- *Corresponding Author:
- V. M. Berlin Grace
Department of Biotechnology, Karunya Institute of Technology and Sciences, Karunya Nagar, Coimbatore, Tamil Nadu 641114, India
E-mail: berlin@karunya.edu
| Date of Submission | 12 May 2020 |
| Date of Revision | 23 September 2021 |
| Date of Acceptance | 21 March 2022 |
| Indian J Pharm Sci 2022;84(2):348-357 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The flowers of Cocos nucifera (L.) have a traditional use as a healer of inflammatory disorders and are available as Ayurveda formulations. But the scientific knowledge on the bioactive phytochemicals and their medicinal effects are lacking. Hence, as a preliminary study, we have extracted the phytochemicals from the flowers of Cocos nucifera(L.) using ethanol and subjected to biochemical tests as well as gas chromatography–mass spectrometry analysis for phytochemical screening. The anti-cancer effect on human lung cancer cell line was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay for 25-200 μg/ml of the extract and the anti-inflammatory activity for different concentrations (1000- 8000 μg/ml) of the extract was evaluated using in vitro egg albumin degradation assay. The biochemical tests have indicated the presence of phytosterols and tannins. A total of 152 phytochemicals were reported in the gas chromatography–mass spectrometry analysis, which includes several phytosterols and few fatty acids, polyphenols and terpenes. A dose dependent growth inhibitory action (96.15 % by 200 μg/ml) was exerted on human lung cancer cell line cells by the extract (half-maximal inhibitory concentration value-90.2 μg/ ml). An effective inhibition on protein degradation (>50 %) as an indication of anti-inflammatory activity was exerted by the extract from 1000 μg/ml and the 4000 μg/ml treatment has shown a similar action as the 500 μg/ml of standard anti-inflammatory drug, diclofenacsodium has shown. The secondary metabolites such as quercetinderivative, eugenol, catechol, stigmasterol, campesterol, t-butylhydroquinone identified might have exerted these medicinal effects. This study results thus gives a clear medicinal impact of the ethanol extract of Cocos nucifera(L.) flower by showing a greater anti-inflammatory and anti-cancer properties for further exploration of its pharmacological importance in the future.
Keywords
Cocos nucifera (L.), phytosterols, gas chromatography–mass spectrometry, lung cancer, antiinflammatory
Nature has been a source of medicinal agents for thousands of years and an impressive number of modern drugs have been isolated from natural resources. The use of medicinal plants to treat human diseases has its roots in pre-historical times. Medicinal plants are used by 80 % of the world population as the only available medicines especially in developing countries[1]. Traditional systems of medicine continue to be widely practiced on many accounts. Population rise, inadequate supply of drugs, prohibitive cost of treatments, side effects of several synthetic drugs and development of resistance to currently used drugs for infectious diseases have led to increased emphasis on the use of plant materials as a source of medicines for a wide variety of human ailments. Several recent studies have recorded that the phytochemicals are effective in controlling the cancer and inflammatory conditions. The Cocos nucifera (L.), commonly known as coconut or coconut of beach is a member of the family Arecaceae and every part of it such as root, husk, water, inflorescence, leaves and fruit adds greater medicinal value to human. It is widely available in India, basically came from Southeast Asia and the islands between the Indian Ocean and Pacific Ocean. The various constituents of Cocos nucifera (L.) were reported to have various pharmacological properties such as antioxidant, antitumor, anti-helminthic, anti-inflammatory, anti-arthritic, anti-diabetic and antimicrobial activities[2]. The review also has highlighted the cardio, nephron and hepatic-protective nature of Cocos nucifera. The coconut powder was proven to be a gluten-free good source of protein, packed with dietary fiber and can be used in the form of cookies for gluten intolerance condition like celiac disease that affects 1 out of 33 people[3,4]. Various preparations of coconut husk fiber is used to reduce renal inflammation, injuries, dermatitis and abscesses in Guatemala[5]. As the constituents of each part of Cocos nucifera varies, the pharmacologic effect also varies. The unripe coconut water was reported to contain more minerals like potassium than the ripe coconut water[6].
The flowers of Cocos nucifera (L.) have a traditional use as a potent healer of many inflammatory disorders like postnatal changes. Food technology based studies are underway to use its powder as an alternate to wheat flour by considering its gluten free nature, nutritional value and natural sweetness. The flower (inflorescence) of Cocos nucifera (L.) may also be a natural and delicious alternative to wheat and grain, which is not explored yet. In India, the coconut flower infusions as tea are used for treating the disorders associated with menstrual cycle[7]. Coconut flower nectar is full of vitamins and minerals which contains 17 amino acids, minerals and vitamin C; has a broad spectrum of B vitamins (vitamin B1, B2, B3 and B6); and is high in potassium, magnesium, zinc and iron[8,9]. The coconut flower nectar syrup is available (ex: Bali Sun) as low glycemic ‘sap’ that was extruded from the coconut blossoms. Coconut flower relieves stress on pancreas and enzyme systems of the body, reducing the risks associated with diabetes and pancreatitis. It reduces problems associated with malabsorption syndrome and cystic fibrosis. However, the knowledge on the bioactive phytochemicals present in the extracts of coconut flower and their pharmacological effects are lacking. The traditional usage of coconut flowers as medicinal agent provoked us to explore the bioactive compounds present in the extract of Cocus nucifera(L.) flower and its medicinal properties in this study.
