- *Corresponding Author:
- G. Singh
Department of Pharmaceutical Sciences, IKG Punjab Technical University, Kapurthala-144 603, India
E-mail: sainigurjeet87@gmail.com
| Date of Submission | 21 August 2016 |
| Date of Revision | 18 February 2017 |
| Date of Acceptance | 30 July 2017 |
| Indian J Pharm Sci 2017;79(5): 674-687 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Solubility is an important determinant in drug liberation and hence drug absorption, which plays a key role in oral bioavailability of formulations. The dissolution rate of a drug directly depends upon its solubility. Most of the new drugs have poor water solubility; thereby pose a difficulty in formulating into drug delivery systems. Therefore, solubility enhancement of poorly water soluble drugs is one of the necessary preformulation steps in the pharmaceutical product development research. Solid dispersion is a unique and promising approach for enhancing the dissolution characteristics and oral bioavailability of poorly water-soluble drugs. The present review highlights portrayal of solid dispersions, its types including eutectic mixtures and solid solutions, method of preparation and also the useful carriers for the preparation of solid dispersions. Furthermore, the wide research conducted hitherto on solid dispersions for enhancing solubility of different efficacious drugs, challenges encountered in the development of solid dispersions and recent advancements to overcome its pitfalls have also been elaborated.
Keywords
Dissolution, solid dispersions, eutectics, solid solution, amorphous precipitates
Absorption of a drug through the oral route involves its dissolution from the formulation into gastric and/ or intestinal fluids followed by its permeation through gastrointestinal cell membranes and finally into the systemic circulation. Oral solid dosage forms are one of the most commonly used formulation types having multiple benefits over other formulations/routes. However, the challenge for a pharmaceutical scientist lies in the fact that dissolution of a drug from an oral solid formulation (a key factor in drug absorption) is dependent on the aqueous solubility of the drug. Therefore, a drug with poor aqueous solubility would exhibit dissolution rate limited absorption and similarly a drug possessing poor membrane permeability undergo permeation rate limited absorption. A drug is highly soluble when highest dose of drug is soluble in ≤250 ml of water over a pH range of 1 to 7.5 and a drug is highly permeable when extent of absorption in humans is to be ≥90% of an administered dose [1]. It has been investigated that most of new chemical entities currently being discovered and intended to be used as a solid dosage form should produce an efficient and reproducible plasma concentration after oral administration. However, most of them tend to have poor water solubility, which limits the therapeutic efficacy of that drug. Moreover, poor solubility results in increased dose and frequent administration leading to higher incidences of side-effects [2-6]. Hence, pharmaceutical research has emphasised on elevating the oral bioavailability of poorly water-soluble drugs by improving their solubility, dissolution rate and membrane permeability.
A number of strategies have been worked upon to overcome the crisis of poor aqueous solubility like chemical modification, alteration of solvent composition, use of carrier system and physical modification including solid dispersion method. However, solid dispersion technology stands out from the rest in being the most promising approach that increases the solubility of poorly soluble drugs. Formulation of poorly soluble drugs as solid dispersions offer a variety of processing and excipient options that enhances the operational flexibility when formulating oral drug delivery systems. Apart from improvement of solubility and bioavailability, the recent research on solid dispersion systems have been directed toward the development of extendedrelease dosage forms. Extensive research that has been conducted on solid dispersion strategy involves the use of drugs that are poorly soluble in water and highly permeable through biological membranes as with these types of drugs dissolution is the rate limiting step to absorption. Therefore, it has been predicted that the rate of absorption will be simultaneously increased with an increase in the rate of dissolution. Usually on the basis of Biopharmaceutical Classification System (BCS), drugs having low aqueous solubility and high membrane permeability are sub-categorized as Class II drugs. Consequently, solid dispersion technique is particularly used for improving the oral absorption and in turn bioavailability of Class II drugs [7-9].
In 1961, Sekiguchi and Obi reported the formation of eutectic mixture of sulfathiazole with urea and demonstrated that the drug existed in microcrystalline state [10]. On the contrary, another study reported that the drug need not exist in a microcrystalline state; a certain fraction of the drug may be molecularly dispersed in the matrix, forming solid solution [11]. When a solid dispersion is dispersed in aqueous medium, the carrier solubilised and released the drug as fine colloidal particles, leading to the enhancement of dissolution rate and bioavailability of poorly water-soluble drugs which could be attributed to enhanced surface area [5]. This article provides a brief review on the types and characterization of solid dispersions, description of different generations, detailed research conducted on solid dispersion and also the challenges encountered in formulation development.
Portrayal of Solid Dispersions Types
On the basis of drug release mechanisms, the solid dispersions can be classified into six groups, simple eutectic mixtures, glass solutions/suspensions, solid solutions, amorphous precipitations of a drug within a crystalline carrier, compound or complex formation and any combinations among these groups. These systems can be determined by various methods like thermal analysis, X-ray diffraction, microscopic methods, dissolution rate and thermodynamic studies [9,12,13].
Simple eutectic mixtures
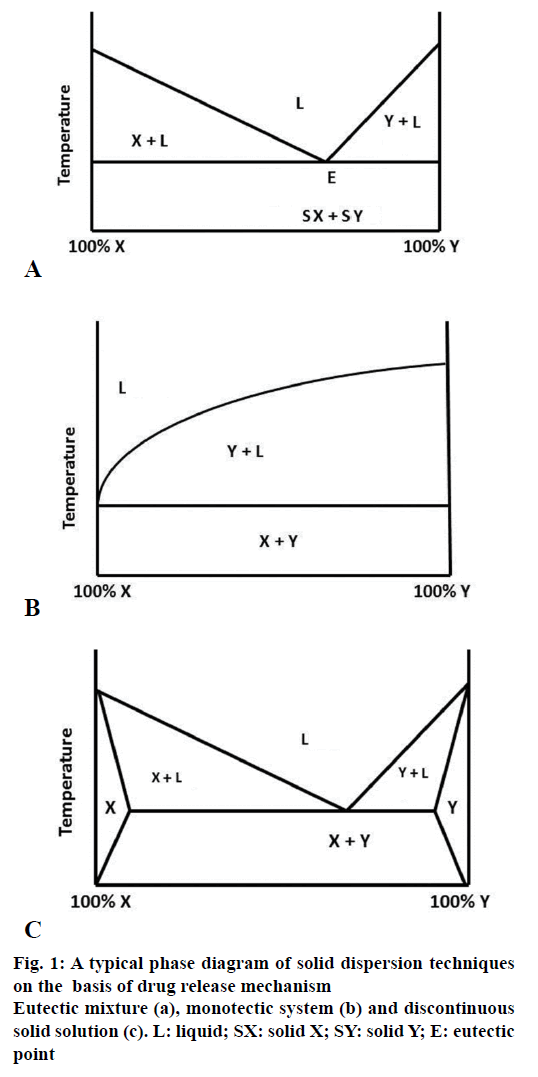
This type of solid dispersion involves mixing of two compounds, which are completely miscible in their liquid state but negligibly in the solid state. They are formed by melting compound X and Y, followed by rapid cooling of the mixture to obtain a physical mixture with fine crystals of both compounds. When mixture of two compounds with composition E is cooled or solidified, X and Y crystallize out simultaneously, whereas in case of other compositions, one of the compounds crystallizes out before the other compound. When the eutectic mixture with composition E is subjected to aqueous medium, the water soluble carrier dissolves and releases fine crystals of drug thus improving its aqueous solubility (Figure 1a) [14]. Sekiguchi and Obi obtained the eutectic mixtures of sulfathiazole and determined that sulfathiazole on formation of eutectic mixture with urea shows improved absorption and excretion after oral administration as compared to ordinary sulfathiazole [10].
