- *Corresponding Author:
- Z. Cheng
Department of Orthopedics, Huanggang Central Hospital, Huangzhou, Huanggang, Hubei 438000, China
E-mail: xxm17301229154@163.com
| Date of Received | 24 March 2023 |
| Date of Revision | 14 August 2022 |
| Date of Acceptance | 24 March 2023 |
| Indian J Pharm Sci 2023;85(2):518-524 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Panax notoginseng saponins are often used to treat a frequently of inflammatory diseases, but the underlying mechanisms of their effects on human osteoarthritis are limited. An osteoarthritis model was established by interleukin-1 beta treatment of chondrocytes for experiments. During the experiment, cell activity was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide method. The enzymelinked immunosorbent assay was applied to measure tumour necrosis factor alpha and interleukin-6 level, malondialdehyde content, superoxide dismutase activity, glutathione peroxidase activity in each group. Toll-like receptor 4 and myeloid differentiation factor 88 protein expression level was measured using Western blot. In this study, compared to the control group, the cell viability and the activity of superoxide dismutase and glutathione peroxidase in interleukin-1 beta group were notably reduced, while the content of malondialdehyde, interleukin-6 and tumour necrosis factor alpha, as well as the protein expression of tolllike receptor 4 and myeloid differentiation factor 88 were sharply increased. Compared with interleukin-1 beta group, cell viability, superoxide dismutase activity, glutathione peroxidase activity, malondialdehyde content were higher, whereas interleukin-6 and tumour necrosis factor alpha content and toll-like receptor 4 and myeloid differentiation factor 88 expression were lower in interleukin-1 beta+Panax notoginseng saponin groups. Moreover, relative to interleukin-1 beta+Panax notoginseng saponin-H+vector group, tolllike receptor 4 level and malondialdehyde, interleukin-6 and tumour necrosis factor alpha content were significantly increased, while cell activity, superoxide dismutase activity, glutathione peroxidase activity were considerably lowered in interleukin-1 beta+Panax notoginseng saponin H+toll-like receptor 4 group. Panax notoginseng saponins promoted interleukin-1 beta-stimulated osteoarthritis chondrocyte proliferation and inhibited cell oxidative damage and inflammatory response by modulating the toll-like receptor 4/myeloid differentiation factor 88 axis.
Keywords
Panax notoginseng saponins, osteoarthritis, chondrocytes, proliferation, oxidative damage, inflammatory response
In clinical orthopedics, Osteoarthritis (OA) is a common degenerative osteoarticular inflammatory disease that affects people in middle-aged and elderly people. Factors such as heredity, joint injury and joint deformation can induce arthritis[1]. Most studies have shown that oxidative damage and inflammatory reaction of chondrocytes are the main pathological mechanisms of OA[2,3]. Traditional Chinese medicine is an essential tool in the treatment of OA[4]. The components of traditional Chinese medicine can effectively alleviate the symptoms of OA, such as Angelica sinensis[5] and Panax notoginseng saponins (PNS) [6], but the relevant regulatory mechanism research is very limited.
PNS is usually used in treatment of central nervous system diseases, cardiovascular and cerebrovascular diseases, and a variety of inflammatory diseases[7-9]. For instance, PNS not only protected the heart in acute myocardial infarction and heart failure by inducing autophagy, but also enhanced platelet inhibition by regulating arachidonic acid metabolism in combination with aspirin to reduce stomach injury in gastric mucosa[10,11]. Moreover, the application of PNS can counteract the effects of adenine in chronic kidney disease and restore the level of inflammatory factors in laboratory mice to the level of healthy mice, mainly by regulating intestinal micro biota microorganisms and inhibiting pro-inflammatory proteins[12]. Therefore, PNS relieves the symptoms of the disease via hindering inflammation. Meanwhile, the important role of PNS in OA is also reported. Zhang et al.[6], discovered that PNS could effectively repress the chondrocytes senescence and apoptosis in OA via modulating the Phosphatidylinositol-3-Kinase (PI3K)- Protein Kinase B (AKT)-mammalian Target of Rapamycin (mTOR) axis, however, there are relatively few reports on the research mechanism of oxidative damage and inflammation in human OA chondrocytes.