The coconut flower is available as Ayurveda formulations and value added products for human consumption. An Ayurvedic preparation named “Thengin Pookula Lehyam”, made from coconut flowers, is widely used by the women of Kerala as a post-natal medicine and an in vivo study has proven a significant treatment effect of aqueous extract of coconut flower on induced Poly Cystic Ovarian Disease (PCOD) in rats, which supports the traditional uses[10]. In traditional medicine, it helps in relieving back pain, promoting lactation, reducing post-natal tension and anxiety. It also helps in recovery from health changes and in the overall development of the baby and the mother. Yet, not much research studies have been carried out on the medicinal properties of the flower extracts of Cocos nucifera (L.). The phytochemicals were analyzed by preliminary tests in different solvent as well as aqueous extracts of the flowers of Cocos nucifera, in which the ethanol extract was found to have phytosterols and tannins[11]. Tannins were reported in both fiber and inflorescence[12-14]. The alcoholic extract and oil of Cocos nucifera flower have shown a curative effect on alloxan-induced pancreatic cytotoxicity with an anti-diabetic action[15]. The polyphenol tannins and the phytosterols are the phytochemicals proven to have an effective anti-inflammatory and anti-cancer properties[16-21]. Hence, the ethanol extract of Cocos nucifera flower may poses a good anti-inflammatory and anti-cancer potency and in this study, therefore we have used the ethanol extract of Cocos nucifera flowers for analysis.
Inflammation is a complex biological response of vascular tissues to harmful stimuli as a protective attempt by the organism to remove the injurious stimuli and initiate the healing process[22,23]. Injury and inflammation is also reported in pregnancy and the fetal inflammation plays a major role in the perinatal period and infancy[24,25]. However prolonged inflammation in colon like in Crohn’s disease and chronic ulcerative colitis, leads to colon carcinogenesis[26]. Recent studies have shown a strong association of inflammation in the promotion and progression of various cancers[27,28]. Hence, the recent trend in research is the search of medicinal agents having both anti-inflammatory and anti-cancer effects. The synthetic anti-inflammatory drugs leads to complications like gastrointestinal disorder[29] severe skin and soft tissue malfunction[30]. Similarly, the synthetic chemotherapeutic agents used to cure cancers are cytotoxic in nature and are harmful to the normal cells, causing various side effects. Hence in recent years, several plant sources are being studied and considered as an alternative medicines to overcome the side effects and increase the quality of life[31,32]. Lung cancer is a malignant lung tumor which if left untreated, it can spread beyond the lung by the process of metastasis into nearby tissue or other parts of the body by mimicking inflammatory process[33-36]. Hence, the phytochemicals having anti-inflammatory activity and anti-cancer activity may be of a better choice to treat lung cancer. The phytochemicals can reduce the side effects by its anti-oxidant potency and improve the cytotoxicity of chemotherapy by decreasing the resistance to drugs. Several plant secondary metabolites have been trialed as adjuvant with chemotherapeutic drugs for treating various cancers[37]. During the past years, several parts of Cocos nucifera (L.) have been tested for different pharmacological properties. The aqueous extract of Cocos nucifera (L.) husk fiber has been proven to have anti-inflammatory and anti-oxidant property in mice study[38] and it was found to be a good anti-neoplastic agent[39]. The ethanol extract of the seeds of Cocos nucifera (L.) and the aqueous kernel extract were found to have anti-inflammatory, anti-ulcer and wound healing potency in rats[40,41]. However, no studies have been carried out for the Cocos nucifera (L.) flower extract till now, in spite of having many medicinal effects in traditional use. Hence, this study was focused to explore the medicinal potency and the phytochemicals of the ethanol extract of Cocus nucifera L. flowers.
Materials and Methods
Collection of sample and authentication:
Fresh flowers of the plant Cocos nucifera (L.) were collected from Coimbatore, India. The plant materials were taxonomically identified and authenticated by the Botanical Survey of India and the voucher specimen (Authentication no: BSI/SRC/5/23/2017 Tech/3223) was retained in our laboratory for future reference.
Preparation of extract:
The flowers of Cocos nucifera (L.) were air dried under the shade for 7 d and ground in an electrical grinder to coarse powder. The powder was packed in the Soxhlet apparatus and extracted with ethanol for 24 h at about 75°. The extract was dried and stored in the refrigerator until analysis[42].
Preliminary screening tests for phytochemicals:
The Cocos nucifera (L.) flowers extract was subjected to various preliminary phytochemical tests by following the reported biochemical test protocols as given below:
Mayer’s test for alkaloids: A small quantity of the extract was treated with few drops of dilute Hydrochloric Acid (HCl) and filtered. The filtrate was tested with Mayer’s reagent for the formation of cream precipitate.
Sodium Hydroxide (NaOH) test for flavonoids: Few drops of NaOH solution was added to the extract and observed for the formation of intense yellow color that becomes colorless on addition of few drops of diluted HCl.
Phenol test: The formation of intense colour upon addition of about 0.5 ml of ferric chloride to the extract was observed.
Salkowski test for phytosterols: Equal volumes of chloroform and concentrated Sulfuric Acid (H2SO4) were added to the extract and shaken well. The appearance of red chloroform layer and greenish yellow fluorescence acid layer was observed.
Ferric chloride test for tannins: The extract was boiled in 20 ml of water and then filtered. A few drops of 0.1 % ferric chloride was added to the filtrate and observed for the appearance of brownish green or blue-black color.
Molisch’s test for carbohydrates: About 2 drops of Molisch’s regent was added to the extract and then 2 ml of concentrated H2SO4 was added carefully along the sides. The formation of violet ring at the junction was observed.
Ninhydrin test for amino acids: The extract was heated after addition of 2 drops of freshly prepared 0.2 % ninhydrin reagent and the appearance of purple blue color was observed.
