- *Corresponding Author:
- Geetha Subramanian
Department of Biotechnology, University College of Engineering, BIT Campus, Anna University, Tiruchirappalli, Tamil Nadu 620024, India
E-mail: jinoaancius@gmail.com
| Date of Received | 04 Jun 2025 |
| Date of Revision | 05 Jun 2025 |
| Date of Accepted | 15 July 2025 |
| Indian J Pharm Sci 2025;87(4):133-140 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Mycosporine like amino acids are small extra-cellular metabolites, which are produced by diverse group of microorganisms including cyanobacteria; possess photo protectant and antioxidant action. The goals of this work include increasing the productivity of Mycosporine like amino acids in cyanobacteria, identification and description of these metabolites using Fourier transform infrared and high-performance liquid chromatography, and evaluation of the cytotoxic activity of the investigated cyanobacterial extracts in vitro. The outcomes extend the current knowledge of Mycosporine like amino acids biomedical utility and lay the foundation for the development of their therapeutic potential.
Keywords
Mycosporine, amino acids, cyanobacteria, photo protectant, antioxidant action
The impact of Ultraviolet (UV) radiation from the sun is destructive to nucleic acids, proteins, and lipids of all living organism. Even though, the detrimental effects of UV radiation cannot be avoided by simply covering the skin, many organisms possess UVabsorbing compounds like the Mycosporine-Like Amino Acids (MAAs)[1]. MAAs are small, water soluble compounds incorporating a cyclohexanone or cyclohexenimine chromophore directly conjugated to a side chain derived from an amino acid or an amino alcohol. They show high absorption in the UV region of the spectrum ranging from 310-365 nm, and act as natural sunblock, shielding the cells against UV deficient damage[2]. Of all the sources of MAAs, cyanobacteria deserve special attention. Cyanobacteria are photosynthetic microorganisms found in various extreme conditions and are thus the most efficient producers of bioactive substances, such as MAAs[3][4]. These organisms have been the focus of many researchers interested in understanding the nature of MAAs synthesis under different stress factors such as UV radiation, high salinity, and low nutrient concentrations[5]. Other practical uses arising from the biotechnological prospect of cyanobacteria include its ease of genetic modification and their fast growth dynamics. MAAs have received much attention because they are involved in many functions besides UV protection[6]. It shows antioxidant; antiinflammatory and anti-aging properties thus making them useful in the production of cosmeceutical products.
The latter has recently also been demonstrated to possess anticancer properties in several investigations[7]. MAAs can suppress cancer cell growth and stimulate cell death through regulating levels of oxidative stress and signalling pathways[8]. These properties can entitle MAAs as potential candidates for designing new untainted anticancer agents[9]. While and their biotechnological applications have been identified in cyanobacteria, the cellular quantities of MAAs often do not meet the requirements for commercial production[10]. Metabolic engineering approaches, improvement of the cultivation conditions, and studying stress-inductive conditions are the major directions for further research[11]. Pharmacogenomics work has revealed enzymes implicated in MAA synthesis including dehydroquinate synthase and O-methyltransferase, which can be genetically adjusted to increase MAA yield.
Identifying the structure and properties of MAAs is therefore crucial towards the elucidation of the biosynthetic routes and the activity profiles. Currently, High-Performance Liquid Chromatography (HPLC), Mass Spectrometry (MS), and Nuclear Magnetic Resonance (NMR), are most frequently used for MAA identification and quantity measurement. Advanced techniques in the analysis have allowed the identification of numerous MAAs with peculiar UV-VIS absorption and biological activities[6]. To date, there seem to be little understanding of the ability of MAAs to possess anticancer activity[2]. Several authors have stated that some MAAs selectively exhibit cytotoxic behaviours towards cancer cells and are harmless toward healthy cells[12]. At the molecular level, MAAs help to control Reactive Oxygen Species (ROS) and, consequently, oxidative stress mediated Deoxyribonucleic Acid (DNA) damage and apoptosis in cancer cells. In cancer cells the same oxidative stress modulation may affect the redox balance and result in cell death[13]. Moreover, MAAs have also been found to regulate important molecular pathways such as Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/AKT) and Mitogen-Activated Protein Kinase (MAPK), which are dysregulated in cancer cells [14]. The focus of the commenced study is to improve the MAA biosynthesis in cyanobacteria through environmental manipulation, to characterize the isolated MAA?s physicochemical properties and to evaluate their in-vitro anticancer properties. The present study could therefore set the tone for the institutionalization of MAAs as affordable and realistic biomolecules in the pharma and cosmetic industries.
