- *Corresponding Author:
- Jianhua Li
Department of Gastroenterology, Shandong Provincial Third Hospital, Cheeloo College of Medicine, Jinan 250000, Shandong Province, China
E-mail: ljhua889@163.com
| Date of Received | 22 August 2020 |
| Date of Revision | 01 March 2021 |
| Date of Acceptance | 23 June 2021 |
| Indian J Pharm Sci 2021;83(3):569-574 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To assess the ameliorating effects of vitamin D3 on inflammatory bowel disease in rats via the Toll-like receptor 4/myeloid differential factor 88/nuclear factor kappa B signaling pathway. Thirty two rats were randomly assigned into Control group, model group (Vehicle group), low-concentration vitamin D3 group (vitamin D3 low group) and high-concentration vitamin D3 group (vitamin D3 high group). The model of inflammatory bowel disease was constructed using 3 % dextran sodium sulfate and given different concentrations of vitamin D3. Disease activity index score was calculated, colon tissues were pathologically scored by hematoxylin-eosin staining, and the mRNA expression levels of Toll-like receptor 4/myeloid differential factor 88/nuclear factor kappa B were measured by quantitative reverse transcriptionpolymerase chain reaction. The expression levels of serum tumor necrosis factor-α, pro-inflammatory factors interleukin 1β and interleukin 6, as well as anti-inflammatory factor interleukin 10 were detected through enzyme-linked immunosorbent assay. The disease activity index score, mRNA expression levels of Toll-like receptor 4/myeloid differential factor 88/nuclear factor kappa B and expression levels of tumor necrosis factor-α, interleukin 1β and interleukin 6 were significantly higher in Vehicle group than those in Control group, while the expression level of interleukin-10 was lower in Vehicle group (p<0.05). After intervention with vitamin D3, vitamin D3 low group and vitamin D3 high group had significantly decreased disease activity index score, messenger RNA expression levels of Toll-like receptor 4/myeloid differential factor 88/nuclear factor kappa B and expression levels of tumor necrosis factor-α, interleukin 1β and interleukin 6, increased interleukin-10 expression level, and relieved damage of colon tissues compared with Vehicle group (p<0.05). Vitamin D3 ameliorates intestinal injury and colonic inflammation in inflammatory bowel disease rats by suppressing the Toll-like receptor 4/myeloid differential factor 88/ nuclear factor kappa B signaling pathway and decreasing the expression of downstream pro-inflammatory factors.
Keywords
Toll-like receptor 4, myeloid differential factor 88, nuclear factor kappa B, vitamin D3, inflammatory bowel disease
Inflammatory bowel disease (IBD), a group of chronic and non-specific intestinal diseases including ulcerative colitis (UC) and Crohn’s disease (CD) is able to affect the entire digestive tract. It exhibits a yearly increasing incidence rate recently, but its pathogenesis remains unclear. IBD may have an association with environmental factors, genetic factors and immune disorders and the intestinal immune response is a vital player in the development, progression and treatment of IBD[1]. The nature of pathology of IBD is the injury of intestinal mucosa and repair of epithelial cells caused by massive inflammatory cells and factors in the intestine[2]. Toll-like receptors (TLRs) refer to cell transmembrane receptors and pathogen pattern recognition receptors in the innate immune system, which mainly mediate the interaction between microorganisms and cells and participate in inflammatory signal transduction. TLR4, a specific receptor for lipopolysaccharides (LPS), is capable of activating genes related to adaptive immunity[3]. As a downstream signal molecule of the TLR signaling pathway, myeloid differential factor 88 (MyD88) contains the TLR domain and functions as an adapter protein[4]. In the MyD88-dependent TLR4 signaling pathway, the pro-inflammatory factors [tumor necrosis factor-α (TNF-α) and interleukin 1β (IL-1β)] are released due to the activation of nuclear factor kappa B (NF-κB), and the inflammatory factors released further activate NF-κB, so the inflammation constantly amplifies and deepens and eventually develops into a cancer[5]. In many existing studies, the therapeutic effects of drugs on IBD are observed through the TLR4/MyD88/NF-κB signaling pathway. Shon et al.[6] found that Ido-1 downregulated the TLR/MyD88/NF-κB signaling pathway to alleviate dextran sodium sulfate (DSS)-induced colitis in mice. Vitamin D (VD3), a fat-soluble vitamin, is easily converted into VD3 under ultraviolet light, of which the active form is 1,25(OH)2D3. VD is involved in the calcium-phosphorus metabolism in the body and also binds to nuclear receptor VD receptor (VDR) to regulate the immune function[7]. Numerous studies have proved that VD3 affects the pathogenesis of IBD. Erben et al.[8] found that the 1,25(OH)2D3 level was lower in the mouse model of IBD and patients with IBD. Arihiro et al.[9] discovered that knocking out the VDR gene in mice induced IBD that was significantly mitigated after supplementing 1,25(OH)2D3. However, whether VD3 can alleviate IBD by regulating the TLR4/ MyD88/NF-κB signaling pathway is rarely reported. In this study, therefore, a rat model of IBD was established to probe into the ameliorating effect of VD3 on IBD in rats through the TLR/MyD88/NF-κB signaling pathway, hoping to provide experimental evidence for clinical treatment of IBD with VD3.