Gas Chromatography–Mass Spectrometry (GC-MS) analysis for volatile phytochemicals:
The Thermo GC-Trace Ultra VER: 5.0 (Bremen, Germany) and Mass Spectroscopy (MS) DSQ II electron ionization mode with ionization energy of 70 eV was used in our GC-MS analysis for the ethanolic extract of Cocos nucifera L. flowers to identify the biologically important compounds. The column temperature was set to 80°-250° at 8°/min rate. The temperatures of 280° and 290° were set for the GC injector and MS transfer respectively. The major carrier gas used was is helium and its flow rate was 1.0 ml/min. Then 1 μl volume of sample was used for the GC-MS analysis. The major compounds were identified by the retention time and mass fragmentation patterns. The National Institute of Standards and Technology (NIST)/Wiley 9.0 library was used[43] for the comparative detection of compounds.
Evaluation of in vitro anti-inflammatory activity:
The study was carried out based on relevant literature[44]. The reaction mixture (5 ml) consisted 2.8 ml of phosphate buffered saline (pH 7.4), 0.2 ml of egg albumin (from fresh hen’s egg) and 2 ml of varying concentrations of ethanolic extract of Cocos nucifera L. flowers so that the final concentrations become 1000, 2000, 4000 and 8000 µg/ml. Similar volume of double-distilled water served as control. Then the mixture was incubated at 37° for 15 min in a Bio-Oxygen Demand (BOD) incubator and then heated at 70° for 5 min. After cooling, the absorbance of the solution was determined to measure the protein denaturation level by using Ultraviolet-Visible (UV-Vis) spectrophotometer at the wavelength of 660 nm by using vehicle as blank. Diclofenac sodium at the final concentration of 500 µg/ml was used as reference drug and treated similarly for determination of absorbance. The experiments were performed in triplicate. The percentage inhibition of protein denaturation as measure of anti-inflammation was calculated by using the following formula:
Percent inhibition=100×(Vt/Vc-1)
Where, Vt=Absorbance of test sample and Vc=Absorbance of control
Evaluation of in vitro anti-cancer activity on human lung cancer cell line:
The anticancer effect of the Cocos nucifera L. flower extract was investigated against the human lung cancer cell line (A549) using 3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) assay[45]. A549 cells were procured from National Centre for Cell Science at Pune and maintained in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10 % Fetal Bovine Serum (FBS), antibiotic 2 % (streptomycin) in a humidified atmosphere of 5 % Carbon Dioxide (CO2) at 37°. The stock cultures were grown in culture flask and experiments were carried out in 96 well plate. Cells were placed on 96 well plates and allowed to grow in CO2 incubator for 24 h (37°, 5 % CO2). The cells were then treated with different concentrations of the ethanolic extract in triplets ranging from 25, 50, 75, 100, 125, 150, 175, 200 µg/ml for 24 h by incubating at 37° and 5 % CO2. After this, 20 μl MTT stock solution (5 mg/ml in phosphate-buffered saline) was added to each well and incubated for 4 h. Then 20 μl Dimethyl Sulfoxide (DMSO) was added to each well to dissolve the MTT metabolic product. Then the plate was shaken at 150 rpm for 5 min and the optical density was measured at 570 nm. The cell viability was calculated as follows:
Percentage cell viability=(OD of control−OD of testcompound/OD of control)×100
Where, OD=Optical Density
Results and Discussion
The results of phytochemical analysis comprehensively validated the presence of therapeutically important and valuable secondary metabolites in Cocos nuciferaL. flower extract. The phytochemical preliminary screening showed the presence of phytosterols and tannins. Surprisingly the extract was negative for carbohydrates, amino acids, flavonoids and phenols as shown in Table 1.
| S.NO | Phytochemicals | Ethanolic extract |
|---|---|---|
| 1 | Alkaloids | - |
| 2 | Flavonoids | - |
| 3 | Phenol | - |
| 4 | Phytosterols | + |
| 5 | Carbohydrates | - |
| 6 | Tannins | + |
| 7 | Aminoacids | - |
Note: Where, (+): Presence and (-): Absence
Table 1: The Phytochemicals Present in Ethanolic Extracts of Cocos nucifera Flowers
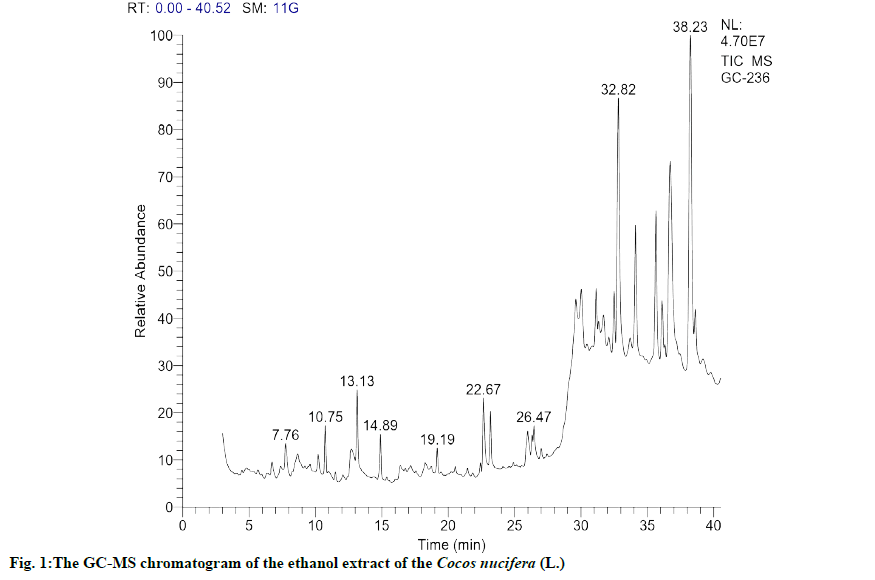
The GC-MS chromatogram as shown in fig. 1 revealed the presence of 152 compounds, which includes several phytosterols and few fatty acids, polyphenols and terpenes. The phenolic terpene and sterol compounds identified with higher probability match with reference compound and peak area are listed as shown in Table 2. The most bioactive secondary metabolites, having anti-inflammatory and anti-cancer activities such as quercetin derivative, eugenol, catechol, stigmasterol, campesterol, tertiary butylhydroquinone were identified in this list and their biological importance, which are the focus of current research are given in Table 3. Furthermore this result shows the presence of more phytosterols, which is correlating with the result of preliminary phytochemical tests.