MonotecticsThe monotectic system is same as eutectics but having eutectic melting point convergent with that of the pure material. Craig et al. 1999, formed solid dispersions of Nortriptyline-PEG, the phase diagrams thus obtained were found to be monotectic in nature, illustrating little or no interaction between the solid components and the drug. The drug was found as a separate phase within the carrier (Figure 1b) [15].
Solid solutions
A solid solution comprising a poorly water-soluble drug or a solid solvent having solid solute dissolved in it which have good water solubility are comparable to the liquid solution, thus improving dissolution of a drug to a greater extent. Solid solutions are resultant single phase, which come into being when two compounds disperse in each other at their molecular level [16]. Both the compounds then crystallize out simultaneously leading to formation of homogenous single phase system. Solid solutions formed by combination of water insoluble carriers with highly water soluble carriers present a view of having better dissolution rate comparable to the eutectic mixtures, the reason being that the drug particle size is reduced to molecular level. The volume of the medium used to dissolve the solid solution should be enough so as to dissolve the drug completely. The solid solutions can be classified on the basis of their miscibility viz. continuous and discontinuous solutions and secondly on the basis of how the solvate molecules are distributed in the solvent viz. substitutional, interstitial or amorphous solid solutions.
Continuous solid solution
In case of solid solution, both components are totally miscible with each other at solid state. The strength of bond between the components of continuous solid solution should be greater than that of either of the pure components. Theoretically, it is possible but there is no established pharmaceutical literature to prove the same [14].
Discontinuous solid solution
In discontinuous solid solutions, solutes tend to have limited solubility in the solid solvents. They dissolve up to a certain limit above the eutectic temperature. Discontinuous solid solution is formed by a binary system having components X and Y as shown in Figure 1c, where the regions X and Y show portions of true solid solutions, where one of the solid components is completely dissolved in the other. But below a certain temperature, the mutual solubility of the two components starts decreasing. Below this temperature, a decrease in solubility is observed. The free energy of this solid solution is less than the pure solvent. Goldberg et al. 1965, reported that to form a solid solution the solubility of one component in other should be greater than 5%. But later it was detected that the solid solutions of concentration less than 5% are also possible but it is applicable for only low dose drugs [14,17].
Substitutional solid solution
In this case, the solute particles are substituted in place of some solvent molecules in the crystal lattice. The particle size of the solute should be as close as possible to that of solid solvent. An extensive solid dispersion can only be obtained if the difference in the diameters of solute and solvent is less than 15%. Depending on the solubility, continuous or discontinuous solid solution can be formed [3,17].
Interstitial solid solution
Here, the solute molecules fit into the interstitial spaces of the solid solvent. In order to obtain extensive interstitial solid solution of metals, the apparent diameter of the solute atom should be less than 0.59 from that of the solvent i.e. the volume of the solute should be less than 20% of the solvent [5].
Glass solutions/glass suspensions
A glass solution is generally a homogenous glassy system, where in a solute dissolves in a solvent that is in glassy state. A glass can be particularly a pure chemical or their mixtures in a glassy/vitreous state, which is usually obtained by an abrupt quenching of the melt. The chemical bonds in the glass differ considerably in both length and strength; moreover there is no particular temperature at which all the bonds break simultaneously, therefore it does not exhibit a sharp melting point. The compounds that can form glasses readily upon cooling the liquid state include sucrose, glucose, ethanol, 3-methylhexane, and sugars. The glass solution formed by combining water insoluble drug with water-soluble carrier shows high dissolution rate as the carrier dissolves quickly upon exposure to aqueous medium. The dissolution rate of glass solution is expected to be higher than that of solid solution. In case of glass suspension, it can be defined as a mixture in which precipitated particles are dispersed in a glassy solvent [5].
Amorphous precipitations in a crystalline carrier
In this case a drug may precipitate out in an amorphous form in the crystalline carrier, which differentiates it from eutectic mixture in which both the drug and the carrier crystallise out simultaneously. Amorphous form of a drug has high dissolution rate than the crystalline form. According to Taylor and Zografi, the enhancement of drug release can usually be achieved by using the drug in its amorphous state, since no energy is needed to break the crystal lattice during the process of dissolution [18]. For example, amorphous form of novobiocin was found to have higher solubility and dissolution rate than its crystalline form [9].
Compound or complex formations
In this kind of system, drug and matrix strongly interact with each other in aqueous environment to form complexes. During the dissolution of a drug in the body from a complex or a compound, the availability of a drug depends on its solubility, dissociation constant, and the intrinsic absorption rate of the complex. In comparison with pure insoluble drugs, the rate of dissolution and GI absorption can be augmented by the formation of a soluble complex having low association constant [9].
Generational Breakdown of Solid Dispersions
On the basis of the composition and preparation of solid dispersions, it can be categorized into four generations.
First generation solid dispersions
Firstly, in a study by Sekiguchi and Obi, the word solid dispersions came into existence, where a eutectic mixture was formed which improved the rate of dissolution and also the oral bioavailability of water insoluble drugs. These solid dispersions were named as first generation solid dispersions and were prepared using crystalline carriers viz. urea, sugars, and organic acids. But these first generation of solid dispersions were associated with formation of crystalline solid dispersions, which were thermodynamically stable but were not able to fasten the release of the drug as that of amorphous dispersions [6,10,14,17].
Second generation solid dispersions
In second generation of solid dispersions, crystalline carriers were replaced by amorphous one. Here, the drug is molecularly dispersed in an amorphous polymeric carrier. These carriers were extensively used for solid dispersions as they were able to form amorphous solid dispersions and are further divided into synthetic and natural product based polymers. Synthetic polymers comprise polyethyleneglycols, povidone, and polymethacrylates. Natural polymers composed of several cellulose derivatives (ethylcellulose or hydroxypropylcellulose) or starch derivates (cyclodextrines) [19-21].
Third generation solid dispersions
Third generation solid dispersions involved the utilization of carrier which has self-emulsifying properties or involve the use of mixture of amorphous polymers and surfactants as carriers, which can improve the dissolution in more efficacious manner. These third generation solid dispersions are intended to attain the enormous bioavailability and to stabilize the solid dispersion by avoiding drug recrystallization. It includes the use of carriers such as Poloxamer 408, Tween 80, Gelucire 44/14 [22-24].
