glucose, blood lipids and other related indicators. The biochemical indexes such as blood glucose, blood lipids (Total Cholesterol (TC), Triglyceride (TG)) and insulin were measured by ELISA detection method. According to the tissue samples, the activities of related metabolic enzymes in liver and adipose tissue were measured.
Hematoxylin and Eosin (H&E) staining:
The mice were killed and the liver tissue was quickly removed. The surrounding fat and connective tissue were removed with surgical instruments, and the liver was cut into thin slices about 2-3 mm thick. Place the cut liver slices in a petri dish containing 4 % neutral buffer formalin solution (or formaldehyde solution) and soak, and fix for at least 24 h. The purpose of this step is to fix the tissue and maintain the cell morphology. The fixed liver slices were removed and put into an increasing concentration of alcohol solution for dehydration. In the process of dehydration, the concentration of alcohol solution gradually increases from 40 % to 100 %. Each concentration stayed for about 1 h and was carried out for 3 times. The thin slices of the dehydrated liver become transparent. The dehydrated liver slices were put into xylene solution and treated transparently. In the process of transparent treatment, xylene solution can remove alcohol and make the tissue transparent. Transparent treatment was for about 1 h. The transparent liver slices were put into hematoxylin solution and stained. During staining, hematoxylin can stain the nucleus and cytoplasm. The staining time was 30 min. Remove the dyed liver slices and rinse with warm water to remove excess dyes. The washed liver slices were put into eosin solution and stained with eosin. Eosin can stain cytoplasm and collagen fibers. The dyeing time is 10 min. Take out the thin slices of the liver stained with eosin and rinse with warm water to remove the excess dye. The flushed liver slices were put into an increasing concentration of alcohol solution for dehydration. During dehydration, the concentration of alcohol solution gradually decreases from 100 % to 70 %. Each concentration stayed for about 10 min and was carried out for 3 times. The dehydrated liver slices were put into xylene solution and treated transparently. Transparent treatment was for about 1 h. The transparent liver slices were taken out and sealed with neutral gum. After the film is sealed, the section is observed under a microscope.
ELISA detection: Mouse serum samples were collected. The blood was placed in a test tube and centrifuged for 10 min to remove red blood cells from the blood to get a serum sample. Take out the ELISA plate, add 100 μl coating solution to each hole, and rest at room temperature for 1 h. Then, the coating liquid of the coating plate is poured out, the remaining liquid in the solution is emptied, and the hole is washed 3 times with washing buffer. Remove the washing buffer and gently pat to dry the ELISA board. The standard and sample are added to the coating plate, and 100 μl is added to each hole. Different concentrations of standards should be in different holes. After adding the specimen, the ELISA plate was sealed and incubated at 37° for 2 h. Put the coating board into the scrubber and wash it with washing buffer for 3 times. After each washing, gently pat it dry with a paper towel. Add the substrate solution, 100 μl per hole, then seal the ELISA plate and incubate at room temperature for 30 min. 50 μl terminator was added to stop the enzyme reaction. Using enzyme labeling instrument, the absorbance of each hole was measured at 450 nm wavelength.
Western blot detection: The mouse liver tissue was collected and homogenized with a homogenizer. The protein was extracted according to the preset method of protein extraction, and the protein concentration was determined. The extracted protein samples were added to the electrophoresis tank, and the proteins with different molecular weights were separated by electrophoresis. The target proteins on the electrophoretic gel were transferred to Polyvinylidene Difluoride (PVDF) or Nitrocellulose (NC) membrane. PVDF membrane or NC membrane was sealed with sealing solution, and then an antibody was added and incubated overnight at 4°. Wash off the sealing solution, add secondary antibodies, incubate at room temperature for 1 h, and detect using gel imaging system. The gray value of the band was read by gel imaging system, and the protein concentration was measured by protein quantitative instrument, and the results were compared.
Statistical method:
The experimental data were processed by Statistical Package for the Social Sciences (SPSS) 19.0 statistical analysis software, and the data were expressed as mean±standard deviation (variance x±s). One-way Analysis of Variance (ANOVA) was used for comparison among groups, and Least Significant Difference (LSD) t-test was used for pairwise comparison between groups. Compared with group A, ap<0.05 and compared with the group B, bp<0.05.