| S. No | Compound | Molecular formula | Molecular weight | Probability | Area % |
|---|---|---|---|---|---|
| 1 | Catechol/1,2-Benzenediol | C6H6O2 | 110 | 88.83 | 1.17 |
| 2 | Eugenol | C10H12O2 | 164 | 14.66 | 0.69 |
| 3 | 3-Allyl-6-methoxyphenol | C10H12O2 | 164 | 11.52 | 0.69 |
| 4 | 2-tert-Butyl-4-isopropyl-5-methylphenol | C14H22O | 206 | 28.17 | 2.67 |
| 5 | 2-Allyl-5-t-butylhydroquinone | C13H18O2 | 206 | 10.06 | 2.67 |
| 6 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | C10H12O3 | 180 | 66.55 | 0.91 |
| 7 | 24,25-Dihydroxycholecalciferol | C27H44O3 | 416 | 25.29 | 4.97 |
| 8 | 9,10-Secocholesta-5,7,10(19)-triene-3,24,25-triol, | C27H44O3 | 416 | 25.29 | 4.97 |
| 9 | Quercetin 7,3',4'-trimethoxy | C18H16O7 | 344 | 9.34 | 1.75 |
| 10 | Dehydroergosterol 3,5-dinitrobenzoate | C35H44N2O6 | 588 | 28.86 | 1.1 |
| 11 | (22E)-Ergosta-5,7,9(11),22-tetraen-3á-ol | C28H42O | 394 | 21.52 | 1.1 |
| 12 | (E)-5,10-secocholest-1(10)-en-3,5-dione | C27H44O2 | 400 | 36.63 | 12.93 |
| 13 | Ergost-5-en-3-ol, (3á,24R)- (CAS) | C28H48O | 400 | 24.4 | 12.93 |
| 14 | Ergost-5-en-3-ol, (3á)- | C28H48O | 400 | 24.4 | 12.93 |
| 15 | Campesterol | C28H48O | 400 | 24.4 | 12.93 |
| 16 | Stigmasterol | C29H48O | 412 | 79.62 | 20.3 |
| 17 | Stigmasta-5,22-dien-3-ol, (3á,22E)- (CAS) | C29H48O | 412 | 79.62 | 20.3 |
| 18 | Stigmasta-5,22-diene, 3-methoxy-, (3á,22E)- | C30H50O | 426 | 5.12 | 20.3 |
| 19 | Stigmasta-5,22-dien-3-ol, (3á,22E)- (CAS) | C29H48O | 412 | 79.62 | 20.3 |
| 20 | 5à-Cholestan-3-one | C27H46O | 386 | 15.2 | 0.6 |
| 21 | 7á-Methylcholesterol | C28H48O | 400 | 6.52 | 12.93 |
| 22 | 19-Methylene-5,10-secocholestan-3,5-dione | C27H44O2 | 400 | 1.21 | 12.93 |
| 23 | Cholesta-22,24-dien-5-ol, 4,4-dimethyl | C29H48O | 412 | 9.39 | 20.3 |
Table 2: Phenolic, Terpene and Sterol Phytochemicals Compounds Identified in GC-MS
| S.No | Compound | Biological activity |
|---|---|---|
| 1 | Quercetin (and derivatives) | Anti-cancer[46]; anti-inflammatory[47] |
| 2 | Eugenol | Anti-inflammatory[48]; anti-cancer[49] |
| 3 | Catechol | Molecular action on lung cancer[50]; anti-inflammatory[51,52] |
| 4 | Stigmasterol | Anti-bacterial and anti-inflammatory[53]; protective against risk of ovarian cancer[54] |
| 5 | Campesterol (and derivatives) | Cytotoxic and anti-inflammatory[55] |
| 6 | Tert-butylhydroquinone | Protection against ulcer[56] |
Table 3: The Selected Compounds Identified in GC-MS Having Research Focus
The results reveal that the flower extract of Cocos nucifera L. has good in vitro anti-inflammatory activity as shown in Table 4. The ethanolic extract of Cocos nuciferaflower at 4000 µg/ml showed a percentage inhibition of 75.02±11.16 which is similar to the percentage inhibition of standard drug diclofenac sodium (75.90±9.02) at 500 µg/ml.