Materials and Methods
Collection of cyanobacteria:
The cyanobacterial species used in the current study were Oscillatoria species (BDU 14219) obtained from the National Facility for Marine Cyanobacteria (NFMC), Bharathidasan University.
Cultivation of cyanobacteria[15]:
Oscillatoria species were isolated under laboratory culture experiments. Preliminary studies on the physiology of MAA synthesis involving light intensity (50 µ mol-2 s-1), temperature (25°), and nutrient composition (modified BG-11) were used. Samples were harvested in the exponential phase of growth, when the cells were producing large quantities of metabolites on which the cultures were fed.
Extraction and purification of MAAs:
The extraction of MAAs was done using the procedure described[16]. Firstly, cyanobacterial crust was washed three times with Millipore water to wash off dust particles and other contaminants. The cleaned crust was lyophilized and the dried mat was then mechanically homogenized to powder with a micro pestle.
The latter was followed by the suspension of 1 g of the powdered biofilm in 20 ml of 100 % HPLC grade methanol at 4° for 12 h. The sample was sonicated for 1 min and centrifuged at 10 000×g for 5 min. The supernatant was left to dry at room temperature [17]. Finally, ultra-pure water was used to dissolve 650 µl of the dried extract; the solution was again subjected to the centrifuge at 10 000×g for 5 min to filter away water insoluble substances. 100 µl of chloroform was added to the solution and the solution was mixed using a vortex. The purified MAAs float in the upper aqueous layer, while the lipophilic pigments and other contaminants are in the lower organic phase, thus, the upper aqueous layer was carefully transferred to the Eppendorf tubes.
Last, the purified extract was filtered using a 0.22 µm micro centrifuge type syringe filter to produce the sample prior to its use in HPLC.
UV-Vis spectroscopy:
The absorbance spectrum of the supernatants was obtained using a UV-Vis spectrophotometer; between a wavelength of 200 nm and 700 nm. Spectra obtained were processed using software which is in-built in the spectrophotometer after data has been transferred to a computer as per the manufacturer?s specifications[18].
The evaluation of the purified MAAs was done relative to their RP-HPLC profile using RP-HPLC system with Empower-2 software. The instrument was coupled with reverse-phase C18 column for the analysis.
Sample preparation of the extract was done by injecting 20 µl of the extract directly into the column via auto sampler. The samples (10 µl) were injected and the sample were analysed isocratically under a mobile phase of 0.02 % (v/v) acetic acid in HPLC-grade water at a flow rate of 1 ml/min. Measurements for MAAs were made at a fixed wavelength of 330 nm[20].
The individual peaks were thereafter scrutinized with the help of software provided by the manufacturer. The collected fractions were lyophilized and then characterized later.
Fourier Transform Infrared (FTIR) analysis:
Characterization of the functional groups in the MAAs was done using the FTIR analysis. The dried MAAs were blended with oven-dried Potassium bromide (KBr) to make a pellet using hydraulic press. The analysis was done using an FTIR spectrophotometer PerkinElmer. Spectra that were recorded were processed using the software that accompanies these devices from the manufacturer[21].
In vitro antioxidant assay:
2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) assay:
333 µl of DPPH was added to 1 ml of sample (concentration range: 20 to 100 µg/ml). L-Ascorbic acid was used as positive control. The mixture was shaken vigorously for a few seconds and then was allowed to stand at room temperature for 30 min. The absorbance was read at 513 nm in a UV-visible spectrophotometer. The absorbance of the reaction mixture is indirectly proportional to the free radical scavenging ability of the samples.