Fourth generation solid dispersion
The fourth generation solid dispersion is also known as controlled release solid dispersion (CRSD), where we use poorly water-soluble drugs having short biological half-life. It includes two objectives namely solubility enhancement and extended release in a controlled manner. In this generation, the molecular dispersion of drug in a carrier will improve the solubility whereas use of water swellable polymers can delay the drug release. Due to which we can administer adequate amount of drug for an extended period of time, which in turn offer many benefits such as reduced dosing frequency leading to patient compliance, decreased side effects, prolonged therapeutic effect for poorly water-soluble and short biological half-life drugs. The polymers which are used include ethyl cellulose, hydroxypropyl cellulose, Eudragit RS, RL, poly (ethylene oxide) and carboxyvinyl polymer [25,26].
Suitable Carriers for Solid Dispersion
Many water soluble excipients are employed as carriers for solid dispersions. Following is the criteria that should be considered during selection of such carriers: higher water solubility, which improve wettability and enhance dissolution; high glass transition point leading to improved stability; minimal water uptake (reduces Tg); soluble in common solvent with drug (solvent evaporation); relatively low melting point (melting process); capable of forming a solid solution with the drug-similar solubility parameters.
Poloxamers
The poloxamers are surface active compounds commonly used in the pharmaceutical industry. They are block polymers of a-b-a type, which generally consists of a central, hydrophobic block of polypropylene oxide edged by two hydrophilic blocks of polyethylene oxide. The polymers are obtained from consecutive polymerization of propylene oxide and ethylene oxide and their properties differ in a wide range, due to the probability of combination of blocks of different molecular weights. Generally they are waxy, white granules of free-flowing nature and are odourless and tasteless in nature. They are also known as pluronics. Aqueous solutions of pluronic remain stable in presence of acids, alkalis, and metal ions. They have been considered as a preferred molecule in the formulation development because of its free solubility in polar and non-polar organic solvents. Because the lengths of the polymer blocks can be customized, many different poloxamers exist those have considerably different properties [27,28]. These copolymers are named with "P" (for poloxamer) followed by three digits, where first two digits multiplied by 100 provide the approximate molecular mass of the polyoxypropylene core. Furthermore, the last digit multiplied by 10 gives the percentage polyoxyethylene content (e.g., P407 can be described as poloxamer with polyoxypropylene molecular mass of 4000 g/mol (40×100) and a 70% (7×10) polyoxyethylene content).
Polyethylene glycol (PEG)
PEG is generally water soluble polymer, which is formed by combining monomers of ethylene oxide having molecular weight usually between 200 to 3 00 000. In the formulation of solid dispersions and solutions, PEGs with molecular weights ranging between 1500-20 000 are generally employed. As the molecular weight increases, they lead to increase in the viscosity. Drug to carrier ratio is one of the integral parts, which affects the properties of a solid dispersion. The melting point of the PEGs of concern lies under 65°. The dissolution rate of number of water insoluble drugs has been enhanced by formulating its solid dispersion in PEG 6000 [29].
Polyvinylpyrrolidone (PVP)
PVP results from polymerization of vinyl pyrrolidone and have molecular weights ranging between 2500 and 30 00 000. PVPs have a limited application for the preparation of solid dispersions by the hot melt method but due to their excellent solubility in an abundant variety of organic solvents, they are appropriate for the preparation of solid dispersions by the solvent method. Improved wettability and thereby an improved dissolution rate from a solid dispersion in PVP has been demonstrated. A profound disadvantage of PVPs is that they show poor aqueous solubility with increase in the chain length, additionally the high molecular weight of PVPs exhibit much higher viscosity at a given concentration. This was found in the case of indomethacin where the slower dissolution of indomethacin was observed from PVP K90 compared to PVP K12. It was attributed to the higher viscosity generated by PVP K90 in the diffusion boundary layer adjacent to the dissolving surface of the dispersion. Similar to PEG, solid dispersions prepared with high proportions of PVP tends to high drug solubility and release rate than those with high proportions of drug. Most studies of PVP solid dispersions reported in the literature have used PVPs of molecular weight 2500±50 000 (K12 to K30) [30,31].
Polyvinylalcohol and polyvinylacetate (PVA)/PVP copolymer
These polymers belong to the polyvinyl group and are water soluble. The use of PVA/PVP copolymers as carriers in solid dispersions has been shown to lead an enormous increase in the drug release rate. When solid dispersions of nifedipine were prepared with carrier mixtures consisting of nicotinamide and PVP, hydroxypropylmethylcellulose (HPMC) or PVA in a drug/nicotinamide/polymer ratio of 1:3:1, those prepared with PVA dissolved 20 times faster than the drug alone. However, the other carriers, HPMC and PVP, yielded even better results [30,31].
Crospovidone
This polymer also belongs to polyvinyl group which swells when dispersed in water. Although crospovidone does not dissolve in water, it can also be used as a carrier to improve drug release rate in solid dispersions. For example, solid dispersion of furosemide with crospovidone led to an increase in the dissolution rate by a factor of 5.8 in comparison to either the drug powder or physical mixture of furosemide with crospovidone. The mechanism of increase in the release rate of furosemide was due to the presence of the drug in the amorphous form in the developed dispersion, which was proved by X-ray diffraction studies [31].
Cellulose derivatives, HPMC
Celluloses are naturally occurring polysaccharides, which can be further derivatized to form different carriers. The molecular weight of HPMC lies between 10 000 to 15 00 000 and are completely soluble in water. They are mixed ethers of cellulose where 16-30% of hydroxyl groups are methylated and about 4-32% are derivatized with hydroxypropyl groups HPMC when used for the preparation of solid dispersion of albendazole enhanced the release rate and bioavailability. It was further determined that HPMC was able to hinder the crystallization of albendazole, and further improvement in release characteristics could be achieved by using HPMC and HPMCP in combination. Other drugs which exhibit faster release from solid dispersion in HPMC include poorly soluble weak acids nilvadipine and benidipine [32].
Hydroxypropylcellulose
Hydroxypropylcellulose solubilise in water (up till 408°), ethanol, methanol and chloroform. The average molecular weight of the hydroxypropylcellulose ranges from 37 000 (Type SSL) to 11 50 000 (Type H). Extensive research has been done to determine the effect of chain length hydroxypropylcellulose and also the concentration of hydroxypropylcellulose on the release of solid dispersion of flurbiprofen. The release rate was enhanced as the amount of hydroxypropylcellulose was increased [32].
Carboxymethylethylcellulose (CMEC)
CMEC belongs to the cellulose ethers, but it is resistant to dissolution under gastric or acidic conditions. It dissolves at pH above 5, with lowest dissolution pH being dependent on the grade of the CMEC. Amorphous solid dispersions of nifedipine and spironolactone showed massive enhancement in the dissolution rate of the drug at pH value of 6.8 [32].
Polyacrylates and polymethacrylates
Both polyacrylates and polymethacrylates are glassy substances which are formed by polymerization of acrylic and methacrylic acid. In pharmaceuticals, they are commonly utilized as coatings to modify the release of drug from the dosage form. Commonly the trade name Eudragit refers to them. Among all Eudragits, Eudragit E is frequently used to improve the release rate as it is soluble in all buffer at pH up to 5 and swells when pH is increased, while Eudragit L can be used when it is desirable to evade release in the stomach. Eudragit L has been effectively used to increase the dissolution of griseofulvin and spironolactone at a pH value of 6.8 [33].