As a classical inflammatory pathway, Toll-Like Receptor 4 (TLR4)/Myeloid Differentiation Factor 88 (MyD88) pathway is strongly associated with OA pathogenesis[13]. For example, improving Fat-mass and Obesity-associated gene (FTO) expression successfully relieved symptom of OA by controlling the TLR4/MyD88/Nuclear Factor Kappa B (NF-κB) signal pathway[14]. Here, this project looked into the potential mechanism of PNS influencing chondrocyte injury in OA triggered by Interleukin-1 beta (IL-1β) and hypothesized that TLR4/MyD88 signaling pathway might be involved.
Materials and Methods
Reagents and antibodies:
Trypsin, collagenase, Bicinchoninic Acid (BCA) kit and electrochemiluminescence solution were purchased from Beijing Solebo Bio. Fetal Bovine Serum (FBS) and Dulbecco’s Modified Eagle Medium (DMEM) were provided by Hyclone Company (United States of America (USA)). Nanjing Zelang Medical Technology Co., Ltd provided PNS to treat cells. Sigma Company (USA) offered IL-1β. Empty vector and overexpressed TLR4 were purchased from Shanghai Jima Company. Lipofectamine 2000 transfection kit was obtained from Invitrogen (USA). 3-(4,5-Dimethylthiazol-2- yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) kit was obtained from Shanghai Jingkang Biological Engineering Co., Ltd. Nanjing Jiancheng Biological Research Institute provided the Superoxide Dismutase (SOD) kit, Glutathione Peroxidase (GSh- Px) kit, Malondialdehyde (MDA) kit, IL-6 kit and Tumor Necrosis Factor Alpha (TNF-α) kit. The primary antibodies anti-TLR4, anti-MyD88 and anti-glyceraldehyde 3-phosphate dehydrogenase were acquired from Abcam (USA). Secondary antibodies horseradish peroxidase-labeled goat anti- Immunoglobulin G (IgG) was obtained from Beijing Biolaibo Technology Co., Ltd.
Cell culture:
Human chondrosarcoma cells (SW1353), similar to chondrocytes, were purchased from American Type Culture Collection (ATCC) (Massas, Virginia, USA). SW1353 were digested with 2 ml 0.25 % trypsin for 30 min at room temperature. After removing trypsin, cells were digested overnight with 2 ml collagenase (0.2 %) and filtered through 200 mesh. The cells were then incubated in a constant temperature incubator with a DMEM medium containing 10 % FBS at 37° and 5 % Carbon dioxide (CO2). The medium was changed every 2 d and the growth confluence rate reached 85 % for digestion and subculture.
Cell groups and transfection:
A density of 3000 chondrocytes cells from the 3rd to 5th generations per well were planted into 96- well plates and cultured overnight. Following IL-1 (10 ng/ml) treatment, PNS at low, medium and high concentrations (100 g/ml, 200 g/ml and 400 g/ml) were added into each well. They were classified as IL-1, IL-1+PNS-L, IL-1+PNS-M and IL-1+PNS-H, with a blank control serving as a control group. Using Lipofectamine 2000, the empty vector and TLR4 overexpression were transfected into chondrocytes. 6 h later, cell solution was added with 10 ng/ml IL- 1β and 400 μg/ml PNS. They were recorded as IL- 1β+PNS-H+vector group and IL-1β+PNS-H+TLR4 group. The cells were cultured continuously for 48 h and subjected to collection for follow-up experiments.
MTT assay:
Cell viability was determined using MTT assay. Briefly, cells in each group were separated by trypsin and added to 96-well plates. The cells were cultured for 24 h, 48 h and 72 h at 37° and 5 % CO2. 20 μl MTT reagents was added into each well and the cell liquid was removed from each well, 150 μl dimethyl sulfoxide was added and the crystals were dissolved by stirring for 10 min. An automatic micro plate reader was used to measure the absorbance (Optical Density (OD) value at 490 nm and the cell activity (%)=OD treatment/OD control group×100 %.
Enzyme-Linked Immunosorbent Assay (ELISA) assay:
In short, collected cells in each group were cultured for 48 h. Then, cell supernatant was collected after 15 min of centrifugation at 12 000 r/min. The ELISA kit was used to detected SOD activity, GSH-Px activity and MDA, IL-6 and TNF-α content.