| S. No | Treatment concentration (µg/ml) | Percentinhibition of protein denaturation |
|---|---|---|
| 1 | Extract-1000 | 54.51±11.45 |
| 2 | Extract-2000 | 60.63±9.20 |
| 3 | Extract-4000 | 75.02±11.16 |
| 4 | Extract-8000 | 88.40±6.38 |
| 5 | Std (Diclofenac)-500 | 75.90±9.02 |
Note: Results are expressed as mean±SD
Table 4: Effect of Cocos nucifera Flower Extract on Inhibition of Protein Denaturation
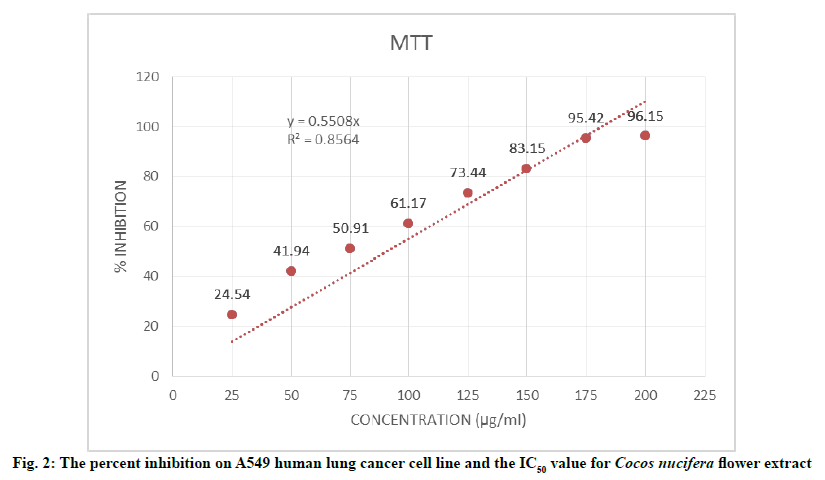
The result of MTT assay performed to investigate the cytotoxic activity of Cocos nuciferaL. flower ethanolic extract showed an increased percent inhibition on the growth of A549 cell line with the increasing concentration of treatments from 25-200 µg/ml as shown in Table 5. The treatment at highest concentration of 200 µg/mL showed a maximum of 96.15 % inhibition on cell growth as shown in fig. 2. The Half-Maximal Inhibitory Concentration (IC50) value was found to be 90.2 μg/ml.
| S.NO | Concentration (µg/ml) | Average OD values | Percent inhibition of cell growth |
|---|---|---|---|
| 1 | 25 | 0.412±0.008 | 24.54 |
| 2 | 50 | 0.317±0.012 | 41.94 |
| 3 | 75 | 0.268±0.008 | 50.91 |
| 4 | 100 | 0.212±0.007 | 61.17 |
| 5 | 125 | 0.141±0.006 | 73.44 |
| 6 | 150 | 0.092±0.010 | 83.15 |
| 7 | 175 | 0.025±0.004 | 95.42 |
| 8 | 200 | 0.021±0.003 | 96.15 |
Note: OD: Optical Density
Table 5: Anti-Cancer Activity of Cocos nucifera L. Flower Extract By MTT Assay on A549 Cells
The phytochemicals derived from medicinal plants have gained significant and considerable recognition as natural non-toxic therapeutic agents in recent past few years. All the valuable phytochemicals proven to have bioactive potency was identified based on the preliminary screening in the crude extracts. The different extracts of various parts of the Cocos nucifera (L.) were studied extensively for various pharmacological properties. On the other hand, the studies on the flowers of Cocos nucifera (L.) are in infancy stage though it has been developed as Ayurveda preparations based on its traditional usage. One study has identified the bioactive phytochemicals having valuable therapeutic index in various extracts of the flowers of Cocos nucifera (L.) by preliminary screening tests.
This study was focused on the ethanol extract of the flowers of Cocos nucifera (L.) for the identification of bioactive compounds by GC-MS analysis and in vitro screening for anti-inflammatory and anti-cancer properties. The preliminary phytochemical screening tests revealed the presence of phytosterols and tannins in the extract which is in line with the report of Dyana et al.[11]. Epidemiological data suggests that the phytosterol content of the diet is associated with a reduction in common cancers including cancers of the colon, breast and prostate[57]. Phytosterols potentially enable the human system for more robust antitumor responses, by boosting the immune recognition of cancer, influencing hormonal dependent growth of endocrine tumors and altering sterol biosynthesis[58,59]. In addition, phytosterols have effects that directly inhibit tumor growth, including the slowdown of cell cycle progression, the induction of apoptosis and the inhibition of tumor metastasis[60]. Several phytosterols have shown a good anti-inflammatory activities as per the review article of recent studies[20]. Tannins (commonly referred to as tannic acid) are water-soluble polyphenols that are present in many plant foods, including buds. Tannins isolated from various plants have shown good anti-inflammatory activity in animal models[16]. Tannins have also shown remarkable activity in cancer prevention[61]. Hence, the anti-inflammatory and anti-cancer effects exerted by the ethanol extract of Cocos nucifera (L.) in this study might be because of the presence of sterols and tannins as reported in the preliminary screening tests.
Furthermore, in this study, a total of 152 phytochemicals were identified by GC-MS analysis, as the first report on the flower extract of Cocos nucifera (L.). The list of compounds include few biologically active fatty acids, fatty esters, simple polyphenolics, flavonoid derivatives and several phytosterols, majorly, stigma sterol derivatives. Surprisingly, no notable tannin compounds have been identified in the GC-MS report. A recent systematic meta-analysis has revealed that the phytosterol intake protects from cancer risk[62]. A case control study has revealed that the highest quintile of stigmasterol intake has associated with a lower risk of developing ovarian cancer[54]. Few compounds having therapeutic index as listed in Table 3 are the much focused phytochemicals as per the recent research reports.
The egg albumin denaturation bioassay was carried out to study the anti-inflammatory property and a concentration dependent per cent inhibition of denaturation was observed for the Cocos nucifera (L.) flower ethanol extract in this study. Furthermore, the concentration of 4000 µg/ml has shown a similar percent inhibition as the standard and strong anti-inflammatory drug diclofenac has shown. This indicates a good anti-inflammatory activity. The phytochemicals present in the Cocos nucifera(L.) extract such as quercetin, eugenol, catechol, stigmasterol, campesterol, tertiary butylhydroquinone and their derivatives as identified by GC-MS analysis as listed in Table 3 and may be responsible for its anti-inflammatory activity. However, further in vitro studies and validations using in vivo models are warranted.