Urea
Urea is the end product of human protein metabolism, it has a light diuretic effect and is regarded as nontoxic. Its solubility in water is good and it also exhibits fine solubility in several common organic solvents. In a bioavailability study of solid dispersions, it was shown that sulphathiazole was better absorbed in rabbits when given as a eutectic with urea. In case of ursodeoxycholic acid, it was found that the release rate from urea solid dispersions prepared by the hot melt method was more rapid as compared to other carriers, which were studied, like PEG 6000 [10].
Sugar, polyols and their polymers
Even though sugars and associated compounds are highly water soluble and have less toxicity issues, yet they are less suitable than other carriers for the preparation of solid dispersions. The sugars have high melting point, therefore restricting the applicability of hot melt method for preparation of solid dispersion. Similarly, due to its poor solubility in most organic solvents, it is difficult to form co-evaporates. In spite of these drawbacks, various attempts have been made to prepare solid dispersions with sugars and their derivatives. Mannitol, is one such example, it has a melting point of 165-168° and decomposes only above 250° and has been employed in some cases to prepare dispersions by hot melt method [34].
Different Methods of Preparation
Kneading technique
In case of kneading method, water is added to the carrier and drug to form a thick paste which is kneaded for particular time. The kneaded mixture is dried and passed through sieve to obtain uniform size of solid dispersion [35].
Melting method
Here drug and carrier are mixed properly with the help of mortar and pestle to form homogenous dispersion and subjecting the mixture to heating above the melting point of both drug and carrier. It is then cooled to obtain a firm mass which is crushed and sieved to obtain uniform dispersion. But this method is associated with various drawbacks as it is not relevant for thermolabile drugs and polymers with high melting point like PVP [14].
Co-precipitation method
In this method, the solution of carrier is formed and appropriate amount of drug is added to it and kept under magnetic stirring. To this an antisolvent is added to endorse precipitation. The precipitate is removed, filtered and dried [35].
Co-grinding method
Appropriate amount of drug and carrier are mixed together using a blender at a particular speed and subjected to grinding in the chamber of a vibration ball. The strong grinding forces increase the activation energy and leads to the deformation of crystal lattice. This leads to reduction in crystallinity of drug when ground with a carrier in a vibrational ball mill and consequent increases dissolution rate and bioavailability [36].
Solvent evaporation method
Drug and carrier are solubilized in a common organic solvent and evaporated to obtain solid mass. The solid mass is then grounded, sieved and dried. The nature of solvent and temperature for evaporation of solvent are the two critical factors, which affect the solid mass. Spray drying technique is the amendment of solvent evaporation method for development of solid dispersions wherein drug and carrier are dissolved in a solvent and subjecting it to heated air flow in the spray dryer for the removal of solvent. Similarly freezedrying technology (lyophilisation) is also a surrogate to solvent evaporation method in which drug and carrier are dissolved in a common solvent, which is frozen and sublimed to get a lyophilized molecular dispersion. Small variations in the conditions for evaporating the solvent in both methods lead to prominent effect on product performance. Even the organic solvent used should be properly removed as most of the organic solvents are associated with toxicity issues [14].
Electrostatic spinning method
In this method solid fibers of submicron diameters are formed by subjecting the polymeric solution or melt through a nozzle of millimetre size. As the solvent gets evaporated, the solid fibres are formed, which can be collected on a spinning mandrel. This process involves the electrostatic forces, which when triumph over the surface tension of solution at the air interface lead to the formation of fibres. The method is simple, economical and has remarkable potential for the preparation of nanofibres and control the release of a drug [3].
Melt extrusion method
This technique for the preparation of solid dispersion involves the use of a rotating twin screw extruder through which drug and carrier mixture is extruded. The speed of screw and water content are the two critical parameters which affect the performance of solid dispersions. The concentration of drug in the dispersions is kept as 40% w/w and a plasticizer in the concentration between 5-30% weights of the extrudate can be added to decrease the viscosity of melt in the extrudate [37].
Melt agglomeration process
This method involves the heating of binder, which also act as carrier, drug and excipient above the melting point of the binder. It may also involve spraying the drug dispersion in molten binder using high shear mixer on the heated excipient. Due to better control of the temperature and high feasibility of binder content to be incorporated in the agglomerates, rotary processor is alternative equipment for melt agglomeration [38].
Supercritical fluid process
This technique is applicable for the preparation of solvent free solid dispersion. Supercritical fluid (SCF) is a substance which exists above its critical point of particular temperature and pressure. On heating the liquid its density starts to decrease, whereas the density of vapour increases. As the critical point is reached, densities of both liquid and gas become equal and there is no phase boundary [39]. Above this critical point (the supercritical region), the fluid possesses the penetrating power of a gas and the solvent of a liquid. Carbon dioxide is used as antisolvent for the solute in the SCF antisolvent technology. The use of supercritical carbon dioxide possesses various advantages due of its low temperature and pressure which makes it suitable for heat labile pharmaceuticals, non-toxicity, low cost and non-inflammability. The potential to rapidly diverge the strength of solvent or antisolvent and thus rate of super saturation of dissolved materials is exploited as an alternative technology for formation of particles under various names viz. precipitation from supercritical solutions i.e. rapid expansion of supercritical solution (RESS), precipitation from saturated solutions using SCF as an antisolvent i.e gas antisolvent (GAS), precipitation with PCA-compressed antisolvent, SAS-supercritical antisolvent, ASES-aerosol solvent extraction system, SEDS-solution enhanced dispersion by SCF process, and also precipitation from gassaturated solutions particles from gas saturated solutions (PGSS) [40].
Characterization of Solid Dispersions
Several molecular structures of the drug in the matrix can be encountered in solid dispersions. Many efforts have been made to investigate that how the molecules are arranged in solid dispersions, also to develop a difference between amorphous and crystalline material scientists have put in many efforts. To detect the amount of crystalline material in the dispersion many techniques are available. Different methods used for characterization of solid dispersions are included in Table 1 [41-44].
| Characteristic Feature |
Significance | Method Used |
|---|---|---|
| Drug-carrier interactions | To find out the solid state interaction between drug and carrier and formation of inclusion complex. | FTIR Raman spectroscopy Solid state NMR studies |
| Surface properties | To study the morphology and degree of crystallinity. | Dynamic vapour sorption Inverse gas chromatography Atomic force microscopy Raman microscopy |
| Dissolution rate | To find out the rate and extent of drug release. | Dissolution studies Intrinsic dissolution Dynamic solubility studies |
| Amorphous content | To find out the amorphous transition. | Polarized light optical microscopy Hot stage microscopy Humidity stage microscopy DSC XRD |
Table 1: Characterization of Solid Dispersions
Research Conducted Hitherto on Solid Dispersions
Many researchers have employed the technology of solid dispersion in order to enhance the solubility of different valuable and efficacious drugs. Different methods and carriers have been employed for increasing the water solubility of substantially waterinsoluble compounds [10,45-80].