Western blot:
Generally, protein lysate was added to cells of each group and the total protein was extracted. The BCA method was used for quantitative analysis of the extracted total protein. The protein was boiled for 5 min, removed to cool and then 30 μg protein per well was loaded on Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE). The cultures were closed until they contained 5 % skim milk powder for 2 h. The membranes were washed three times after an overnight incubation with TLR4 and MyD88 at 4°. The secondary antibodies were then added at a dilution of 1:2500. The membranes were incubated at room temperature for 2 h and exposed to an electrochemiluminescence reagent. The gray value of protein was observed using ImageJ software.
Statistical analysis:
All data was analyzed using Statistical Package for the Social Sciences (SPSS) 21.0 software in this study. Statistical significance boundary value was set at p<0.05. Data were compared using Analysis of Variance (ANOVA) for five groups or t-test for two groups. Experiments were exhibited as mean±standard deviation.
Results and Discussion
Relative to control group, cell viability was significantly reduced in IL-1β group at 24 h, 48 h and 72 h (p<0.05) (Table 1), indicating the repression of IL-1β on chondrocyte proliferation. Furthermore, as presented in Table 1, cell viability in IL-1β+PNS groups was significantly high compared to IL- 1β group at 24 h, 48 h and 72 h (p<0.05). More than that, the cell activity of IL-1β stimulated OA chondrocyte also increased gradually with increasing PNS concentration. As a result, PNS could effectively enhance IL-1β stimulated OA chondrocyte proliferation.
| Group | Cell viability (%) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Control | 101.37±6.54 | 102.54±9.23 | 98.27±7.19 |
| IL-1β | 59.66±5.31* | 52.66±4.36* | 46.32±4.11* |
| IL-1β+PNS-L | 65.39±5.24# | 61.38±5.47# | 53.45±3.28# |
| IL-1β+PNS-M | 77.39±7.52# | 70.69±5.92# | 62.87±6.51# |
| IL-1β+PNS-H | 89.65±7.83# | 84.13±7.25# | 75.39±6.82# |
| F | 61.129 | 78.255 | 112.085 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 as compared to control group and #p<0.05 as compared to IL-1β group
Table 1: Effect of PNS on IL-1β Stimulated OA Chondrocyte Viability (x̄±S, n=9)
As shown in Table 2, after IL-1β treatment, SOD and GSH-Px activities of chondrocyte were significantly reduced, while MDA content was obviously enhanced (p<0.05). Otherwise, SOD and GSH-Px activity in IL-1β+PNS groups were obviously increased vs. IL-1β group, whereas MDA content was notably reduced (p<0.05). And the higher the concentration of PNS, the more obvious the change of SOD and GSH-Px activity, and MAD content. Therefore, PNS could weaken oxidative stress in OA chondrocytes induced by IL-1β.
| Group | SOD (U/ml) | GSH-Px (U/l) | MDA (μmol/l) |
|---|---|---|---|
| Control | 21.32±2.01 | 79.32±6.85 | 2.81±0.22 |
| IL-1β | 6.32±0.54* | 26.15±2.90* | 9.53±0.45* |
| IL-1β+PNS-L | 8.66±0.35# | 38.97±3.21# | 8.01±0.33# |
| IL-1β+PNS-M | 12.11±1.01# | 50.04±4.85# | 6.24±0.54# |
| IL-1β+PNS-H | 16.38±1.42# | 67.93±6.22# | 4.26±0.35# |
| F | 217.602 | 161.985 | 430.547 |
| p | 0.000 | 0.000 | 0.000 |
Note: *p<0.05 as compared to control group and #p<0.05 as compared to IL-1β group
Table 2: Influence of PNS on SOD, GSH-Px and MDA in IL-1β Stimulated OA Chondrocytes (x̄±S, n=9)
As important inflammatory factors, IL-6 and TNF-α could reflect cellular immunity. In Table 3, IL-6 and TNF-α levels in the IL-1 group enhanced dramatically when compared to control group (p<0.05). Otherwise, compared with IL-1β group, IL-6 and TNF-α content in IL-1β+PNS groups were lower (p<0.05). Not only that, IL-6 and TNF-α levels also diminished gradually with increasing PNS concentration in IL-1β stimulated OA chondrocyte. Therefore, PNS could effectively reduce inflammatory process in OA chondrocytes stimulated by IL-1β.