In the MTT assay, the ethanol extract of Cocos nucifera (L.) flower has shown a 96.15 % cell inhibition on growth of A549 cells, which is a good anti-cancer activity when compared to the anti-cancer effects shown by the ethanol extracts of other plant sources such as Piper betel leaf (80 %) and Abutilon indicum to (72.1 %) at 200 µg/ml[63]. This implies that the flower extract of Cocos nucifera L. has comparatively a good anti-cancer activity against human lung cancer cell line. The anti-cancer potency may be contributed by the significant phytochemicals present in the extract as identified by GC-MS analysis such as flavones such as quercetin derivative; simple phenolic compounds like eugenol, catechol and, phytosterols such as stigmasterol and campesterol.
The other parts of Cocos nucifera (L.) also have shown such medicinal properties. A review article has highlighted the phytochemicals of the husk fiber extract of the plant Cocos nucifera (L.) and their various medicinal properties[2] which also support our study results. In an in vivo study, the aqueous extract of coconut flower has shown an effective treatment action on PCOD. An in vivo anti-tumor activity was reported by a previous study for the coconut flower extract[64]. The chemopreventive action exerted in mice by the ethanol extract of coconut fruit powder was due to the presence of flavonoids and tannins[65]. The polyphenol rich phenolic extract of coconut kernel has shown anti-cancer molecular effects on human prostate cancer cell lines[66]. The anti-cancer properties of sterols present in coconut products were indicated by an article[67].
The phytochemical screening tests of the ethanol extract of Cocos nucifera(L.) flowers have demonstrated the presence of phytosterols and tannins, which are having many medicinal properties. The phytochemicals identification by GC-MS analysis also done first time for the ethanol extract of Cocos nucifera(L.) flowers and reported the presence of bioactive phytosterols. In this study, the extract has shown a very good anti-inflammatory and anti-cancer potency against A549 cell line in the in vitro assays. The bioactive compounds identified in GC-MS analysis such as quercetin, eugenol, catechol, stigmasterol, campesterol, tertiary butylhydroquinone and their derivatives, which are having anti-inflammatory and anti-cancer potency, are implicated as the reason for these pharmacological actions. Thus, the traditional knowledge on the healing potency of coconut flower needs to be explored for the development of drug formulations with scientific validation. This can lead to the development of effective anti-inflammatory and anti-cancer drugs from the natural sources. However, further in vivo and clinical investigations on the phytosterol fraction of the flower of Cocos nucifera(L.) are warranted to explore the pharmacologic potency.
Acknowledgements
The authors would like to acknowledge the contribution of the basic lab facilities established with the funding for cancer nano-therapy research by DST-SERB and the DBT, New Delhi, India in the successful completion of this study. The authors would like to acknowledge the technical help provided by the SITRA (The South Indian Textile Research Association), Coimbatore for GC-MS analysis and the editing help rendered by the English Professor, Dr. D. David Wilson, Karunya Institute of Technology and Sciences.
Conflict of interests:
The authors declare that they have no conflict of interest.
References
- Bhagwat DA, Killedar SG, Adnaik RS. Anti-diabetic activity of leaf extract of Tridax procumbens. Int J Green Pharm 2008;2(2):131-6.
- Lima EB, Sousa CN, Meneses LN, Ximenes NC, Santos MA, Vasconcelos GS, et al. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz J Med Biol Res 2015;48:953-64.
[Crossref] [Google Scholar] [PubMed]
- Dhankhar P, Tech M. A study on development of coconut based gluten free cookies. Int J Eng Sci Invention 2013;2(12):10-9.
- Hirakawa K, Suzuki H, Oikawa S, Kawanishi S. Sequence-specific DNA damage induced by ultraviolet A-irradiated folic acid via its photolysis product. Arch Biochem Biophys 2003;410(2):261-8.
[Crossref] [Google Scholar] [PubMed]
- Cáceres A, Girón LM, Alvarado SR, Torres MF. Screening of antimicrobial activity of plants popularly used in Guatemala for the treatment of dermatomucosal diseases. J Ethnopharmacol 1987;20(3):223-37.
[Crossref] [Google Scholar] [PubMed]
- Adegoke AO, Bamigbowu EO, George-Opuda MI, Edomwande P. Electrolyte and glucose contents of ripe and unripe coconut liquid as source of oral rehydration solution. Int J Appl Res Nat Prod 2012;5(1):18-21.
- Bhandary MJ, Chandrashekar KR, Kaveriappa KM. Medical ethnobotany of the siddis of Uttara Kannada district, Karnataka, India. J Ethnopharmacol 1995;47(3):149-58.
[Crossref] [Google Scholar] [PubMed]
- Ebuna RM, Magat SS, Maravilla JN, Santos GA, Baylon GB. Performance of selected coconut varieties/hybrids under sequential coconut toddy and nut production scheme or SCTNP. Coconut Res Dev 2002;18(1):34.
- Hussein AO, Mohammed GJ, Hadi MY, Hameed IH. Phytochemical screening of methanolic dried galls extract of Quercus infectoria using gas chromatography-mass spectrometry (GC-MS) and Fourier transform-infrared (FT-IR). J Pharmacogn Phytother 2016;8(3):49-59.
- Soumya V, Muzib YI, Venkatesh P, Hariprasath K. GC-MS analysis of Cocus nucifera flower extract and its effects on heterogeneous symptoms of polycystic ovarian disease in female Wistar rats. Chin J Nat Med 2014;12(9):677-84.
[Crossref] [Google Scholar] [PubMed]
- Dyana JP, Kanchana G. Preliminary phytochemical screening of Cocos nucifera L. flowers. Int J Curr Pharm Res 2012;4(3):3-4.