Various Challenges in Development of Solid Dispersions
Solid dispersion has a great potential for increasing the bioavailability of drug as well as developing controlled release formulations. Despite of long research on solid dispersions, its commercial application is very limited. Much better solubility enhancement of the drug can be obtained with solid dispersion technology, but unfortunately, there are only few products made by this technology that have been marketed so far [81]. Some of them are mentioned in Table 2 [13].
| Commercial products | Polymer used | Manufacturer Company |
|---|---|---|
| Gris-PEG® (griseofulvin) | PEGs21qzz | Valeant Pharmaceuticals, Canada |
| Cesamet® (nabilone) | PVP | Valeant Pharmaceuticals, Canada |
| Sporanox® (itraconazole) | HPMC | Janssen Pharmaceuticals, Belgium |
| Intelence® (etravirin) | HPMC | Tibotec, Yardley, PA, USA |
| Kaletra® (lopinavir& ritonavir) | PVP/VA | Abbott Laboratories, USA |
| Prograf® (tacrolimus) | HPMC | Fujisawa Pharmaceuticals, Spain |
| Rezulin® (troglitazone) | PVP | Sankyo Pharma, Japan |
| Nivadil® (nivaldipine) | HPMC | Fujisawa Pharmaceuticals, Japan |
| Certican® (everolimus) | HPMC | Novartis International, Canada |
| Isoptin® (verapamil) | HPMC/PC | Abbott Laboratories, Canada |
Table 2: Commercially Available Solid Dispersions
Commercial application of the solid dispersion is limited due to: method of preparation: laborious and expensive; reproducibility of physiochemical properties; formulation into dosage forms; scale-up of manufacturing processes; physical and chemical stability of drug and vehicle.
In spite of numerous advantages of solid dispersion, the above mentioned reasons limit its use in commercial dosage forms. The conventional methods for preparation of solid dispersions pose many problems, which limit its use. Processing variables of the melt method influence the physicochemical properties and stability of solid dispersions. Organic solvents used during preparation should be completely removed, which is a difficult process. Dosage form development of solid dispersion into tablet or capsule is also problematic. The soft and waxy nature of the carriers used in development of solid dispersions also possesses some drastic problems, which have been stated here. During compression of powder into tablet, the carrier could plasticize, soften or melt, filling the pores within the tablets and thus making them non-disintegrating. Due to soft and tacky nature of solid dispersions, pulverization and sieving is very difficult. This nature of solid dispersions also causes unique stability problems that might not be seen in other types of solid dosage forms. The conversion of amorphous state to crystalline on storage has resulted in physical instability, which ultimately has impact on dissolution profiles and also bioavailability of drug formulated into solid dispersion. During the preparation of solid dispersions, the drug is molecularly dispersed in an inherently amorphous carrier forming amorphous system. But during storage nucleation and recrystallization of a crystalline drug takes place in solid dispersions [82]. Furthermore, there are many other pitfalls which also requires a profound studies and it include the inability to scale up the solid dosage formulation from a small scale melt quench or a solvent-evaporation technique, insufficient knowledge of the mechanism of dissolution of drug from the dosage form, prevention of crystallization of some drug which takes place in the gastric fluids and the poor understanding of the in vitro-in vivo correlation between these dosage forms [83,84].
Rationale of physical instability and advancement to overcome it
As discussed, the physical instability associated with solid dispersions leads to phase separation and crystallization, which limits the commercial use of solid dispersions. The storage temperature and relative humidity are the crucial factors which affect the physicochemical properties of solid dispersion and causes physical instability; hence protection from moisture is mandatory. However, the use of appropriate polymer is the most established technique for the stabilization of amorphous system. Polymers used in solid dispersions are capable of inhibiting drug crystallization either by enhancing the glass transition temperature (Tg) of the miscible mixture and thereby reducing the molecular mobility or by interacting with functional groups of amorphous drug. To inhibit drug crystallization, the polymer should remain miscible with the drug. Crystallization of drug could occur if drug-carrier miscibility is adversely affected by heat or humidity. Hence, it is imperative to understand the drug and carrier miscibility in order to define strategies for ensuring the physical stability of amorphous solid dispersions. The mechanism behind the instability of solid dispersion has been broadly described by Vasanthavada et al. [85]. The prepared dispersion possesses relatively high Tg values and the molecular mobility is low at the storage temperature. The drug remains in a kinetically frozen state of miscibility. But during exposure to moisture, the dispersion gets plasticized increasing the molecular mobility. Water weakens the hydrogen bond interaction by bridging with structural units of polymer and drug, or it increases the molecular mobility by plasticizing the mixture. In both the cases, diffusion of drug through the polymeric matrix can lead to separation of drug into an amorphous phase, which then crystallizes. In case of temperature, the thermal expansion of the drug and polymer matrix occur and it may reduce the degree of interaction between the components which affects the hydrogen bonding and thus decrease the miscibility limit leading to recrystallization [86]. Additionally, at elevated temperature i.e. above Tg, with the conversion from glass to supercooled liquid phase, the structural relaxations occurs leading to phase separation or crystallization [85]. Different polymers/copolymers have the tendency to act as recrystallization inhibitors in solid dispersions. The appropriate selection of polymer and the drug load are imperative aspects that affect the solid dispersion properties and its physical stability. One can modify the glass transition temperature, which has the impact on rate of crystallization. Furthermore, the polymers having good hydrogen bond acceptors also show greater impact on the stabilization of the amorphous system [87]. During a study on solid dispersions of acetaminophen, Miyazaki et al. 2004, determined that both solid dispersions of acetaminophen prepared with polyacrylic acid and PVP possessed similar Tg values but the former showed slower crystallization rate at the temperature range of 45–60° which was due to the due to stronger hydrogen bonding [88]. Hence the appropriate selection of polymer is very crucial step which affects the physical stability of solid dispersions. In a study, the polymer concentration that resulted in a minor increase in Tg, enhanced the drug stability to a significant level [89].
In addition to the polymer, the use of surfactant can have significant effect on physical stability of solid dispersions. They have also been employed as recrystallization inhibitors. Fule and Amin determined the combined effect of polymer and surfactant on the stability of solid dispersions of lafutidine. The prepared solid dispersions were characterized using different advanced technologies including modulated differential scanning calorimetry (M-DSC), Raman spectroscopy, atomic force microscopy (AFM) and 1H–COSY NMR. AFM studies showed drug and polymer molecular miscibility and surface interaction at the micro level. However, 1H-COSY NMR spectroscopy revealed miscibility/interaction between drug and polymer with chemical shift drifting and line broadening. The combined effect of polymer and surfactant reduced the molecular mobility and inhibited recrystallization during storage of solid dispersions [90].