Scavenging effect (%) = (Absorbance of test - Absorbance of control) / (Absorbance of control) × 100
Hydrogen peroxide (H2O2) inhibition assay:
43 mM solution of H2O2 in 0.1M phosphate buffer (pH 7.4) and the samples of varying concentrations (20 to 100 µg/ml) was mixed in equal volumes. L-Ascorbic acid was used as positive control. Sodium phosphate buffer without H2O2 served as blank. The samples were measured at 230 nm. The percentage of inhibition was calculated using the following formula,
H2O2 inhibition activity (%) = (Absorbance of test - Absorbance of control) / (Absorbance of control) × 100
In vitro anticancer assay:
The cytotoxicity of MAAs was studied on A431 (skin cancer cell line) and L929 (Fibroblast cell line) using (3-(4, 5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) assay. Varying concentration of the sample (1 µg/ml to 500 µg/ml) were added to the cells (1×105 cells/well) and incubated for 24 h. Later 10 µg of MTT was added to each well and incubated again for 4 h. Finally, the entire media was aspirated without disturbing the formazan crystals to which 100 µl of Dimethyl Sulfoxide (DMSO) was added and mixed gently to dissolve the crystals. After 20 min the plate was read at 570 nm. The recorded absorbance was directly proportional to the cell viability. The half maximal inhibitory concentration (IC50) value was calculated using GraphPad prism software.
Results and Dis cussion
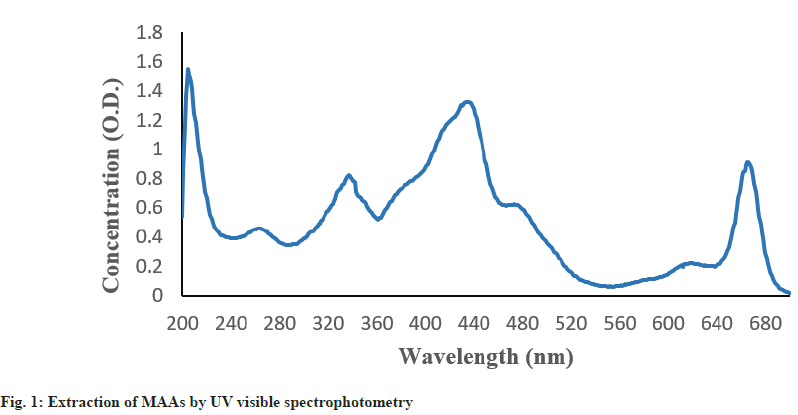
MAAs containing methanolic extracts of Oscillatoria sp. strain were detected using the absorbance spectra of the compound in the UV-Vis region and is illustrated in the fig. 1. The presence of MAAs in the organism was validated by the absorption peak shown by the spectrum which ranged between 334 and 335 nm.
Moreover, the spectrum showed typical peaks representing carotenoids (440-480 nm). The presence of such various pigments in the methanolic extract of Oscillatoria sp., is assisted by this work.
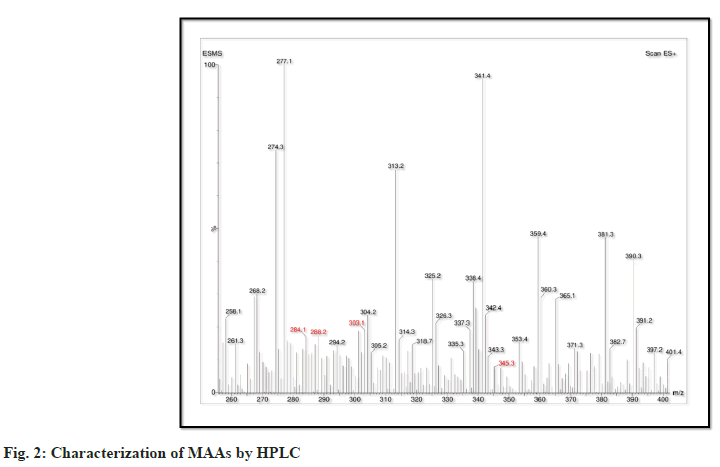
Thus, there are differences in the HPLC pattern and molecular properties of MAAs isolated from cyanobacterium Oscillatoria sp., presented below. The MAAs isolated are Palythene (C13H20N2O5 M/Z=284.1), Asterina-330 (C12H20N2O6 M/Z=288.2) Palythinol (C13H23N2O6 M/Z=303.1) and Porphyra-334 (C14H22N2O8 M/Z=345.3). In these, porphyra-334 has the highest molecular weight indicating its larger structure may be partly responsible for the improvement of its ability to absorb UV radiation (fig. 2). On the other hand, palythene had the lowest M.W consistent with giving it a simpler molecular structure for distinct purposes.
