- *Corresponding Author:
- Bhagyashree Mahanta
Department of Zoology, Morigaon College, Morigaon 782105, India
E-mail: mahantabhagyashree@rediffmail.com
| Date of Received | 14 March 2020 |
| Date of Revision | 12 October 2021 |
| Date of Acceptance | 03 August 2022 |
| Indian J Pharm Sci 2022;84(4):999-1005 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Solanum torvum Sw. is a native plant of India found abundantly in North East. It has many medicinal properties and its berries are used to treat fungal infections by the natives of the study area. The efficacy of Solanum torvum Sw. was evaluated using both in vitro antimicrobial methods and in vivo model of vaginal Candida albicans infection. Two in vitro models, disc diffusion assay and micro dilution broth assay was used and in in vivo study laboratory female rats were used. In the disc diffusion assay highest concentration i.e. 200 mg/ml Solanum torvum Sw. methanolic extract dose shows larger inhibition zone of fungal growth than the other concentrations of extract. In the microdilution broth assay, 150 mg/ml concentration of extract shows highest fungal growth inhibition. Histological study was done by loading fungal conidia on vaginas of in vivo model (rat) where 600 mg/kg extract treated group showed complete reduction of fungal growth over vaginal epithelium and vaginal cavity. In result, this plant proves its efficacy in reducing the fungal load from vaginas of infected rat as well as showing good minimum inhibitory concentration and zone of inhibition at in vitro study. From the above study, it was clear that Solanum torvum Sw. showed promising positive result against Candida albicans.
Keywords
Solanum torvum Sw., Candida albicans, minimum inhibitory concentration, zone of inhibition
All over the world, the Candida albicans (C. albicans) infection is the common and frequently reported infection[1,2]. It has been considered as the major nosocomial cause of mortality in immunocompromised patients[3,4]. It infect patients in different ways from mucosal candidiasis, including oropharyngeal and oesophageal candidiasis, observed in immunocompromised patients, to vulvovaginal candidiasis, which affects a large number of otherwise healthy women[2]. A vulvovaginal candidiasis is considered the most common infection affecting around 75 % of women at least once in their life time, which could also present frequent episodes of recurrence[5,6]. Very often this infection including mucosal and invasive candidiasis is associated with biofilm formation. Biofilm serving as a protective environment against external insults[7]. C. albicans vaginitis has been increasing in medical importance, since a significant proportion of women suffer from acute episodes and recurrent infections may often occur after therapy. Vulvovaginal candidiasis is frequently associated with conditions such as diabetes mellitus, anti-biotic therapy and pregnancy; although in many cases there are no clear predisposing factors[8,9]. In recent years azole agents have become the drugs of choice for treating vulvovaginal candidiasis[10,11]. Azole antifungals such as fluconazole and ketoconazole are being extensively used for treatment of systemic candidiasis and the extensive use has led to the rapid development of drug resistance among Candida species[10]. The high incidence of the different forms of candidiasis is an increasing number of compromise patients and the development of resistance against these conventional antifungal agents point to the urgent need to discover and develop novel therapeutics against infection caused by Candida species[6,11-13].
Phytochemicals are always been very effective against different disease. In this study Solanum torvum Sw. berry extract was used to inhibit the growth of C. albicans both in vitro and in vivo models.
Solanum torvum Sw. is a perennial plant native to India[14,15]. It is reportedly to be widely distributed in Southern India, Himalayan foot hills and North Eastern India[16,17]. Solanum torvum Sw. exhibit pharmacological effect as sedative and diuretic[18]. The fruit decoction is used in cough, liver and spleen enlargement. The methanolic extract of fruit and leaves were reported to have anti-microbial activity against human and animal clinical isolated[19]. The aqueous extracts from various parts of Solanum torvum Sw. exhibit potential antiinflammatory and analgesic properties[20]. Depending on the scientific reports about Solanum torvum Sw. as a potential medicinal plant and information about the use of berries to treat leucorrhoea (vaginal candidiasis) by the herbal healers of Mayong where survey for medicinal plants was conducted by authors, the plant was selected for the present experiments.