- Freitas JC, Nunes-Pinheiro D, Pessoa AW, Silva LC, Girão VC, Lopes-Neto BE, et al. Effect of ethyl acetate extract from husk fiber water of Cocos nucifera in Leishmania braziliensis infected hamsters. Rev Bras Farmacogn 2011;21(6):1006-11.
- Lilley BD, Brewer JH. The selective antibacterial action of phenylethyl alcohol. J Am Pharm Assoc 1953;42(1):6-8.
[Crossref] [Google Scholar] [PubMed]
- Renjith RS, Chikku AM, Rajamohan T. Cytoprotective, antihyperglycemic and phytochemical properties of Cocos nucifera (L.) inflorescence. Asian Pac J Trop Med 2013;6(10):804-10.
[Crossref] [Google Scholar] [PubMed]
- Kabra MP, Rachhadiya RM, Desai NV, Sharma S. Hypoglycemic effect of Cocos nucifera flower alcoholic extract and oil in normal and alloxanised hyperglycemic rats. Int J Pharm Phytopharm Res 2012:29-31.
- Soyocak A, Kurt HÜ, Cosan DT, Saydam FA, Calis IU, Kolac UK, et al. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum Exp Toxicol 2019;38(11):1296-301.
[Crossref] [Google Scholar] [PubMed]
- Guénette SA, Hélie P, Beaudry F, Vachon P. Eugenol for anesthesia of African clawed frogs (Xenopuslaevis). Vet AnaesthAnalg 2007;34(3):164-70.
- Cai Y, Zhang J, Chen NG, Shi Z, Qiu J, He C, et al. Recent advances in anticancer activities and drug delivery systems of tannins. Med Res Rev 2017;37(4):665-701.
[Crossref] [Google Scholar] [PubMed]
- Woyengo TA, Ramprasath VR, Jones PJ. Anticancer effects of phytosterols. Eur J Clin Nutr 2009;63(7):813-20.
[Crossref] [Google Scholar] [PubMed]
- Vilahur G, Ben-Aicha S, Diaz-Riera E, Badimon L, Padró T. Phytosterols and inflammation. Curr Med Chem 2019;26(37):6724-34.
[Crossref] [Google Scholar] [PubMed]
- Kocaçalışkan I, Talan I, Terzi I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z Naturforsch C J Biosci 2006;61(9-10):639-42.
[Crossref] [Google Scholar] [PubMed]
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin S. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol 2007;147(2):227-35.
[Crossref] [Google Scholar] [PubMed]
- Maldini M, Sosa S, Montoro P, Giangaspero A, Balick MJ, Pizza C, et al. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonimacrassifolia Kunth, Sweetiapanamensis Yakovlev and the leaves of Sphagneticolatrilobata Hitchcock. J Ethnopharmacol 2009;122(3):430-3.
[Crossref] [Google Scholar] [PubMed]
- Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications and fetal injury. Nutr Rev 2007;65(3):S194-202.
[Crossref] [Google Scholar] [PubMed]
- Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitexnegundo. Afr J Biochem Res 2010;4(7):191-5.
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860-7.
- Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med 2019;18(3):121-6.
- Greten FR, Grivennikov SI. Inflammation and cancer: Triggers, mechanisms and consequences. Immunity 2019;51(1):27-41.
[Crossref] [Google Scholar] [PubMed]
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001;120(3):594-606.
[Crossref] [Google Scholar] [PubMed]
- Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti‐inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol 2008;65(2):203-9.
[Crossref] [Google Scholar] [PubMed]
- Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract2012;2012.
[Crossref] [Google Scholar] [PubMed]
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012;2(2):303-36.
[Crossref] [Google Scholar] [PubMed]
- Yu N, Su X, Wang Z, Dai B, Kang J. Association of dietary vitamin A and β-carotene intake with the risk of lung cancer: A meta-analysis of 19 publications. Nutrients 2015;7(11):9309-24.
[Crossref] [Google Scholar] [PubMed]
- Heggen E, Granlund L, Pedersen JI, Holme I, Ceglarek U, Thiery J, et al. Plant sterols from rape seed and tall oils: Effects on lipids, fat-soluble vitamins and plant sterol concentrations. Nutr Metab Cardiovasc Dis 2010;20(4):258-65.
[Crossref] [Google Scholar] [PubMed]
- Ragasa CY, EbajoJr VD, Reyes RG, Brkljača R, Urban S. Sterols and lipids from Pleurotus florida. Der PharmaChemica 2015;7(10):331-6.
- Pokharkar VB, Shekhawat PB, Dhapte VV, Mandpe LP. Development and optimization of eugenol loaded nanostructured lipid carriers for periodontal delivery. Int J Pharm Pharm Sci 2011;3(4):138-43.
- Mohan L. Plant-based drugs as an adjuvant to cancer chemotherapy. Altern Med 2021:331.
- Silva RR, Fontes HR, Alviano CS, Fernandes PD, Alviano DS. Anti-inflammatory, antioxidant and antimicrobial activities of Cocos nucifera var. typica. BMC Complement Altern Med 2013;13(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Koschek PR, Alviano DS, Alviano CS, Gattass CR. The husk fiber of Cocos nuciferaL. (Palmae) is a source of anti-neoplastic activity. Braz J Med Biol Res 2007;40:1339-43.
[Crossref] [Google Scholar] [PubMed]
- Anosike CA, Obidoa O. Anti-inflammatory and anti-ulcerogenic effect of ethanol extract of coconut (Cocos nucifera) on experimental rats. African J Food Agric Nutr Dev 2010;10(10):4286-300.