Materials and Methods
Laboratory animals:
A total of 32 Specific-Pathogen Free-grade male Sprague Dawley rats aged 10-12 w and weighing (200±20) g were purchased from Nankai University [production license number: SYXK (Tianjin) 2019- 0003], fed with normal feed at 25° (room temperature) and 60 % (standard humidity) on a 12 h light-12 h dark schedule (lighting) with water available ad libitum in separate cages for 1 w and then used in experiments.
Main reagents and instruments:
The main reagents used in this study included VD3 (Shanghai Roche Pharmaceuticals Co., Ltd., China), DSS (Sigma, USA), TLR4, MyD88 and NF-κB antibodies (Abcam, UK), secondary antibodies (Beijing Bersee Science and Technology Co., Ltd., China), hematoxylin-eosin (HE) staining solution and RIPA lysis buffer (Shanghai Beyotime Biotechnology Co., Ltd., China), BCA protein concentration determination kit and DAB color development kit (Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd., China), and hematoxylin and eosin (Beijing Solarbio Science & Technology Co., Ltd., China).
The following instruments were adopted in this study: A embedding machine (Leica, Germany), a fluorescence quantitative polymerase chain reaction (PCR) instrument (Roche, Switzerland), an E800 microscope (Nikon, Japan), a table-top freezing centrifuge (Beckman, USA), a protein electrophoresis apparatus and a transfer instrument (Bio-Rad, USA), a microplate reader (BioTek, USA), and an ultrasonic cell disruptor (Sonics, USA).
Modeling and grouping:
The 32 rats enrolled were randomly divided into Control group, model group (Vehicle group), lowconcentration VD3 group (VD3 low group), and highconcentration VD3 group (VD3 high group). (The dosage of administration of VD3 is 50000 IU/w based on the deficiency in adults, which is converted into a dose of 1250 IU/w in rats, and the VD3 concentration was 180 IU/d in VD3 low group and 540 IU/d in VD3 high group).
The rat model of IBD was established using modified classic method of Cooper et al.[10]. The rats in Control group had free access to distilled water for 7 d and were given a gavage of distilled water at 10 o’clock every day, while those in Vehicle group drank 3 % DSS freely for 7 d and were given a gavage of distilled water every day at 10 o’clock. In VD3 low group and VD3 high group, rats were provided with 3 % DSS freely for 7 d and intragastrically given low-concentration VD3 and high-concentration VD3 separately at 10 o’clock every day.
The weight, stool, diet, drinking and movement of rats were observed and recorded every day. All rats were killed under anesthesia at 7 d after modeling, and their colon specimens were collected and stored in liquid nitrogen.