Materials and Methods
Plant sample:
Fresh raw fruit of Solanum torvum Sw. was collected in the month of July and August from different villages of Mayong circle, Morigaon, Assam. Mayong is situated in the latitude of 26.25° North and longitude of 92.04° East. After collection, the berries were washed in 70 % alcohol and cut in small pieces and then shade dried for 20 d and made in coarse powder by an electric mixture grinder machine. The coarse powder was taken for preparation of extract.
The identity of the above-mentioned plant was confirmed by comparison with reference herbarium specimen available in the Department of Botany, Gauhati University, Guwahati, Assam, India and with the help of relevant literature[21]. The voucher specimen was preserved for future reference.
Methods for extraction:
Coarsely powdered material of the plant species was extracted separately at room temperature by cold maceration process by using methanol as a solvent. The extract thus obtained was filtered and evaporated to dryness under reduced pressure using rotary vacuum evaporator (Buchi, Switzerland). The extracts were dried in vacuum desiccators above anhydrous calcium chloride for 96 h and the percentage of extract obtained was recorded.
Experimental animals:
For in vivo experiments, 9-11 w old female Wister rats were used. All the animals required for experiments were obtained from the Animal House Facilities of the Department of Zoology, Gauhati University, India. Experiments on animals were approved by the Institutional Animal Ethics Committee (IAEC No. 655902/ac/05/CPCSEA) and accepted according to veterinary medical practice.
in vivo treatment administration:
The preliminary experiments were carried out with two types of vehicles to administer the crude plant extract to animals, including 80 % ethanol or a mixture of 5 % Tween 80 and 5 % ethanol in distilled water. The second vehicle gives the optimal result. The rats were divided in four groups (vehicle treated group, 300 mg/kg Body Weight (BW) extract treated group, 600 mg/kg BW extract treated group and 30 mg/kg BW ketoconazole treated group). In each extract treated groups, Solanum torvum methanolic extracts (300 mg/kg and 600 mg/kg) were administered by intra gastric gavage. Ketoconazole was used as a positive control at doses determines to be optimal in preliminary study (30 mg/kg BW). Appropriate vehicle controls were also used in parallel respecting strictly identical experimental protocol.
Experimental fungal strain:
A pure culture of C. albicans was purchased from Microbial Type Culture Centre (MTCC) of Chandigarh, India (MTCC No. 3455) in a freeze-dried form.
Preparation of inoculum:
The inoculum size of C. albicans was standardized by harvesting the fungal conidia using an aseptic wire loop after addition of 10 ml sterile saline containing 0.1 % Tween 80 to the culture plates. About 7 ml of the fungal suspension was then centrifuged at 270 g for 20 min at 4° to remove hyphal fragments. The concentration of the conidial suspension was adjusted to a concentration of 1.0×106 conidia per ml by counting with a haemocytometer.
Disc diffusion antimicrobial assay:
Sterile paper discs (Whatman No. 1, 6 mm diameter) were loaded with 100 ml of each of the extract concentration (12.5, 25, 50, 100, 150, 200, 250 and 300 mg/ml) to give a final concentration of 1 mg/disc. An even spread of microorganism was prepared by transferring 50 ml of microbial suspension on sterilized nutrient agar plates using sterile cotton buds. The extract discs were then positioned on the inoculated agar surface. Each extract was assayed in triplicate. Ketoconazole (30 mg/disc) was used as standard antifungal agent, whereas sterilized saline water was used as a negative control. The plates were then incubated at 37° for 24 h. The screening for antifungal activity was done by measuring the diameter of a clear inhibition zone around the disc. The mean diameter of inhibition zone was measured to the nearest millimetre (mm) based on three readings of the diameter zones of each target fungus using the vernier callipers[22].