| Group | IL-6 (ng/l) | TNF-α (ng/l) |
|---|---|---|
| Control | 32.65±3.89 | 59.67±4.82 |
| IL-1β | 126.97±10.45* | 169.35±14.33* |
| IL-1β+PNS-L | 98.34±7.65# | 142.19±10.24# |
| IL-1β+PNS-M | 73.66±7.05# | 106.97±10.11# |
| IL-1β+PNS-H | 48.53±4.28# | 85.32±7.99# |
| F | 257.231 | 172.831 |
| p | 0.000 | 0.000 |
Note: *p<0.05 as compared to control group and #p<0.05 as compared to IL-1β group
Table 3: Effect of PNS on Inflammation in IL-1β Stimulated OA Chondrocytes (x±S, n=9)
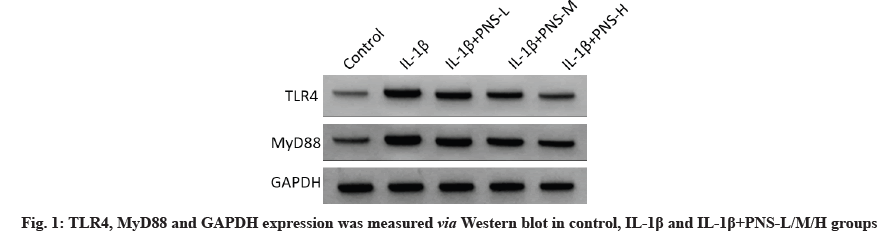
Compared to control group, TLR4 and MyD88 protein expression in IL-1β group was substantially enhanced (fig. 1) (p<0.05). Furthermore, relative to IL-1β group, TLR4 and MyD88 protein expression was markedly reduced in IL-1β+PNS groups (p<0.05) and higher PNS concentration, the lower TLR4 and MyD88 protein expression (Table 4). Sum to up, PNS inhibited the protein expression of TLR4/ MyD88 axis in IL-1β-stimulated OA chondrocytes.
| Group | TLR4 | MyD88 |
|---|---|---|
| Control | 0.24±0.03 | 0.32±0.04 |
| IL-1β | 0.82±0.07* | 0.89±0.07* |
| IL-1β+PNS-L | 0.69±0.06# | 0.75±0.05# |
| IL-1β+PNS-M | 0.57±0.05# | 0.63±0.05# |
| IL-1β+PNS-H | 0.35±0.05# | 0.49±0.04# |
| F | 177.906 | 168.939 |
| p | 0.000 | 0.000 |
Note: *p<0.05 as compared to control group and #p<0.05 as compared to IL-1β group
Table 4: Effect of PNS on TLR4 and MYD88 Proteins in IL-1β Stimulated OA Chondrocytes (x±s, n=9)
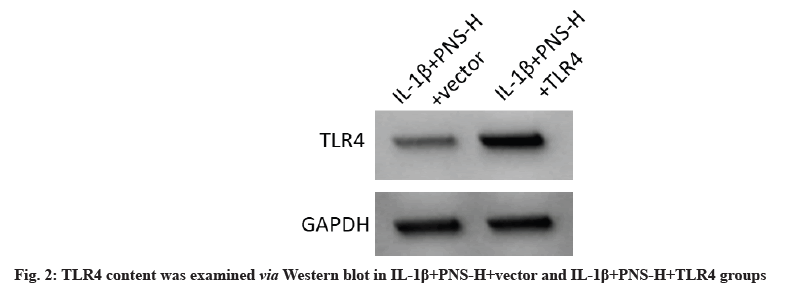
TLR4 protein expression was dramatically increased in the IL-1+PNS-H+TLR4 group compared to the IL-1+PNS-H+vector group (fig. 2), while cell viability was significantly decreased at 24 h, 48 h and 72 h (p<0.05) (Table 5). In addition, relative to IL- 1β+PNS-H+vector groups, SOD and GSH-Px content were significantly reduced, while MDA content and IL-6, and TNF-α were apparent level enhanced in IL-1β+PNS-H+TLR4 group (p<0.05) (Table 6). Therefore, PNS in IL-1β treated OA chondrocytes can promote cell proliferation and oxidative stress recovery while inhibiting inflammatory process by regulating TLR4/MyD88 axis.