Results and Discussion
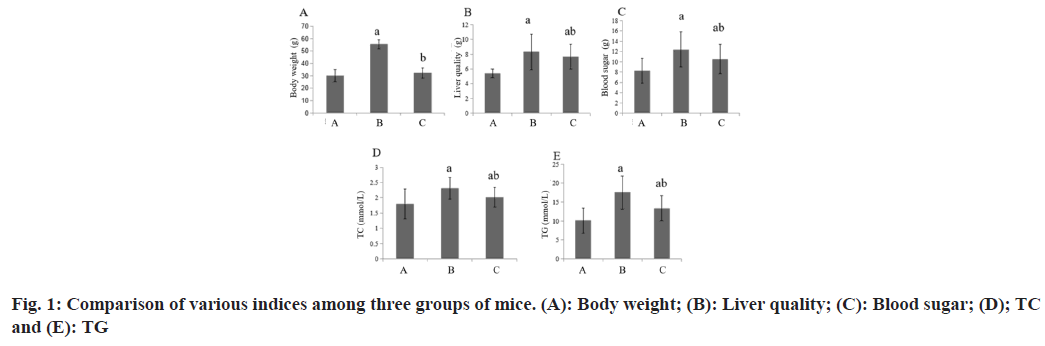
Compared with the group A, the body weight, liver weight, blood glucose and blood lipid of the group B increased, while these in the group C decreased (Table 1 and fig. 1).
| Group | n | BW (g) | Liver quality (g) | Blood sugar (mmol/l) | Blood lipids | |
|---|---|---|---|---|---|---|
| TC (mmol/l) | TG (mmol/l) | |||||
| A | 8 | 30.23±4.96 | 5.43±0.56 | 8.25±2.36 | 1.80±0.49 | 10.09±3.33 |
| B | 8 | 55.39±3.83a | 8.33±2.39a | 12.39±3.41a | 2.31±0.36a | 17.56±4.38a |
| C | 8 | 32.30±4.12b | 7.68±1.67ab | 10.54±2.83ab | 2.01±0.32ab | 13.38±3.29ab |
| F | 83.24 | 6.31 | 4.1 | 3.34 | 8.19 | |
| p | 0.001 | 0.007 | 0.031 | 0.05 | 0.002 | |
Note: Compared with group B, ap<0.05 and compared with the group C, bp<0.05
Table 1: Various Indexes among the Three Groups of Mice (x͞±s)
Compared with the group A, the liver tissue of the group B and the group C showed steatosis and the lipid droplet content increased under the microscope. Compared with the group B, these in group C had steatosis, but the lipid droplet content decreased. The liver tissue of the group A was normal and there was no obvious lipid droplet accumulation.
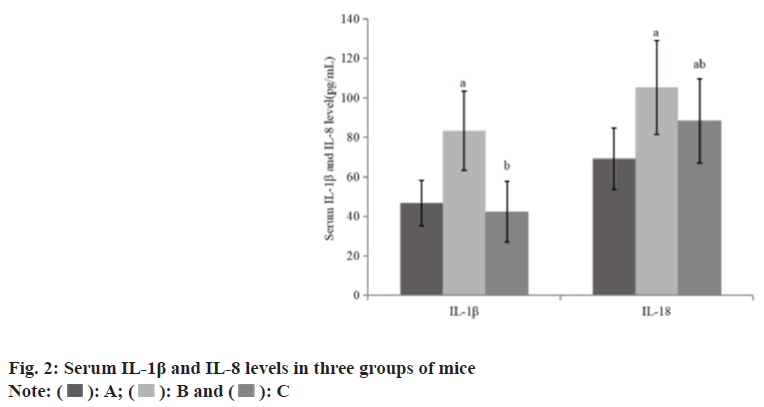
Compared with the group A, the levels of serum IL-1β and IL-8 in the group B increased, while these in the group C decreased than group B (Table 2 and fig. 2).
| Group | n | IL-1β (pg/ml) | IL-18 (pg/ml) |
|---|---|---|---|
| A | 8 | 46.61±11.50 | 69.21±15.53 |
| B | 8 | 83.28±20.09a | 105.28±23.69a |
| C | 8 | 42.28±15.39b | 88.32±21.28ab |
| F | 15.76 | 9.48 | |
| p | 0.001 | 0.009 |
Note: Compared with group B, ap<0.05 and compared with the group C, bp<0.05
Table 2: Serum IL-1β and IL-8 Levels in Three Groups of Mice (x͞±s)
Compared with the group A, the expression levels of NLRP3, ASC, pro-caspase-1 and GSDMD protein in the group B and the group C were increased, while these in the liver of the group C were reduced than those of the group B (Table 3 and fig. 3).