Micro dilution broth assay:
The Minimum Inhibitory Concentration (MIC) value of the extracts was determined against C. albicans using microdilution method. Different concentration of plant extract was prepared (12.5, 25, 50, 100, 150, 200, 250 and 300 mg/ml) in sterile nutrient broth using sterilized test tubes. Ketoconazole (30 mg/ml) was used as positive control; whereas sterilized saline water was used as negative control. Each test tube was seeded by 100 μl standardized fungal inoculums (106 spores per ml) and incubated in 37° for 24 h. The MIC value was taken as the lowest concentration of the extracts in the test tubes that showed no turbidity after 24 h of incubation at 37°. The turbidity of the tubes was interpreted as visible growth of the fungus[23].
Fungal inoculation on animals:
Rats were immunosuppressed by treating them with 30 mg/kg cylcosporin by intra peritoneal route every day for 7 d. Then, rats were inoculated intravaginally with 106 cells (in 150 μl of sterile saline solution) of washed blastoconidia of C. albicans[24-26]. 3 d before inoculation and before the beginning of any treatment, the animals were sampled to confirm the absence of C. albicans organisms in the vaginal cavity.
Vaginal lavage collection:
The vaginal lavage that released by the vagina of rats due to Candida infection was collected by rolling a sterile cotton swab over the vaginal cavity. The swab was then suspended in 1 ml of sterile saline buffer. This operation was repeated on 3rd and 10th d post-infection to observe the course of infection. Determination of the number of Candida organisms was conducted in duplicate after serial 10 fold dilution of washing fluid and plating on nutrient agar. All plates were incubated at 30° for 24 h for each series of dilutions. The number of viable cells (Colony Forming Unit (CFU)) was determined using the drop count method. The percentage of CFU reduction was calculated by using following formula.
Percentage of CFU reduction=(CFU/ml of control-CFU/ ml of treated)/CFU/ml of control
Sacrifice of experimental model:
After completion of the treatment as required by the respective protocol, rats were killed under mild dose of anaesthesia (diethyl-ether) followed by cervical dislocation. The rats receiving daily intra vaginal treatment were sacrificed 24 h after the last dose. Body weight of all the animals was recorded just before killing.
Histological studies of vagina:
Tissue preparation and sectioning: Vaginas were removed from the sacrificed animals and then fixed in toto by immersion in Bouin’s solution for at least 48 h. After fixation the tissues were washed 4-5 times with distilled water (a pinch amount of lithium carbonate was used to remove colour of picric acid of Bouin’s faster). Afterwards tissues were processed for dehydration through a series of ethanol grade (30 %, 50 %, 70 %, 90 % and 100 %) and cleaned with xylene (absolute alcohol/xylene, xylene), before being infiltrated with hot paraffin wax for 2-3 h. Subsequently, tissues were embedded transversely in paraffin wax and cut into 5-6 μm sections using a microtome (Ernst Leitz Wetzlar GMBH, Germany).
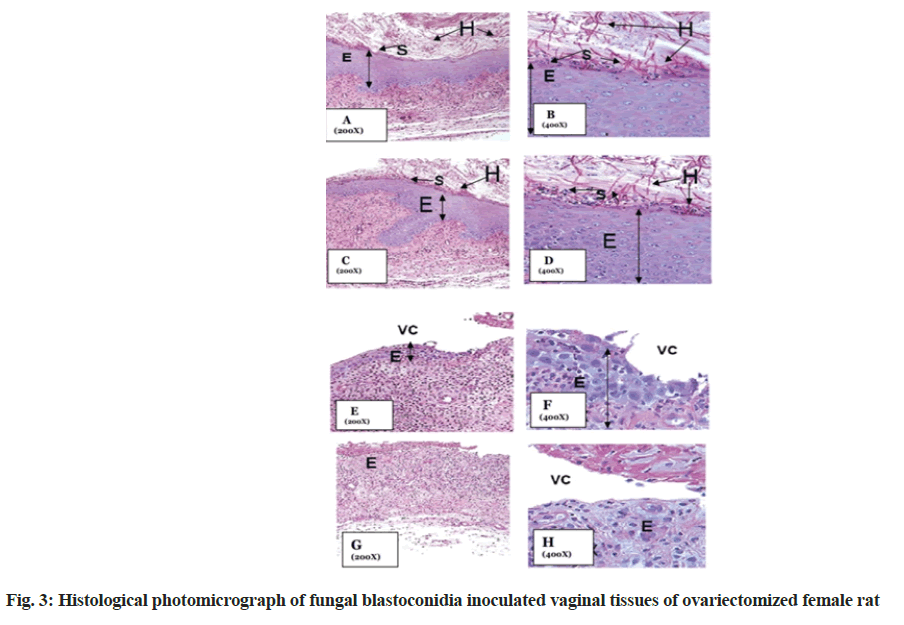
Staining and mounting: The sections were stained with Periodic Acid-Schiff (PAS). Prior to staining, the sections were dewaxed by immersion in xylene. The xylene was removed by immersion in a series of descending grade of ethanol and then in distilled water. The tissues were stained by PAS for detection of fungal growth. The sections were subsequently dehydrated again by ascending grade of alcohol and finally in xylene before mounting by dibutylphthalate polystyrene xylene and covered with cover slips. Slides were then observed under microscope (Leitz, Ortholux II, Germany) for histological study about the growth inhibition of fungal hyphae. The growth inhibition of fungal hyphae was compared among negative control, extract treated and positive control group.