| Group | TLR4 | Cell viability (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| IL-1β+PNS-H+vector | 0.38±0.04 | 88.32±8.69 | 85.66±7.85 | 73.87±7.20 |
| IL-1β+PNS-H+TLR4 | 0.56±0.05 | 75.24±5.37* | 71.32±6.82* | 57.33±6.24* |
| t | 8.433 | 3.841 | 4.137 | 5.208 |
| p | 0.000 | 0.001 | 0.001 | 0.000 |
Note: *p<0.05; IL-1β+PNS-H+vector group was considered as control
Table 5: Effect of PNS on IL-1β Stimulated OA Chondrocyte Viability Via Regulating TLR4/MYD88 Axis (x̄±s, n=9)
| Group | SOD (U/ml) | GSH-Px (U/l) | MDA (μmol/l) | IL-6 (ng/l) | TNF-α (ng/l) |
|---|---|---|---|---|---|
| IL-1β+PNS-H+vector | 18.39±1.78 | 70.36±7.29 | 4.55±0.54 | 46.75±4.37 | 81.24±7.53 |
| IL-1β+PNS-H+TLR4 | 12.22±1.32* | 56.21±4.83* | 7.38±0.44* | 64.58±6.05* | 95.83±8.24* |
| t | 8.353 | 4.854 | 12.188 | 7.167 | 3.921 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
Note: *p<0.05; IL-1β+PNS-H+vector group was considered as control
Table 6: Effect of PNS on Oxidative Stress and Inflammation in IL-1β Stimulated OA Chondrocytes Via Regulating TLR4/MYD88 (x̄±s, n=9)
IL-1β is a multifunctional inflammatory cytokine that belongs to the IL-1 family. IL-1β inhibits antioxidant enzymes, promotes ROS production and promotes the secretion of a variety of inflammatory factors after stimulating OA chondrocytes, thereby exacerbating the development of OA[15-17]. In this research, chondrocytes were treated with IL-1β to obtain an OA model and it found a decrease in cell viability, SOD activity and GSH-Px activity and an increase in MDA, IL-6 and TNF-α content, which was coincident with previous studies[18].
Derived from the root or rhizome of Panax notoginseng, PNS has the effects of dissipating blood stasis and stopping bleeding, as well as reducing swelling and pain[19,20]. The main components of PNS include ginsenoside Rb1 and Rg1 and PNS R1, which have anti-inflammatory, anti-oxidation, regulation of immune diseases and anti-tumor effects[20-23]. Some studies have shown that PNS combined with Tripterygium wilfordii polyglycosides can effectively inhibit inflammatory damage in rats with collageninduced arthritis[24]. According to Zhang et al., PNS could prevent the apoptosis of OA chondrocytes induced by TNF-α and delay matrix degradation. However, it is still unknown how PNS affects OA chondrocyte’s proliferative, oxidative stress-induced and inflammatory responses. Therefore, in this study, OA chondrocytes were treated with PNS at low, medium and high concentrations of 100, 200 and 400 μg/ml, and the results revealed that, PNS at low, medium and high doses could increase the cell proliferation, SOD activity and GSH-Px activity of OA chondrocytes stimulated by IL-1β, decline MDA content and reduce IL-6 and TNF-α levels, suggesting that PNS could enhance the cell proliferation of OA chondrocyte stimulated by IL-1β, inhibit oxidative stress and inflammatory response.
Immune and inflammatory responses are greatly influenced by the TLR4/MyD88 signaling pathway. TLR4 is a member of TLR family. After being recognized by receptors, TLR4 can signal to MyD88, thereby activating NF-κB, Interferon Regulatory Factor 3 (IRF3) and more involved cells in the inflammatory response and promoting the development of arthritis[25-27]. Inhibition of TLR4/ MyD88 axis could attenuate chronic mechanical pain in endometriosis[28]. In addition, astragaloside IV protected against acute myocardial infraction via modulating TLR4/MyD88/NF-κB axis[29]. For instance, the TLR4/MyD88/NF-κB signaling pathway was regulated by the microRNA-382- 3p/CX43 axis in OA chondrocytes, which had an impact on the inflammatory injury[30]. Intriguingly, our finding revealed that TLR4 and MyD88 protein expression was up-regulated after IL-1β treatment of OA chondrocytes, whereas PNS reduced TLR4 and MyD88 protein expression, suggesting that TLR4/MyD88 signaling pathway may be linked to the mechanism of PNS on IL-1β stimulated OA chondrocytes. Moreover, TLR4 overexpression repressed cell activity, SOD activity and GSHPx activity, while increasing MDA, IL-6 and TNF-α level. These findings suggested that PNS may influence IL-1β stimulated OA chondrocyte development by regulating TLR4/MyD88 pathway.