- Zakaria ZA, Reezal I, Mat Jais AM, Somchit MN, Sulaiman MR, Marmin AH, et al.The anti-inflammatory, anti-pyretic and wound healing activities of Cocos nucifera L. (MATAG types) fresh juice and kernel extract in experimental animals. J Pharmacol Toxicol 2006;1(6):516-26.
- Leelaprakash G, Dass SM. In vitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int J Drug Dev Res 2011;3(3):189-96.
- Suluvoy JK, Berlin Grace VM. Phytochemical profile and free radical nitric oxide (NO) scavenging activity of Averrhoa bilimbi L. fruit extract. 3 Biotech 2017;7(1):1.
[Crossref] [Google Scholar] [PubMed]
- Chandra S, Chatterjee P, Dey P, Bhattacharya S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac J Trop Biomed 2012;2(1):S178-80.
- Cole SP. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother Pharmacol 1986;17(3):259-63.
[Crossref] [Google Scholar] [PubMed]
- Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci 2020;10(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Saeedi-Boroujeni A, Mahmoudian-Sani MR. Anti-inflammatory potential of quercetin in COVID-19 treatment. J Inflamm 2021;18(1):1-9.
- Barboza JN, da Silva Maia BezerraFilho C, Silva RO, Medeiros JV, de Sousa DP. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid Med Cell Longev 2018;2018:3957262.
[Crossref] [Google Scholar] [PubMed]
- Jaganathan SK, Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules 2012;17(6):6290-304.
[Crossref] [Google Scholar] [PubMed]
- Do Young Lim SH, Lee MH, Malakhova M, Kurinov I, Wu Q, Xu J, et al. A natural small molecule, catechol, induces c-Myc degradation by directly targeting ERK2 in lung cancer. Oncotarget 2016;7(23):35001.
[Crossref] [Google Scholar] [PubMed]
- Zheng LT, Ryu GM, Kwon BM, Lee WH, Suk K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: Inhibition of microglial neurotoxicity. Eur J Pharmacol 2008;588(1):106-13.
[Crossref] [Google Scholar] [PubMed]
- Funakoshi-Tago M, Nonaka Y, Tago K, Takeda M, Ishihara Y, Sakai A, et al. Pyrocatechol, a component of coffee, suppresses LPS-induced inflammatory responses by inhibiting NF-κB and activating Nrf2. Sci Rep 2020;10(1):1-7.
- Correa G, Abreu VD, Martins DA, Takahashi JA, Fontoura HD, Cara DC, et al. Anti-inflammatory and antimicrobial activities of steroids and triterpenes isolated from aerial parts of Justicia acuminatissima (Acanthaceae). Int J Pharm Pharm Sci 2014;6(6):75-81.
- McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr 2003;133(6):1937-42.
[Crossref] [Google Scholar] [PubMed]
- Moreno-Anzúrez NE, Marquina S, Alvarez L, Zamilpa A, Castillo-España P, Perea-Arango I, et al. A cytotoxic and anti-inflammatory campesterol derivative from genetically transformed hairy roots of Lopezia racemosa Cav. (Onagraceae). Molecules 2017;22(1):118.
- Rahman Z, Dwivedi DK, Jena GB. Ethanol-induced gastric ulcer in rats and intervention of tert-butylhydroquinone: Involvement of Nrf2/HO-1 signalling pathway. Hum Exp Toxicol 2020;39(4):547-62.
[Crossref] [Google Scholar] [PubMed]
- Suttiarporn P, Chumpolsri W, Mahatheeranont S, Luangkamin S, Teepsawang S, Leardkamolkarn V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015;7(3):1672-87.
[Crossref] [Google Scholar] [PubMed]
- Nashed B, Yeganeh B, HayGlass KT, Moghadasian MH. Antiatherogenic effects of dietary plant sterols are associated with inhibition of proinflammatory cytokine production in Apo E-KO mice. J Nutr 2005;135(10):2438-44.
[Crossref] [Google Scholar] [PubMed]
- Awad AB, Downie AC, Fink CS. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int J Mol Med 2000;5(5):541-6.
[Crossref] [Google Scholar] [PubMed]
- Bradford PG, Awad AB. Phytosterols as anticancer compounds. Mol Nutr Food Res 2007;51(2):161-70.
[Crossref] [Google Scholar] [PubMed]
- Li H, Wang Z, Liu Y. Review in the studies on tannins activity of cancer prevention and anticancer. Zhong Yao Cai 2003;26(6):444-8.
[Google Scholar] [PubMed]
- Jiang L, Zhao X, Xu J, Li C, Yu Y, Wang W, et al. The protective effect of dietary phytosterols on cancer risk: A systematic meta-analysis. J Oncol 2019;2019.
[Crossref] [Google Scholar] [PubMed]
- Banerjee DE, Shah B. Anti-proliferative activity of Piper betel leaf extracts on human lung cancer cell line (A549). Int J Pharm Pharm Sci 2014;6(1):432-5.
- Kavitha SP, Beena J. Evaluation of antimicrobial, antioxidant and antitumor activity of fresh and dry Cocos nucifera female flower extracts. Int J Curr Pharm Res 2012;4(3):169-74.
- Dev S, Shefa AA, Mandal A, Gayen PR, Asma K, Al Bari MA, et al. Evaluation of antioxidant and chemopreventive effect of Cocos nucifera L. Jahangirnagar Univ J Biol Sci 2017;6(2):47-58.
- Dhanyakrishnan R, Sunitha MC, Prakash Kumar B, Sandya S, Nevin KG. Morphological and molecular effects of phenolic extract from coconut kernel on human prostate cancer cell growth in vitro. Mediterr J Nutr Metab 2018;11(1):21-36.
- Ghosh A. Coconut: Natural source of potential anti-cancer agent. CORD 2016;32(1):9.