Disease activity index (DAI) score:
The weight, stool viscosity, bloody stool and other conditions of rats were observed and recorded every day, based on which the DAI score was calculated (Table 1). DAI=weight loss score+stool viscosity score+bloody stool score[10].
| Score | Weight loss (%) | Stool viscosity | Bloody stool |
|---|---|---|---|
| 0 | Unchanged | Normal | Normal |
| 1 | 1-5 | ||
| 2 | 6-10 | Loose | Occult blood positive |
| 3 | 11-15 | ||
| 4 | >15 | Loose stool | Gross bloody stool |
Table 1: Dai Scoring Standards
HE staining and pathological scoring of colon tissues:
Frozen colon tissues were taken and prepared into paraffin sections after fixation and paraffin embedding. Then, the sections were deparaffinized with xylene, dehydrated with graded ethanol and stained with hematoxylin and eosin. Thereafter, the sections were gradiently dehydrated with ethanol, permeabilized with xylene and mounted with neutral balsam, followed by observation using a microscope (200×).
The colon tissues were pathologically scored[10]. Specifically, 5 fields of view were randomly selected for each section and scored according to the pathology scoring standards (Table 2).
| Score | Inflammation | Depth | Crypt injury | Lesion size (%) |
|---|---|---|---|---|
| 0 | No | No | No | 0 |
| 1 | Mild | Mucosa | Destruction of 1/3 basal crypts | 1-25 |
| 2 | Severe | Submucosa | Destruction of 2/3 basal crypts | 26-50 |
| 3 | Substrate | Intact superficial epithelium only | 51-75 | |
| 4 | Serosa | Destruction of crypts and epithelia | 76-100 |
Table 2: Pathological Scores
Measurement of mRNA expression levels of TLR4, MyD88 and NF-κB through quantitative reverse transcription-polymerase chain reaction (qRTPCR):
Total RNAs were extracted from frozen colon tissues using TRIzol method and reversely transcribed into cDNAs with a reverse transcription kit. Next, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal reference, the amplification was performed using the SYBR Green method under the following conditions: 95° for 5 min, 95° for 20 s and 60° for 30 s for a total of 40 cycles. After that, the relative expression levels of TLR4, MyD88 and NF-κB were calculated using the 2-ΔΔCT formula.
The PCR primer sequences were as follows:
TLR4:upstream: 5’-CAGAATGAGGACTGGGTG-3’ and downstream:
5’-GTGAAGGCAGAGGTGAAAG-3’,
MyD88: upstream: 5’-CTCGCAGTTTGTTGGATG-3’ and downstream:
5’-TACCTAAGTATTTCTGGCAGT-3’,
NF-κB: upstream:
5’-ACTGCCGGGATGGCTACTAT-3’ and downstream: 5’-TCTGGATTCGCTGGCTAATGG-3’ and
GAPDH: upstream: 5’-TGTTTCCTCGTCCCGTAG-3’ and downstream: 5’-CAATCTCCACTTTGCCACT-3’
Detection of expression levels of pro-inflammatory factors TNF-α, IL-1β and IL-6 and the antiinflammatory factor IL-10 in the serum by enzymelinked immunosorbent assay (ELISA):
The blood was drawn from the abdominal aorta of rats and centrifuged at 4° and 3500 r/min for 5 min and then diluted as per the instructions of the ELISA kit and a standard curve was plotted. Next, the content of serum TNF-α, IL-1β, IL-6 and IL-10 in rats was detected.
Statistical analysis:
SPSS 16.0 software was employed for statistical analysis, and GraphPad 5.01 software was adopted for plotting. The measurement data were expressed as (x?±s) and compared between groups using independent t test and among groups through single-factor analysis of variance. p<0.05 indicated that a difference was statistically significant.