Results and Discussion
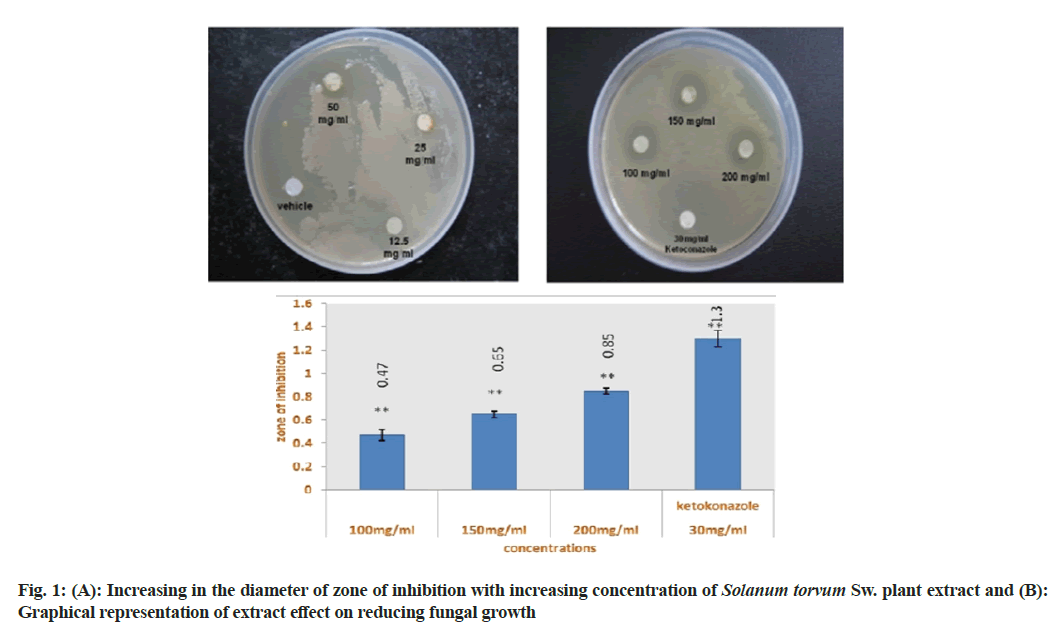
The disc diffusion assay was carried out by loading different concentration of Solanum torvum SW. extract (12.5, 25, 50, 100, 150, 200, 250 and 300 mg/ml) on sterilized filter paper discs. Ketoconazole (30 mg/disc) was used as standard antifungal agent, whereas sterilized saline water was used as a negative control. The extract loaded discs were then positioned on the C. albicans inoculated agar surface and incubated at 37° for 24 h. After incubation clear zone of inhibition of fungal growth was found surrounding the filter paper disc at 100, 150 and 200 mg/ml concentration of plant extract as well as ketoconazole loaded disc. 12.5, 25 and 50 mg/ml concentration of extract displayed no zone of inhibition (fig. 1A and fig. 1B).