| Group | n | NLRP3 | ASC | Pro-caspase-1 | GSDMD |
|---|---|---|---|---|---|
| A | 8 | 1.13±0.32 | 1.66±0.49 | 1.36±0.21 | 1.96±0.36 |
| B | 8 | 3.52±0.46a | 5.03±0.39a | 1.83±0.36a | 3.03±0.38a |
| C | 8 | 2.59±0.23ab | 2.69±0.13ab | 1.45±0.32b | 2.13±0.29b |
| F | 94.94 | 174.95 | 5.41 | 22.16 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Compared with group B, ap<0.05 and compared with the group C, bp<0.05
Table 3: Protein Levels of NLRP3, SC, Pro-Caspase-1 and GSDMD (x͞±s)
VD can regulate the synthesis and decomposition of lipids, promote the decomposition of fat by activating enzymes in adipocytes, reduce the synthesis of fat[7], regulate the genes related to lipid metabolism, such as regulating the expression of fatty acid synthase and inhibiting the synthesis of fatty acids. Other studies have shown that VD can effectively improve the disorder of lipid metabolism induced by HFD[8]. By regulating the expression of genes and enzymes related to lipid metabolism, VD can reduce the concentration of blood lipids, reduce fat accumulation and prevent lipid metabolism disorders. VD promotes fat decomposition and reduces fat synthesis by activating enzymes in adipocytes. VD can regulate the expression of genes related to lipid metabolism, such as regulating the expression of fatty acid synthase and inhibiting the synthesis of fatty acids. VD can effectively improve the disorder of lipid metabolism induced by HFD. By regulating the expression of genes and enzymes related to lipid metabolism[9], VD can reduce the concentration of blood lipids, reduce fat accumulation and prevent lipid metabolism disorders. VD Receptor (VDR) exists widely in a variety of tissues, such as intestinal tract, bone, adipocytes and so on. It plays a vital role in metabolic regulation[10]. First of all, VDR can regulate the absorption of calcium ions, maintain the stability of blood calcium, and then affect insulin secretion and glucose metabolism. Secondly, VDR can also regulate the development and differentiation of adipocytes and affect the synthesis and decomposition of fat. In addition, VDR can also inhibit the release of inflammatory factors and reduce the inflammatory response. VD receptor is the key molecule for VD to exert its biological effects. When VD is deficient, the expression and activity of VD receptor are decreased, which affects the signal transduction of VD in vivo. In addition, the metabolites of VD are also involved in regulating metabolic functions, such as 1,25-dihydroxyvitamins can inhibit inflammation and promote glucose metabolism. Lack of VD results in blocking of these regulatory mechanisms, which in turn aggravates metabolic diseases induced by HFD. VDR agonists can effectively improve metabolic diseases induced by HFD. By activating VDR[11], it can enhance the sensitivity of insulin, improve the efficiency of glucose metabolism, reduce fat accumulation and inhibit inflammation, so as to prevent and manage metabolic diseases. Studies have shown that VD supplementation can improve the stability of blood glucose levels[12], reduce the concentration of cholesterol and TG, and reduce the accumulation of adipocytes. In this study, the experimental mice induced by HFD and VDD diet increased body weight, liver mass, blood glucose and blood lipids, and fatty degeneration of liver tissue. The changes in these indicators suggest that a HFD may lead to metabolic diseases, which are aggravated by VD deficiency.
The deposition of saturated fatty acids induced by high fat can increase the expression of genes related to NLRP3 inflammatory bodies and promote local inflammatory response in the liver[13]. At the same time, NLRP3/caspase-1 signal pathway mediates IR-HepG2 cell death and participates in metabolic diseases such as insulin resistance and type 2 diabetes. At the same time, GSDMD participates in the regulation of cell membrane permeability in inflammatory reaction. During digestion, GSDMD proteins help vitamin molecules enter the cells by regulating the permeability of the cell membrane, which is then absorbed and utilized by the human body. In addition, it is also closely related to vitamin metabolism and bioutilization. The GSDMD protein helps maintain the permeability of the cell membrane and ensures that vitamin molecules can successfully participate in the enzymatic reaction. In this study, it was found that the expression levels of NLRP3, ASC, pro-caspase-1 and GSDMD in the liver of mice in the group C were decreased. In addition, the levels of IL-1β and IL-8 in the serum of mice in the group C were decreased, suggesting that NLRP3, ASC, pro-caspase-1, GSDMD-related pathways and inflammation may play critical role in the aggravation of metabolic diseases induced by HFD in VD deficiency.
To sum up, VD deficiency can aggravate metabolic diseases induced by HFD in young male rats. VD deficiency mediates the occurrence and development of metabolic diseases induced by HFD by regulating blood glucose, blood lipids and promoting the expression of NLRP3, ASC, pro-caspase-1 and GSDMD in liver.