Novel Technologies Associated with Solid Dispersions
Recent research related to solid dispersions is focused mainly towards the use of novel polymers and scalable manufacturing techniques. The attention is given to enhance the solubility and bioavailability of insoluble and high melting point drugs by formation of dispersion at molecular level using specially crafted polymers. In lieu to this, Enose A et al. used amphiphillic polymer Polyvinyl caprolactam-polyvinyl acetate-PEG graft copolymer (Soluplus) for enhancing the solubility of a poorly water soluble drug, telmisartan. The prepared solid dipersions were characterized by DSC and powder X Ray diffractometry and were found to be stable and possessed higher drug release as compared to free drug [91]. However, Zawar et al. proved novel microwave induced solid dispersion technology as an most effective, solvent free and better alternative to other methods of preparations to effectively enhance the solubility of a poorly water soluble drug [92]. Recently, Singh et al. in their study proved the effectiveness of using natural polymers for enhancing the solubility of drug as compared to synthetic polymers. The results were better analysed by determining the particle size of prepared dispersions by using particle size analyser and which were found to be in nanometric size and possessed utmost solubility upon usage of locust bean gum as a carrier [45].
Apart from enhancing the solubility of poorly soluble drugs, research is in progress to determine the applicability of solid dispersions in various other fields. Usmanova et al. in their study formulated magnetically active solid dispersions for effective and targeted delivery of phenacetin using PEG as a polymer [93]. The supermagnetic nanoparticles were formulated and dispersed in polymeric matrix to form solid dispersion and the formation was confirmed using atomic force and magnetic force microscopy. This can be more effective as it combine the advantage of higher solubility and targeted delivery [93]. However, Duarte et al. prepared nano solid dispersions using a novel solvent controlled precipitation process based on microfluidization. They formulated both amorphous as well as crystalline nano solid dispersions which were found to have faster dissolution rates and improved bioavailability as compared to micron sized amorphous powder. They concluded that the reduction of particle size into nanometric range plays more important role as compared to amorphization of the drug in case of solid dispersions [94]. The concept of amorphization of a crystalline drug, a widely reported mechanism for enhancing the solubility has been triumph over by using these novel techniques. Similarly, in a study during the preparation of solid dispersions of carvedilol, it was proved that for enhancing the solubility of a crystalline drug, it is not mandatory to convert it into amorphous form. However, by attaching the hydrophilic carriers to the surface of drug, we can convert the hydrophobic drug to a hydrophilic form without changing the crystalline form by using novel surface attached spray-dried solid dispersion technology. The prepared solid dispersions were found to have 11 500-fold and two fold higher drug solubility and dissolution rate, respectively [95].
Future Prospects
Solid dispersions for poorly soluble drug will flaunt due to recent advancements in the technology with respect to method of preparation, scale-up, and development of newer methods for better predictability of solid state structure. Future research on solid dispersions should adopt new techniques to study the solubility and molecular state of the drug and its interaction with the polymer to remove the difficulty in design of new carriers that can prevent crystallization of drug. The effects of storage conditions on the properties of drug, carrier used, drug release profile and bioavailability of drug need to be addressed more extensively. Moreover, it is a possible assertion that substitution of a hydrophobic carrier for a hydrophilic carrier may result in a well-controlled solid dispersion process. The method could be easily adopted to attain the sustained release of drug or to alter solid state properties. It is imperative that the solid dispersion technique offers vast potential for future research and subsequent growth which may result in development of novel applications for oral drug delivery.
Finally, the question comes to the mind that whether the application of solid dispersion technology can be considered as a universal approach for enhancing the solubility of poorly water-soluble compounds. Currently, not any of the strategies used for increasing the solubility of drug in aqueous media is appropriate for all drugs and dosing requirements. However, determining the stability of the amorphous state of solid dispersions remains an area of interest, which seeks the attention of researchers and there is still more to be worked upon to get the extreme benefit of this effective technique. But among all the techniques used in the industries to enhance dissolution of poorly water soluble drug, solid dispersion emerge to be the most versatile and is applicable to majority of compounds and their dosing requirements.
Conflict of interest
The authors confirm that this article content has no conflicts of interest
Financial support and sponsorship
Nil.
References
- http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm128219.htm.
- Aulton ME. Pharmaceutics: The science of dosage form design. 1st ed. London; Churchill Livingstone, 1996.
- Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersions: A Review. Pak J Pharm Sci 2009;22:234-46.
- Tiwari R, Tiwari G, Srivastava B, Rai AK, Singh P. Solid Dispersions: An overview to modify bioavailability of poorly water soluble drugs. Int J Pharm Tech Res 2009;1:1338-49.
- Serajuddin AT. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci1999;88:1058-66.
- Craig DQM. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm 2002;231:131-44.
- Lobenberg R, Amidon GL. Modern bioavailability and bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm 2000;50:3-12.
- Bergstrom CA, Strafford M, Lazorava L, Avdeef A, Luthman K, Artursson P, et al. Absorption classification of oral drugs based on molecular surface properties. J Med Chem 2003;46:558-70.
- Chiou WL, Riegelman S. Pharmaceutical applications of solid dispersion systems. J Pharm Sci 1971;60:1281-302.
- Sekiguchi K, Obi N. Studies on absorption of eutectic mixtures. I. A comparison of the behaviour of eutectic mixtures of sulfathiazole and that of ordinary sulfathiazole in man. Chem Pharm Bull 1961;9:866-72.
- Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures II. Experimental evaluation of eutectic mixture: urea-acetaminophen system. J Pharm Sci1966;55:482-7.
- Jafari MR, Danti AG, Ahmed I. Comparison of polyethylene glycol, polyvinylpyrolidone and urea as excipients for solid dispersion systems of miconazole nitrate. Int J Pharm 1988;48:207-15.
- Sethia S, Squillante E. Solid dispersions-Revival with greater possibilities and applications in oral drug delivery. Crit Rev Therap Drug Carrier Syst 2003;20:215-47.
- Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm 2000;50:47-60.
- Craig DQM, Newton JM. Characterization of polyethylene solid dispersions using differential scanning calorimetry and solution calorimetry. Int J Pharm 1999;76:17-24.
- Gaurav T. Solid dispersions: an overview to modify bioavailability of poorly water soluble drugs. Int J Pharm Tech Res 2009;1:1338-49.
- Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures I - theoretical considerations and discussion of the literature. J Pharm Sci 1965;54:1145-8.
- Taylor LS, Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm Res 1997;14:1691-8.
- Okonogi S, Oguchi T, Yonemochi E, Puttipipatkhachorn S, YamamotoK. Improved dissolution of ofloxacin via solid dispersion. Int J Pharm 1997;156:175-80.
- Drooge DV, Hinrichs W, Visser M, Frijlink H. Characterization of the molecular distribution of drugs in glassy solid dispersions at the nano-meter scale, using differential scanning calorimetry and gravimetric water vapour sorption techniques. Int J Pharm 2006;310:220-29.
- Urbanetz NA. Stabilization of solid dispersions of nimodipine and polyethylene glycol 2000. Eur J Phar Sci 2006;28:67-76.
- Joshi HN, Tejwani RW, Davidovich M, Sahasrabudhe VP, Jemal M, Bathala MS, et al. Bioavailability enhancement of a poorly water-soluble drug by solid dispersion in polyethylene glycol-polysorbate 80 mixture. Int J Pharm 2004;269:251-8.