Different concentration of plant extract was prepared (12.5, 25, 50, 100, 150, 200, 250 and 300 mg/ml) in sterile nutrient broth using sterilized test tubes for determining the MIC value. Ketoconazole which is a standard antifungal drug (30 mg/ml) was used as positive control; whereas sterilized saline water was used as negative control. The result of micro dilution broth method revealed that 200 mg/ml concentration of Solanum torvum Sw. extract is the MIC which showed clearly visible inhibition of fungal growth. 12.5 to 100 mg/ml concentration of extract showed no inhibition, where 150 mg/ml concentration showed a little inhibition of fungal growth (Table 1).
| Concentration of ketoconazole | Observation |
|---|---|
| 12.5 mg/ml | - |
| 25 mg/ml | - |
| 50 mg/ml | - |
| 100 mg/ml | - |
| 150 mg/ml | + |
| 200 mg/ml | +++ |
| 30 mg/ml ketoconazole | ++++ |
Note: (-): No inhibition; (+): Very little inhibition; (++): Little inhibition; (+++): Clearly visible inhibition and (++++): Complete inhibition
Table 1: Mic Values of Solanum Torvum Sw. Plant Extract
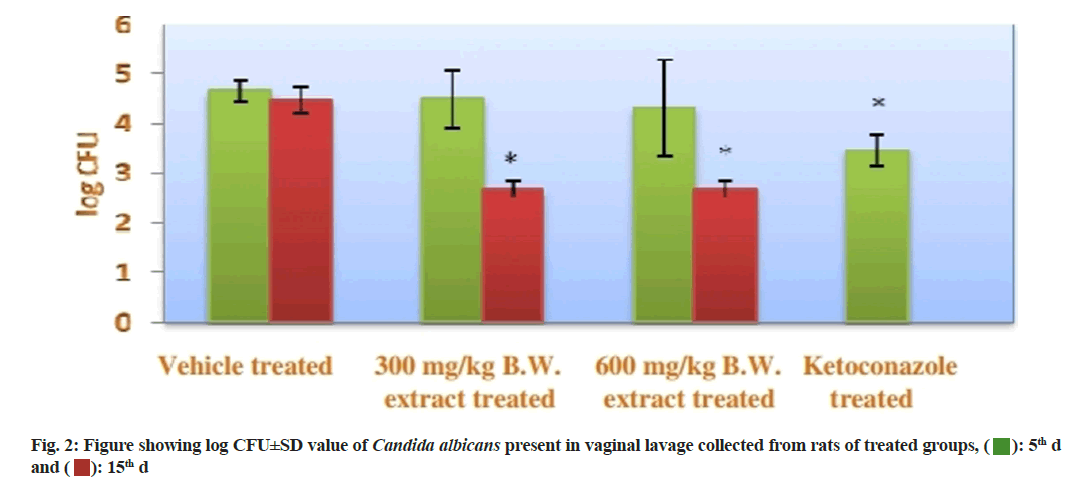
CFU of C. albicans present in the vaginal lavage collected from each group at 5th d and 15th d, it was found that the vehicle treated group showed 0 % reduction on CFU of C. albicans in both 5th and 15th d. On the other hand, the both extracts treated groups (group II and group III) displayed reduction in percentage of CFU at 5th and 15th d. At 5th d, group II showed a little bit lesser percentage of reduction of CFU then the group II, but at 15th d both the group showed same result by reducing the percentage of CFU up to 98.89 %. The positive control group showed maximum reduction at 15th d (100 %). The statistical data of this experiment was recorded in the fig. 2.
Striking changes in the presence of fungal hyphae and spores were observed in the vehicle treated ovariectomized rat (normal control group) (fig. 3A, fig. 3B) when compared with treated groups (fig. 3C-fig. 3H). Presence of a lot of fungal hyphae and spores were found over the epithelium was found in normal control group. Reduction of fungal hyphae and spores over epithelium were also not seen in the first treated group, while complete reduction of the growth of fungal hyphae and spores were seen in both 2nd treated group as well as positive control group.