Results and Discussion
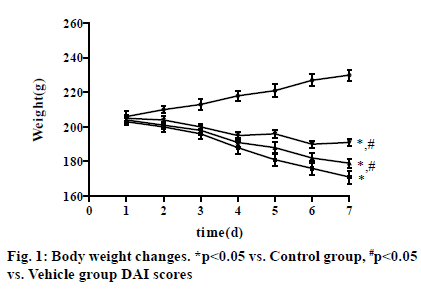
The rats in Control group had glossy hair, constant weight gain and normal urination and defecation, as well as normal drinking, eating and movement. In Vehicle group, the rats had unsmooth and shaggy hair, weight loss, loose stools, decreased drinking, eating and movement from the 3rd d, bloody stools on the 4th d, and gross bloody stools on the 6th d, and the above conditions reached the peak on the 7th d. After intervention with VD3, the above conditions were improved in VD3 low group and VD3 high group compared with those in Vehicle group, with the most obvious improvement in rats in VD3 high group. On the 7th d, the weight of rats was (230.21±3.21) in Control group, showing an upward trend, and (171.18±3.83) in Vehicle group, (179.24±2.62) in VD3 low group and (191.65±2.17) in VD3 high group, displaying a downward tendency (p<0.05) (fig. 1).
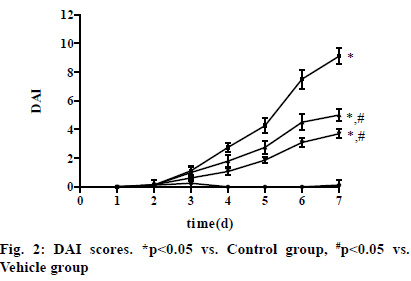
In Vehicle group, the DAI score of rats rose from the 3rd d and reached the peak on the 7th d. After intervention with VD3, the DAI score declined in VD3 low group and VD3 high group compared with that in Vehicle group, with the most obvious decrease in VD3 high group. The score on the 7th d was (0.13±0.35), (9.13±0.54), (5.04±0.48) and (3.71±0.32) in Control group, Vehicle group, VD3 low group and VD3 high group, respectively. The score was significantly higher in Vehicle group, VD3 low group and VD3 high group than that in Control group, while it was significantly lower in VD3 low group and VD3 high group than that in Vehicle group (p<0.05) (fig. 2).
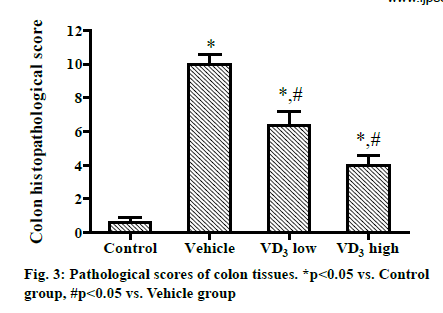
The pathological score of colon tissues was (0.62±0.53) in Control group, (10.21±1.02) in Vehicle group, (6.33±1.52) in VD3 low group, and (4.12±1.10) in VD3 high group. Compared with that in Control group, the score was significantly increased in Vehicle group, VD3 low group and VD3 high group. Compared with Vehicle group, VD3 low group and VD3 high group had significantly reduced scores, and the score decreased markedly with rising concentration of VD3 (P<0.05) (fig. 3).
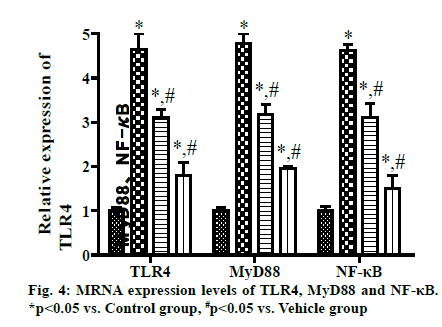
The qRT-PCR results exhibited that the mRNA expression levels of TLR4, MyD88 and NF-κB were significantly higher in Vehicle group, VD3 low group and VD3 high group than those in Control group, while they were significantly lower in VD3 low group and VD3 high group than those in Vehicle group. With increasing concentration of VD3, the mRNA expression levels decreased more significantly (p<0.05) (fig. 4).