- Ghebremeskel AN, Vemavarapu C, Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: selection of polymer-surfactant combinations using solubility parameters and testing the process ability. Int J Pharm 2007;328:119-29.
- Goddeeris C, Willems T, Houthoofd K, Martens J, Mooter GV. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary solid dispersion with TPGS 1000 and Eudragit E100. Eur J Pharm Biopharm 2008;70:861-8.
- Miyazaki T, Aso Y, Yoshioka S, Kawanishi T. Differences incrystallizationrate of nitrendipine enantiomers in amorphous solid dispersions with HPMC and HPMCP. Int J Pharm 2011;407:111-8.
- Huang J, Wigent RJ, Schwartz JB. Nifedipine molecular dispersion in microparticles of ammonio methacrylate copolymer and ethylcellulose binary blends for controlled drug delivery: effect of matrix composition. Drug DevInd Pharm 2006;32:1185-97.
- https://products.basf.com/documents/pim;view/en/8806188788181.ExAct%20(issue%203).pdf.
- Karmarkar AB, Gonjari ID, Hosmani AH. Poloxamers and their applications. Int J Pharma 2008.
- Price JC. Polyethylene glycol. In: Wade A, Weller PJ, editors. Handbook of pharmaceutical excipients. Washington, DC/London: American Pharmaceutical Association/The Pharmaceutical Press; 1994. p. 355-61.
- Buehler V. Soluble Kollidon Grades (Povidone, Polyvidone): Tablet Coatings, Kollidon: Polyvinylpyrrolidone for the Pharmaceutical Industry. Ludwigshafen: BASF; 1999. p. 106-15.
- Walking WD. Povidone. In: Wade A, Weller PJ, editors. Handbook of pharmaceutical excipients. Washington, DC/London: American Pharmaceutical Association/The Pharmaceutical Press; 1994. p. 392-99.
- Harwood RJ, Johnson JL. Hydroxypropylmethylcellulose. In: Wade A, Weller PJ, editors. Handbook of pharmaceutical excipients. Washington, DC/London: American Pharmaceutical Association/The Pharmaceutical Press; 1994. p. 223, 229.
- Hasegawa A, Kawamura R, Nakagawa H, Sugimoto I. Physical properties of solid dispersions of poorly water-soluble drugs with enteric coating agents. Chem Pharm Bull 1985;33:3429-35.
- Kai T, Akiyama Y, Nomura S, Sato M. Oral absorption improvement of poorly soluble drug using solid dispersion technique. Chem Pharm Bull 1996;44:568-71.
- Adley AN, Jose LS, Roberto AC, Pedro JR. Alternative technology to improve solubility of poorly water soluble drug. Lat Am J Pharm 2008;27:789-97.
- Yamamoto K, Nakamo M, Arita T, Nakai Y. Preparation and thermal characterization of poly (ethyl oxide)/griseofulvin solid dispersions for biomedical application. J Pharm Biopharm 1974;2:487-95.
- Choksi R, Zia H. Hot-melt extrusion technique: A review. J Pharm Res 2004;3:107-17.
- Vilhelmsen T, Eliasen H, Schaefer T. Effect of a melt agglomeration process on agglomerates containing solid dispersions. Int J Pharm 2005;303:132-42.
- Subramanian B, Rajewski RA, Snavely K. Pharmaceutical processing with supercritical carbon dioxide. J Pharm Sci 1997;86:885-90.
- Sethia S, Squillante E. Physicochemical characterization of solid dispersions of carbamazepine formulated by supercritical carbon dioxide and conventional solvent evaporation method. J Pharm Sci 2002;91:1948-57.
- Arunachalam A, Ashutoshkumar S, Karthikeyan M, Konam K, Prasad PH, Sethuraman S, et al. Solid Dispersions: A Review. Curr Pharm Research 2010;1:82-90.
- Bugay DE. Characterization of solid-state: spectroscopic technique. Adv Drug Deliv Rev 2001;48:43-65.
- De MP, Rahier H, Van MB. The use of modulated temperature differential scanning calorimetry for the characterization of food systems. Int J Pharm 1999;192:77-84.
- Taylor LS, Zografi G. The quantitative analysis of crystallinity using FT-Raman spectroscopy. Pharm Res 1998;15:755-61.
- Singh G, Sharma S, Gupta GD. Extensive diminution of particle size and amorphization of a crystalline drug attained via eminent technology of solid dispersion: A comparative study. AAPS PharmSciTech2017;18:1770-84.
- Tsunashima D, Yamashita K, Ogawara, Sako K, Higak K. Preparation of extended release solid dispersion formulations of tacrolimus using ethylcellulose and hydroxypropylmethylcellulose by solvent evaporation method. J Pharm Pharmacol 2016;68:316-23.
- Ramesh K, Chandra SB, Khadgapathi P, Bhikshapathi DVRN, Renuka K. Development, characterization and in vivoevaluation of tovaptan solid dispersions via solvent evaporation technique. Int J Drug Delivery 2015;7:32-43.
- Daravath B, Tadikonda R. Formulation and in vitro evaluation of flurbiprofen-polyethylene glycol 20000 solid dispersions. J App Pharm Sci 2014;4:76-81.
- Frizon F, Eloy JO, Donaduzzi CM, Mitsui ML, Marchetti JM. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol 2013;235:532-9.
- Zakir F, Choudhary A, Rana AC. Development and characterization of an atorvastatin solid dispersion formulation using skimmed milk for improved oral bioavailability. Acta Pharm Sinica B 2012;2:421-8.
- Papadimitriou SA, Barmpalexis P, Karavas E, Bikiaris DN. Optimizing the ability of PVP/PEG mixtures to be used as appropriate carriers for the preparation of drug solid dispersions by melt mixing technique using artificial neural networks: I. Eur J Pharm Biopharm 2012;82:175-86.
- Yan ZC, Jie DY. Genomic study of the absorption mechanism of cantharidin and its solid dispersion. Colloids Surf A PhysicochemEng Asp 2012;415:295-301.
- Aggarwal AK, Jain S. Physicochemical characterization and dissolution study of solid dispersions of ketoconazole with nicotinamide. Chem Pharm Bull 2011;59:629-38.
- Patel T, Patel LD, Timir P. Dissolution enhancement of fenofibrate by solid dispersion technique. Curr Pharm Res 2011;1:127-34.
- Lin SY, Hsu CH, Sheu MT. Curve-fitting FTIR studies of loratadine/HP-β-CD inclusion complex induced by co-grinding process. J Pharm Biomed Anal 2010;53:799-803.
- Castro SG, Bruni SS, Lanusse CE, Allemandi DA, Palma SD. Improved albendazole dissolution rate in pluronic 188 solid dispersions. AAPS Pharm Sci Tech 2010;11:1518-25.
- Patel TB, Patel LD. Formulation and characterization of solid dispersions containing glibenclamide. Int J Pharm PharmSci 2010;2:138-41.
- Ghareeb MM, Abdulrasool A, Hussein A, Noordin M. Kneading technique for preparation of binary solid dispersion of meloxicam with poloxamer 188. AAPS Pharm Sci Tech 2009;10:1206-15.