C. albicans is the most common Candida species residing in our oral and vaginal cavity in both health and disease and is the agent of most Candida infection. Several effective antifungal agents are available for the management of candidiasis. But isolates may exhibit intrinsic or secondary resistance to the drug during therapy[27]. So, the use of natural products as alternative agents for the control of fungal disease is considered as an interesting alternative to synthetic fungicides[28].
In this present study, Solanum torvum Sw. methanolic extract possess a promising antifungal activity against C. albicans at both in vitro and in vivo condition. These results support the traditional use of Solanum torvum Sw. berry to treat leucorrhoea which is useful and potential in prevention and treatment of candidal vaginitis. The efficacy of plants and their extracts is due to the presence of several primary or secondary metabolites such as phenols, polyphenol, tannins, flavonoids, alkaloids and terpenoids etc.[29]. Although phytochemicals are antimicrobial in nature but they also produce other biological activities in human body like induction of immunity which indirectly reduces the risk of many disease[30]. Further study to reveal the proper fungicidal mechanism and responsible antimicrobial phytochemical fraction of Solanum torvum Sw. berries is still needed in near future.
Acknowledgements:
I am very thankful to Dr. Krishna Sarma and Dr. Rakesh kr. Sharma from Department of Microbiology, College of Veterinary Science, AAU, Assam for their kind help and encouragement.
Conflict of interests:
The authors declared no conflict of interests.
References
- de Bernardis F, Arancia S, Morelli L, Hube B, Sanglard D, Schäfer W, et al. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J Infect Dis 1999;179(1):201-8.
[Crossref] [Google Scholar] [PubMed]
- Sobel JD, Hasegawa A, Debernardis F, Adriani D, Pellegrini G, Cassone A, et al. Selected animal models: Vaginal candidosis, Pneumocystis pneumonia, dermatophytosis and trichosporosis. Med Mycol 1998;36:129-36.
[Google Scholar] [PubMed]
- Kothavade RJ, Kura MM, Valand AG, Panthaki MH. Candida tropicalis: Its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol 2010;59(8):873-80.
[Crossref] [Google Scholar] [PubMed]
- Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MM. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol 2013;62(1):10-24.
[Crossref] [Google Scholar] [PubMed]
- Ghelardi E, Tavanti A, Lupetti A, Celandroni F, Boldrini E, Campa M, et al. Control of Candida albicans murine vaginitis by topical administration of polycarbophil-econazole complex. Antimicrob Agents Chemother 1998;42(9):2434-6.
[Crossref] [Google Scholar] [PubMed]
- dos Santos Ramos MA, Calixto G, de Toledo LG, Bonifácio BV, dos Santos LC, de Almeida MT, et al. Liquid crystal precursor mucoadhesive system as a strategy to improve the prophylactic action of Syngonanthus nitens (Bong.) Ruhland against infection by Candida krusei. Int J Nanomedicine 2015;10:7455-66.
[Crossref] [Google Scholar] [PubMed]
- Uppuluri P, Pierce CG, López-Ribot JL. Candida albicans biofilm formation and its clinical consequences. Future Microbiol 2009;4(10):1235-7.
[Crossref] [Google Scholar] [PubMed]
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev 1999;12(1):40-79.
[Crossref] [Google Scholar] [PubMed]
- Quereda C, Polanco AM, Giner C, Sanchez-Sousa A, Pereira E, Navas E, et al. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in HIV-infected patients. Eur J Clin Microbiol Infect Dis 1996;15(1):30-7.
[Crossref] [Google Scholar] [PubMed]
- Khodavandi A, Harmal NS, Alizadeh F, Scully OJ, Sidik SM, Othman F, et al. Comparison between allicin and fluconazole in Candida albicans biofilm inhibition and in suppression of HWP1 gene expression. Phytomedicine 2011;19(1):56-63.
[Crossref] [Google Scholar] [PubMed]
- Díaz-Guerra TM, Martinez-Suarez JV, Laguna F, Rodriguez-Tudela JL. Comparison of four molecular typing methods for evaluating genetic diversity among Candida albicans isolates from human immunodeficiency virus-positive patients with oral candidiasis. J Clin Microbiol 1997;35(4):856-61.