However, our study is limited by the lack of experiments on animal modes. In the future, we will build an OA model in mice for relevant research verification. Moreover, the regulatory mechanism of cell response to stimulus is very complex and the regulatory mechanism in the treatment of OA with PNS can be further explored.
In conclusion, PNS promoted IL-1β stimulated OA chondrocyte proliferation, and weaken cell oxidative damage and inflammatory response by modulating the TLR4/MyD88 axis.
Author’s contributions:
Lin Huang and Wei Xue have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Abramoff B, Caldera FE. Osteoarthritis: Pathology, diagnosis and treatment options. Med Clin 2020;104(2):293-311.
[Crossref] [Google Scholar] [PubMed]
- Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev 2021;66:101249.
[Crossref] [Google Scholar] [PubMed]
- Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed Pharmacother 2020;129:110452.
[Crossref] [Google Scholar] [PubMed]
- Liang Y, Xu Y, Zhu Y, Ye H, Wang Q, Xu G. Efficacy and safety of Chinese herbal medicine for knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2022;100:154029.
[Crossref] [Google Scholar] [PubMed]
- Zhuang C, Wang Y, Zhang Y, Xu N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from Angelica sinensis. Int J Biol Macromol 2018 ;115:281-6.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Cai W, Han G, Zhou S, Li J, Chen M, et al. Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in osteoarthritic chondrocytes. Int J Mol Med 2020;45(4):1225-36.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Wang Y, Qiu L, Yu Y, Wang C. Saponins of Panax notoginseng: Chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin Investig Drugs 2014;23(4):523-39.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Guo F, Zhou R, Xiang C, Zhang Y, Gao J, et al. Proteomics and transcriptome reveal the key transcription factors mediating the protection of Panax notoginseng saponins (PNS) against cerebral ischemia/reperfusion injury. Phytomedicine 2021;92:153613.
[Crossref] [Google Scholar] [PubMed]
- Xue-Ming L, Ding-Yi Y, Ya-Hui L, Lei Z, Hong-Kun Q, Yu-Bing Y, et al. Panaxnotoginseng saponins prevent colitis-associated colorectal cancer via inhibition IDO1 mediated immune regulation. Chin J Nat Med 2022;20(4):258-69.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Lv L, Xu Y, Jiang K, Chen F, Qian J, et al. Cardioprotection of Panax notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed Pharmacother 2021;136:111287.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Yang L, Song L, Guo M, Li C, Yang B, et al. Combination of Panax notoginseng saponins and aspirin potentiates platelet inhibition with alleviated gastric injury via modulating arachidonic acid metabolism. Biomed Pharmacother 2021;134:111165.
[Crossref] [Google Scholar] [PubMed]
- Xie J, Ma X, Zheng Y, Mao N, Ren S, Fan J. Panax notoginseng saponins alleviate damage to the intestinal barrier and regulate levels of intestinal microbes in a rat model of chronic kidney disease. Renal Fail 2022;44(1):1948-60.
[Crossref] [Google Scholar] [PubMed]
- Kim SH, Bang J, Son CN, Baek WK, Kim JM. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-κB signaling pathway. Korean J Intern Med 2018;33(3):612-21.
[Crossref] [Google Scholar] [PubMed]
- Cai D, Zhang J, Yang J, Lv Q, Zhong C. Overexpression of FTO alleviates osteoarthritis by regulating the processing of miR-515-5p and the TLR4/MyD88/NF-κB axis. Int Immunopharmacol 2023;114:109524.
[Crossref] [Google Scholar] [PubMed]
- Sun F, Hu P, Xiong Y, Bao J, Qian J, Wu L. Tricetin protects rat chondrocytes against IL-1β induced inflammation and apoptosis. Oxid Med Cell Longev 2019;2019:4695381.