- VyasV, Sancheti P, Karekar P, Shah M, Pore Y. Physicochemical characterization of solid dispersion systems of tadalafil with poloxamer 407. Acta Pharm 2009;59:453-61.
- Biswal S, Sahoo J, Murthy PN. Physicochemical properties of solid dispersions of gliclazide in polyvinylpyrrolidone K90. AAPS Pharm Sci Tech 2009;10:329-34.
- Laitinen R, Suihko E, Toukola K. Intraorally fast-dissolving particles of a poorly soluble drug: Preparation and in vitro characterization. Eur J Pharm Biopharm 2009;71:271-81.
- Patel AR, Joshi VY. Evaluation of SLS: APG mixed surfactant systems as carrier for solid dispersion. AAPS Pharm Sci Tech 2008;9:583-90.
- Chen J, Qiu L, Hu M, Jin Y, Han J. Preparation, characterization and in vitroevaluation of solid dispersions containing docetaxel. Drug DevInd Pharm 2008;34:588-94.
- Ye G, Wang S, Heng PW, Chen L, Wang C. Development and optimization of solid dispersion containing pellets of itraconazole prepared by high shear pelletization. Int J Pharm 2007;337:80-7.
- Shah TJ, Amin AV, Parikh JR, Parikh RH. Process optimization and characterization of poloxamer solid dispersions of a poorly water-soluble drug. AAPS Pharm Sci Tech 2007;8:E18-E24.
- Valizadeh H,Zakeri-MilaniP,Barzegar-Jalali M,Mohammadi G,Danesh-Bahreini MA,Adibkia K,et al. Preparation and characterization of solid dispersions of piroxicam with hydrophilic carriers. Drug DevInd Pharm 2007;33:45-56.
- Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS Pharm Sci Tech 2006;7:E87-E92.
- Badry M, Fathy M. Permeation rates of meloxicam by formation of its freeze-dried solid dispersions in polyvinylpyrrolidone K-30. Drug DevInd Pharm 2006;32:141-50.
- Kim EJ, ChunMK, JangJS, LeeIH, Lee KR, Choi HK. Preparation of a solid dispersion of felodipine using a solvent wetting method. Eur J Pharm Biopharm 2006;64:200-5.
- Zajc N, Obreza A, Bele M. Physical properties and dissolution behaviour of nifedipine/mannitol solid dispersions prepared by hot melt method. Int J Pharm 2005;291:51-8.
- Karavas E, Ktistis G, Xenakis A, Georgarakis E. Miscibility behavior and formation mechanism of stabilized felodipine-polyvinylpyrrolidone amorphous solid dispersions. Drug DevInd Pharm 2005;31:473-89.
- Sethia S, Squillante E. Solid dispersion of carbamazepine in PVP K30 by conventional solvent evaporation and supercritical methods. Int J Pharm 2004;272:1-10.
- Verreck G, Six K, Van den Mooter G, Baert L, Peeters J, Brewster ME,et al. Characterization of solid dispersions of itraconazole and HPMC prepared by melt extrusion. Int J Pharm 2003;251:165-74.
- VerreckC. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm Res 2003;20:810-7.
- Vippagunta SR, Maul KA, Tallavajhala S, Grant DJ. Solid-state characterization of nifedipine solid dispersions. Int J Pharm 2002;236:111-23.
- Jung JY, Yoo SD, Lee SH, Kim KH, Yoon DS. Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique. Int J Pharm 1999;187:209-18.
- Perng CY, Kearney AS, Patel K, Palepu NR, Zuber G. Investigation of formulation approaches to improve the dissolution of SB-210661, a poorly water soluble 5-lipoxygenase inhibitor. Int J Pharm 1998;176:31-8.
- Ho H, Su HL, Tsai T, Sheu MT. The preparation and characterization of solid dispersions on pellets using a fluidized-bed system. Int J Pharm 1996;139:223-9.
- Betageri GV, Makarla KR. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm 1995;126:155-60.
- Save T, Venkitachalam P. Studies on solid dispersions of nifedipine. Drug DevInd Pharm 1992;18:1663-79.
- Janssens S, Mooter GV. Review: physical chemistry of solid dispersions. J Pharm Pharmacol 2009;61:1571-86.
- Sathigari SK, Radhakrishnan VK, Davis VA.Amorphous-state characterization of efavirenz-polymer hot-melt extrusion systems for dissolution enhancement. J Pharm Sci 2012;101:3456-64.
- Serajuddin TM. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci 1999;88:1058-66.
- Sheen PC, Khetarpal VK, Cariola CM, Rawlings CE. Formulation studies of a poorly water-soluble drug in solid dispersions to improve bioavailability. Int J Pharm 1995;118:221-27.
- Vasanthavada M, Tong WQ, Joshi Y, Kislalioglu MS. Phase behaviour of amorphous molecular dispersions i: determination of the degree and mechanism of solid solubility. Pharm Res 2004;21:1598-606.
- Shibata Y, Fujii M, Suzuki A, Koizumi N, Kanada K, Yamada M, et al. Effect of storage conditions on the recrystallization of drugs in solid dispersions with crospovidone. Pharm DevTechnol 2014;19:468-74.
- Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev 2001;48:27-42.
- Miyazaki T, Yoshioka S, Aso Y, Kojima S. Ability of polyvinylpyrrolidone and polyacrylic acid to inhibit the crystallization of amorphous acetaminophen. J Pharm Sci 2004;93:2710-17.
- Konno H, Taylor LS. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J Pharm Sci 2006;95:2692-705.
- FuleR, Amin P. Development and evaluation of lafutidine solid dispersion via hot melt extrusion: Investigating drug-polymer miscibility with advanced characterization. Asian J Pharm Sci 2014;9:92-106.
- Enose AA, Dasan P, Sivaramakrishanan H, Kakkar V. Formulation, characterization and pharmacokinetic evaluation of telmisartan solid dispersions. J Mol Pharm Org Process Res 2016;4:1-8.
- Zawar L, Bari S. Microwave induced solid dispersion as a novel technique for enhancing dissolution rate of repaglinide. AdvPharmacol Pharm 2013;1:95-101.
- UsmanovaLS, ZiganshinMA, GorbatchukV, ZiganshinaSA, BizyaevDA, BukharaevA, et al. A study of the formation of magnetically active solid dispersions of phenacetin using atomic and magnetic force microscopy. J Adv Pharm Tech Res 2017;8:2-7.
- Duarte I, Corvo ML, Serodio P, Vicente J, Pinto JF, Temtem M, et al. Production of nano-solid dispersions using a novel solvent-controlled precipitation process-Benchmarking their in vivo performance with an amorphous micro-sized solid dispersion produced by spray drying. Eur J Pharm Sci 2016;93:203-14.
- Lee SN, Poudel BK, Tran TH, Marasini N, Pradhan R, Lee YI,etal.A novel surface-attached carvedilol solid dispersion with enhanced solubility and dissolution. Arch Pharm Res 2013;36:79-85.