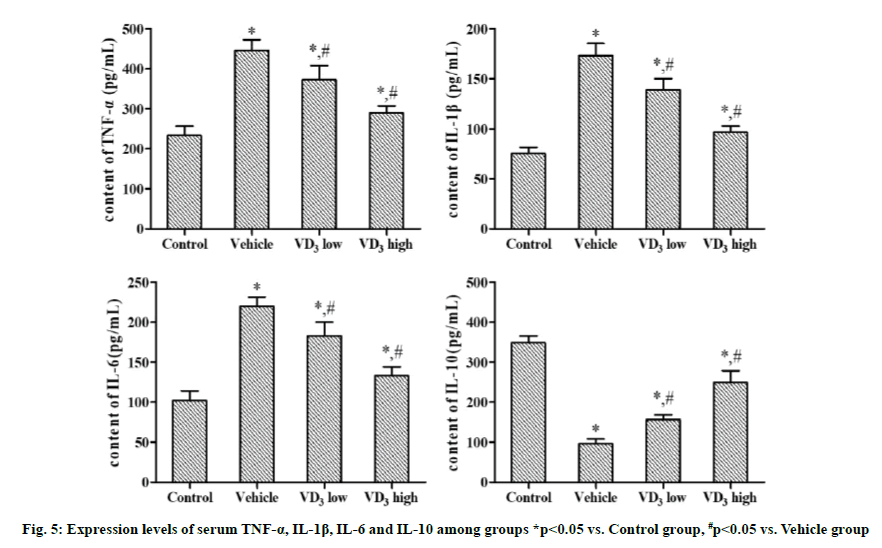
Compared with Control group, Vehicle group, VD3 low group and VD3 high group had significantly increased serum expression levels of TNF-α, IL-1β, and IL-6, and a significantly reduced IL-10 expression level. In comparison with Vehicle group, VD3 low group and VD3 high group displayed significantly lowered expression levels of TNF-α, IL-1β and IL-6 and a significantly elevated IL-10 expression level. With rising VD3 concentration, the decrease in the expression level of pro-inflammatory factors and the increase in the expression level of anti-inflammatory factors were more obvious (p<0.05).
IBD is most often diagnosed in adolescents who have higher risks of extra-intestinal complications and malnutrition than adults[11]. Patients with IBD have varying degrees of VD deficiency, and the content of VD is negatively correlated with disease activity[12]. The rat model of DSS-induced colitis shows similar clinical manifestations and pathological and immunological changes to human IBD, i.e. both rat model of DSSinduced colitis and human IBD will have symptoms such as weight loss, loss of appetite, infiltration of inflammatory cells, diarrhea and hematochezia. Therefore, in this study, rats with DSS-induced colitis were selected as the research model.
In this study, the rats freely drank 3 % DSS solution for 7 d, and the pathological score in Vehicle group was increased. There was a significant difference in above index between Control group and Vehicle group, suggesting the successful establishment of the rat model of IBD, which is consistent with the results of previous studies[13,14]. Besides, IBD manifestations were also observed in VD3 low group and VD3 high group, but the severity was lower than that in Vehicle group, and a higher VD3 concentration indicated lower severity of IBD. These results indicate that VD3 can relieve the colonic inflammation in rats with DSS-induced colitis.
In IBD patients, endotoxins will be produced due to intestinal flora disorders and infections. LPS will activate the TLR signaling pathway, and TLRs will conduct signal transduction through the MyD88-dependent pathways after recognizing LPS. The activated MyD88 is capable of activating TNF receptor associated factor 6 (TRAF6) that binds to NF-κB inhibitor kinase inhibitor (IKK) to activate NF-κB. Activated NF-κB enters the nucleus from cells and activates the expression of related inflammatory genes[15,16]. For this reason, the TLR4/ MyD88/NF-κB signaling pathway is deemed as one of the pathogeneses of IBD. In this study, the results of qRT-PCR uncovered that the mRNA expression levels of TLR4, MyD88 and NF-κB were significantly higher in Vehicle group than those in Control group, whereas they declined after intervention with VD3, being in line with the results of previous research. It suggests that VD3 can alleviate the inflammatory response in rats through the TLR4/MyD88/NF-κB signaling pathway.