[Crossref] [Google Scholar] [PubMed]
- Zou L, Yu L, Zhao X, Liu J, Lu H, Liu G, et al. MiR-375 mediates chondrocyte metabolism and oxidative stress in osteoarthritis mouse models through the JAK2/STAT3 signaling pathway. Cells Tissues Organs 2019;208(1-2):13-24.
[Crossref] [Google Scholar] [PubMed]
- Gu R, Shi Y, Huang W, Lao C, Zou Z, Pan S, et al. Theobromine mitigates IL-1β induced oxidative stress, inflammatory response and degradation of type II collagen in human chondrocytes. Int Immunopharmacol 2020;82:106226.
- Lv M, Cai Y, Hou W, Peng K, Xu K, Lu C, et al. The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. Inflamm Res 2022;71(4):461-72.
[Crossref] [Google Scholar] [PubMed]
- Ji C, Zhang Q, Shi R, Li J, Wang X, Wu Z, et al. Determination of the authenticity and origin of Panax notoginseng: A review. J AOAC Int 2022;105(6):1708-18.
[Crossref] [Google Scholar] [PubMed]
- Liu H, Lu X, Hu Y, Fan X. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol Res 2020;161:105263.
[Crossref] [Google Scholar] [PubMed]
- Guo Q, Li P, Wang Z, Cheng Y, Wu H, Yang B, et al. Brain distribution pharmacokinetics and integrated pharmacokinetics of Panax Notoginsenoside R1, Ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal administration of Panax notoginseng saponins assessed by UPLC/MS/MS. J Chromatogr B 2014;969:264-71.
[Crossref] [Google Scholar] [PubMed]
- Hu S, Wu Y, Zhao B, Hu H, Zhu B, Sun Z, et al. Panax notoginseng saponins protect cerebral micro vascular endothelial cells against oxygen-glucose deprivation/reperfusion-induced barrier dysfunction via activation of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules 2018;23(11):2781.
[Crossref] [Google Scholar] [PubMed]
- Wang P, Cui J, Du X, Yang Q, Jia C, Xiong M, et al. Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J Ethnopharmacol 2014;154(3):663-71.
[Crossref] [Google Scholar] [PubMed]
- Liu D, Y Xiao, Jilin WU. Effect of notoginseng total saponins combined with Tripterygium wilfordii glycosides on expression of serum IL-1β, TNF-α and synovial neovascularization in collagen induced arthritis rats. Chin Med Eng 2018;12:24-8.
- Wang Q, Zhuang D, Feng W, Ma B, Qin L, Jin L. Fraxetin inhibits interleukin-1β induced apoptosis, inflammation and matrix degradation in chondrocytes and protects rat cartilage in vivo. Saudi Pharm J 2020;28(12):1499-506.
[Crossref] [Google Scholar] [PubMed]
- Zhao Z, Li F, Ning J, Peng R, Shang J, Liu H, et al. Novel compound FLZ alleviates rotenone-induced PD mouse model by suppressing TLR4/MyD88/NF-κB pathway through microbiota–gut–brain axis. Acta Pharm Sin B 2021;11(9):2859-79.
- Dos Santos RM, Belardi BE, Tsosura TV, Chiba FY, Mattera MS, Machado NE, et al. Melatonin decreases IRF-3 protein expression in the gastrocnemius muscle, reduces IL-1β and LPS plasma concentrations, and improves the lipid profile in rats with apical periodontitis fed on a high-fat diet. Odontology 2022.
[Crossref] [Google Scholar] [PubMed]
- Su W, Cui H, Wu D, Yu J, Ma L, Zhang X, et al. Suppression of TLR4-MyD88 signaling pathway attenuated chronic mechanical pain in a rat model of endometriosis. J Neuroinflamm 2021;18(1):65.
[Crossref] [Google Scholar] [PubMed]
- Shi H, Zhou P, Gao G, Liu PP, Wang SS, Song R, et al. Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J Food Biochem 2021;45(7):e13757.
[Crossref] [Google Scholar] [PubMed]
- Lei J, Fu Y, Zhuang Y, Zhang K, Lu D. miR-382-3p suppressed IL-1β induced inflammatory response of chondrocytes via the TLR4/MyD88/NF-κB signaling pathway by directly targeting CX43. J Cell Physiol 2019;234(12):23160-8.
[Crossref] [Google Scholar] [PubMed]