[Crossref] [Google Scholar] [PubMed]
- Ngo HX, Garneau-Tsodikova S, Green KD. A complex game of hide and seek: The search for new antifungals. Med Chem Comm 2016;7(7):1285-306.
[Crossref] [Google Scholar] [PubMed]
- Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: Prevalence, mechanisms and management. Lancet Infect Dis 2017;17(12):e383-92.
[Crossref] [Google Scholar] [PubMed]
- Gousset C, Collonnier C, Mulya K, Mariska I, Rotino GL, Besse P, et al. Solanum torvum as a useful source of resistance against bacterial and fungal diseases for improvement of eggplant (S. melongena L.). Plant Sci 2005;168(2):319-27.
- Kumchai J, Wei YC, Lee CY, Chen FC, Chin SW. Production of interspecific hybrids between commercial cultivars of the eggplant (Solanum melongena L.) and its wild relative S. torvum. Genet Mol Res 2013;12(1):755-64.
[Crossref] [Google Scholar] [PubMed]
- Ram D, Rai M, Singh M, Peter KV, Abraham Z. Temperate and subtropical vegetables. Biodivers Hortic Crop 2007:71-108.
- Deka BC, Thirugnanavel A, Patel RK, Nath A, Deshmukh N. Horticultural diversity in Northeast India and its improvement for value addition. Ind J Genet Plant Breed 2012;72(2):157-67.
- Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity of two traditional Indian vegetables: Solanum nigrum L. and Solanum torvum L. Food Sci Biotechnol 2010;19(1):121-7.
- Chah KF, Muko KN, Oboegbulem SI. Antimicrobial activity of methanolic extract of Solanum torvum fruit. Fitoterapia 2000;71(2):187-9.
[Crossref] [Google Scholar] [PubMed]
- Ndebia EJ, Kamgang R, Nkeh-ChungagAnye BN. Analgesic and anti-inflammatory properties of aqueous extract from leaves of Solanum torvum (Solanaceae). Afr J Tradit Complement Altern Med 2007;4(2):240-4.
[Crossref] [Google Scholar] [PubMed]
- Lopez-Ribot JL, McAtee RK, Perea S, Kirkpatrick WR, Rinaldi MG, Patterson TF. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 1999;43(7):1621-30.
[Crossref] [Google Scholar] [PubMed]
- Brown R. Prodromus florae novae hollandiae. J. Johnson & Co. London; 1810.
- Uyanik A, Öktemer A, Loğoğlu E. Antimicrobial and antifungal activity study of poly substituted benzene derivatives. Commun Fac Sci Univ Ank Series B 2009;55(1):17-22.
- Arash M, Mohammad K, Maryam S, Zahra K, Zahra J, Ramin A. Evaluation of antibacterial activity of three Iranian medicinal plants. Afr J Microbiol Res 2012;6(9):2048-52.
- Cassone A, Boccanera M, Adriani DA, Santoni G, de Bernardis FL. Rats clearing a vaginal infection by Candida albicans acquire specific, antibody-mediated resistance to vaginal reinfection. Infect Immun 1995;63(7):2619-24.
[Crossref] [Google Scholar] [PubMed]
- de Bernardis F, Mühlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun 1998;66(7):3317-25.
[Crossref] [Google Scholar] [PubMed]
- Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol 2018;9:1639.27.
[Crossref] [Google Scholar] [PubMed]
- Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol 2018;9:1639.
[Google Scholar] [PubMed]
- Wang Y, Bandara HM, Mikkelsen D, Samaranayake LP. Effects of tea extracts on the colonization behaviour of Candida species: Attachment inhibition and biofilm enhancement. J Med Microbiol 2017;66(8):1244-52.
- Whyte D, Sharma K, Tarver P. The impact of feeding a supplement based on aloe and Manuka honey on milk yield from dairy cows. J Appl Animal Nutr 2017;5.
 ): 5th d
and (
): 5th d
and ( ): 15th d
): 15th d