These differences in molecular structures of these MAAs indicate their different chemical functions and functions related to the protection against the UV induced oxidative damage. It is suggested that owing to the increased molecular weights compared to palythene and asterina-330, Porphyra-334 and palythinol would possess superior stability and antioxidative activity. The high separation efficiency of the reverse-phase HPLC system shows the strong analytical method as applied to the identification of these compounds showing high molecular weight and polarity differences. Detailed characterisation of MAAs has given an understanding on the possibility of these components in pharmacological and cosmeceutical industries, noting their significance in protection against UV radiation and oxidative stress.
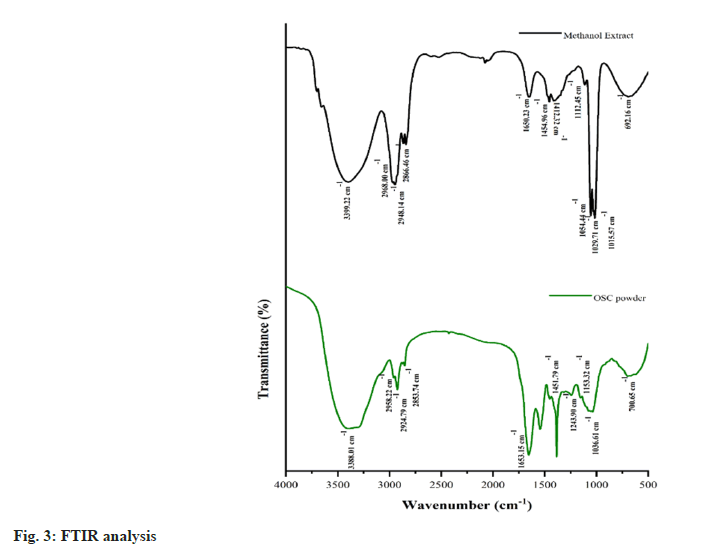
FTIR indicators for MAA derivatives are presented in the following table, with the solid line representing characteristic absorption peaks corresponding to key functional groups (fig. 3). A peak at 3399.22 cm-1 is assigned to the N-H stretch confirming the presence of primary or secondary amines. C-H stretch in the region 2968.00?2853.74 cm-1 corresponds to the region of alkanes the region 2350.00?2215.78 cm-1 corresponds to O-H stretch primary and secondary alcohols. A peak at 1650.23 cm-1 is assigned to the C=C stretch, a feature present in alkenes and the range 1454.96-1412.32 cm-1 refers to the aromatic C-C stretch, another feature of aromatic compounds. All these peaks collectively proved the existence of amines, alkanes, alkenes, and aromatic groups in MAAs and give an elaborate analysis of the chemical structure.
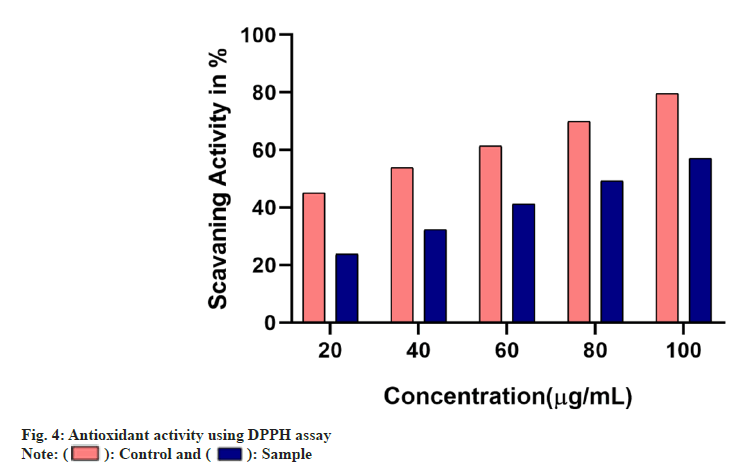
The antioxidant ability of the samples was studied using different assays. The free radical scavenging ability of the sample was found to increase in a dose dependant manner in comparison with the standard used whose IC50 was found to be below 40 µg/ml. The IC50 of the sample in DPPH assay was found to be 80 µg/ml whereas in H2O2 assay it was found to be 100 µg/ml (fig. 4 and fig. 5).
Cell survival analysis by the MTT assay showed that MAAs were toxic to cancer cell lines, reducing their viability at an IC50 concentration of 47.43 µg/ml. This value implies that at low concentrations, MAAs displayed a highly effective cytotoxicity against A431 cell lines. The compound of interest did not exhibit any cytotoxicity on the normal cell lines used for the study.