Acknowledgement:
This work was supported by Henan Province medical education research project (No: WJLX2023155) research project on ideological and political work at Pingdingshan college (No: PXY-SZYJ-202008) 2021 Pingdingshan smart nursing key laboratory.
Conflict of interests:
The authors declared no conflict of interests.
References
- Lee SM, Meyer MB, Benkusky NA, O’Brien CA, Pike JW. The impact of VDR expression and regulation in vivo. J Steroid Biochem Mol Biol 2018;177:36-45.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhang X, Yang Y, Zhao J, Yu Y. Correlation analysis of serum vitamin D levels and postoperative cognitive disorder in elderly patients with gastrointestinal tumor. Front Psychiatry 2022;13:893309.
[Crossref] [Google Scholar] [PubMed]
- Stavast CJ, Erkeland SJ. The non-canonical aspects of microRNAs: Many roads to gene regulation. Cells 2019;8(11):1465.
[Crossref] [Google Scholar] [PubMed]
- Chen B, Chen Y, Xu Y. Vitamin D deficiency in pregnant women: Influenced by multiple risk factors and increase the risks of spontaneous abortion and small-for-gestational age. Medicine 2021;100(41):e27505.
[Crossref] [Google Scholar] [PubMed]
- Chen T, Zhang Z, Lei H, Fen Z, Yuan Y, Jin X, et al. The relationship between serum 25-hydroxyvitamin-D level and sweat function in patients with type 2 diabetes mellitus. J Endocrinol Invest 2022;45(2):361-8.
[Crossref] [Google Scholar] [PubMed]
- Zittermann A, Trummer C, Theiler-Schwetz V, Lerchbaum E, März W, Pilz S. Vitamin D and cardiovascular disease: An updated narrative review. Int J Mol Sci 2021;22(6):2896.
[Crossref] [Google Scholar] [PubMed]
- Su SB, Qin SY, Xian XL, Huang FF, Huang QL, ZhangDi HJ, et al. Interleukin-22 regulating Kupffer cell polarization through STAT3/Erk/Akt crosstalk pathways to extenuate liver fibrosis. Life Sci 2021;264:118677.
[Crossref] [Google Scholar] [PubMed]
- Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. New Engl J Med 2019;381(6):520-30.
[Crossref] [Google Scholar] [PubMed]
- Yang Y, Zhang X, Bao M, Liu L, Xian Y, Wu J, et al. Effect of serum 25-hydroxyvitamin D3 on insulin resistance and β-cell function in newly diagnosed type 2 diabetes patients. J Diabetes Investig 2016;7(2):226-32.
[Crossref] [Google Scholar] [PubMed]
- Christofi C, Lamnis L, Stark A, Palm H, Römer K, Vogt T, et al. Cross-talk of Aryl Hydrocarbon Receptor (AHR)-and Vitamin D Receptor (VDR)-signaling in human keratinocytes. Anticancer Res 2022;42(10):5049-67.
[Crossref] [Google Scholar] [PubMed]
- Zheng Y, Zhou H, Ooi LL, Snir AD, Dunstan CR, Seibel MJ. Vitamin D deficiency promotes prostate cancer growth in bone. Prostate 2011;71(9):1012-21.
[Crossref] [Google Scholar] [PubMed]
- Teng Z, Wei M. Correlation between serum 25-hydroxyvitamin D level and coronary heart disease. Am J Transl Res 2021;13(7):8379.
[Google Scholar] [PubMed]
- Li P, Chen T, Xue J. Vitamin D signaling orchestrates epithelial-mesenchymal interaction during pulmonary fibrogenesis. Cell Death Differ 2020;27(1):201-16.
 C
C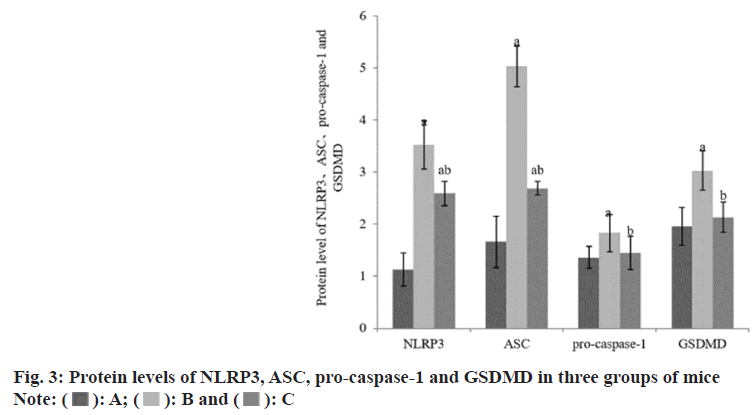
 C
C