1,25-(OH)2D3 can act on Th1 and Th17 cells to inhibit their secretion of pro-inflammatory factors such as IL-6, IL-1β, TNF-α and IFN-γ and up-regulate the anti-inflammatory factors like IL-4 and IL-10, thus suppressing the inflammatory response in the body [17]. NF-κB is also dominant in the modulation of inflammatory response signaling pathways, and induces the transcription and translation of inflammatory factors to release them into the blood[18]. The results of this study revealed that expression levels of serum IL-6, IL-1β and TNF-α were significantly higher in Vehicle group than those in Control group, while the IL-10 expression level was significantly lower in Vehicle group than that in Control group. After intervention with VD3, the expression levels of IL-6, IL-1β and TNF-α declined, whereas the expression level of IL-10 rose compared with those in Vehicle group. This implies that VD3 is able to attenuate the inflammatory response in rats by regulating the expression of inflammatory factors.
In conclusion, VD3 impedes the TLR4/MyD88/NF- κB signaling pathway and reduces the expression of downstream inflammatory factors to mitigate intestinal injury and colonic inflammation in IBD rats. However, in-depth studies should be carried out on the mechanism by which VD relieves IBD, because IBD has a complex pathogenesis and involves many inflammatory response pathways.
Author’s contribution:
Weiwei Liu and Xueping Liu equally contributed to this work.
References
- de Souza HS. Etiopathogenesis of inflammatory bowel disease: today and tomorrow. Curr Opinion Gastroenterol 2017;33(4):222-9.
- Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Translational Res 2016;8(6):2490-7.
- Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J. Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem 2018;47(4):1497-508.
- Makkar S, Riehl TE, Chen B, Yan Y, Alvarado DM, Ciorba MA, Stenson WF. Hyaluronic acid binding to TLR4 promotes proliferation and blocks apoptosis in colon cancer. Molecular cancer Ther 2019;18(12):2446-56.
- Lee J, Park JR, Lee H, Jang S, Ryu SM, Kim H, et al. l-carnosine induces apoptosis/cell cycle arrest via suppression of NF-κB/STAT1 pathway in HCT116 colorectal cancer cells. In Vitro Cellular & Developmental Biology-Animal. 2018;54(7):505-12.
- Shon WJ, Lee YK, Shin JH, Choi EY, Shin DM. Severity of DSS-induced colitis is reduced in Ido1-deficient mice with down-regulation of TLR-MyD88-NF-kB transcriptional networks. Sci Rep 2015;5(1):1-2.
- Meeker S, Seamons A, Maggio-Price L, Paik J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol 2016;22(3):933-36.
- Erben U, Loddenkennper C, Spieckermann S, Heimesaat MM, Siegmund B, Kuehl AA. Histomorphology of intestinal inflammation in inflammatory bowel diseases (IBD) mouse models and its relevance for IBD in men. Int J Clin Exp Med 2016;9:408-42.
- Arihiro S, Nakashima A, Matsuoka M, Suto S, Uchiyama K, Kato T, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflam Bowel Dis 2019;25(6):1088-95.
- Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993;69(2):238-49.
- Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr 2015;169(11):1053-60.
- Fletcher J, Cooper SC, Ghosh S, Hewison M. The role of vitamin D in inflammatory bowel disease: mechanism to management. Nutrients 2019;11(5):1019.
- Hart ML, Ericsson AC, Franklin CL. Differing Complex Microbiota Alter Disease Severity of the IL-10−/− Mouse Model of Inflammatory Bowel Disease. Front Microbiol 2017;8:792.
- Shouval DS, Biswas A, Kang YH, Griffith AE, Konnikova L, Mascanfroni ID, et al. Interleukin 1β mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterol 2016;151(6):1100-4.
- Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang C, et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMed 2018;35:345-60.
- Qiao CX, Xu S, Wang DD, Gao SY, Zhao SF, Zhang ML, et al. MicroRNA-19b alleviates lipopolysaccharide-induced inflammatory injury in human intestinal cells by up-regulation of Runx3. Eur Rev Med Pharmacol Sci 2018;22(16):5284-94.
- Verlinden L, Leyssens C, Beullens I, Marcelis S, Mathieu C, De Clercq P, et al. The vitamin D analog TX527 ameliorates disease symptoms in a chemically induced model of inflammatory bowel disease. J Steroid Biochem Mol Biol 2013;136:107-11.
- Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, et al. An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Communication 2017;8(1):1-3.