Furthermore, a significant cytotoxic effect in a dose dependent manner was found. It can be observed from the fig. 6 that; as the concentration of MAAs rose, cell viability dropped notably especially when the concentration exceeded 200 µg/ml. This suggests that increased levels of MAAs lead to even greater levels of cytotoxicity thus making them useful as therapeutic agents in cancer. The obtained results suggest that MAAs can be potentially beneficial for further exploration as part of anticancer agents.
Cyanobacteria has evolved as strong potential sources of bioactive metabolites including UV-absorbing agents-MAAs. Of these, the Oscillatoria species have particular importance as they contain a range of pigments along with MAAs exhibiting photo-protective and antioxidant properties[18].
This discussion critically assesses the information obtained on Oscillatoria sp. against the background of closely related Lyngbya sp. strain HKAR-15 and correlates the novel findings of the study.
The UV-Vis spectral analysis of Oscillatoria sp. showed maximum absorbance at 334 nm which corresponds to the absorbance of MAAs. This observation confirms the presence of UV-screening compounds in this species. Also, in the upwards scan the shoulder was observed in the range of 440-480 nm due to the presence of carotenoids[22].
Apart from photo protection, these pigments are also involved in antioxidative defense systems of organisms. The presence of such peaks proves the ability of Oscillatoria sp. in synthesizing a number of pigments which gives an organism many-fold protection against UV radiation[1].
In contrast, Lyngbya sp. strain HKAR-15 showed specific MAA-related wavelengths of around 315? 340 nm and additional peaks for carotenoids (around 470 nm) chlorophyll ?a? (around 420 and 665 nm) and phycobiliproteins (around 616 nm). Although both species synthesize MAAs and carotenoids pigments, the range of pigments in Oscillatoria sp. suggests that it was capable of photoprotection and photosynthesis under different conditions[23,24].
The Oscillatoria sp. was further analysed using HPLC that led to the recognition of various MAAs including; palythene (MW=284.1 Da), asterina-330 (MW=288.2 Da), polythinol (MW=303.1 Da) and porphyra 334 (MW=345.3 Da). Of the presented ones, porphyra-334 had the highest molecular weight, its structure being composed of two substructures, thus possessing enhanced functions of UV absorption[10].
Although palythene molecular weight is higher, its structural formula seems to be less complex rendering them perfect for specific biochemical functions.
Lyngbya sp. also exhibited a similar trend, with HPLC analysis revealing palythine (245.02) and porphyra-334 (347.1) as the most abundant MAAs. These findings are further supported by molecular profiling conducted in Oscillatoria sp., highlighting a shared biochemical strategy among cyanobacteria for MAA biosynthesis. Notably, Oscillatoria species demonstrated a broader spectrum of MAAs, suggesting a robust adaptive mechanism to varying UV radiation conditions. Based on these advantages, Oscillatoria species were selected for further investigation[8].
The functional group of Oscillatoria sp. shown by the FTIR analysis includes N-H stretch corresponding to primary and secondary amine, C-H corresponding to alkanes and C=C corresponding to aromatic amines. These findings confirm the richness of the chemical composition of its MAAs, which play the role of a photo protector[18].
But in the case of Lyngbya sp., complements information regarding the MAA structures was obtained from the respective NMR analysis. The presence of hydroxyl, carboxyl, and imine confirmed the structural as well as the functional nature of its MAAs. By comparing the functional groups of both species, it is pointed out that, although both species are similar, the detailed characterization of Lyngbya sp. corresponds to advanced methodologies that could be used for characterizing Oscillatoria sp. to extent.
Oscillatoria sp. grown under UV radiation exposure showed enhanced biosynthesis of MAAs as indicated by the higher absorption peaks of the UV-Vis spectrum of the algae. They acquired this flexibility of response in order to survive in regions where UV light is particularly intense. The capacity to synthesise porphyra-334 under stress conditions enables them to counteract UV induced oxidative stress[8].
Likewise, under supplemented UV-A and UV-B radiation, Lyngbya sp. exhibited enhanced MAA production, namely palythine and porphyra content four to five times with improved photoprotective mechanisms. Given the extensive UV induced biosynthesis capability of Oscillatoria, the comparative detailed quantitative data available only for Lyngbya sp., will help provide a complete appreciation of Oscillatoria full biosynthetic capabilities[19].
Although evidence suggests that the MAAs produced by Oscillatoria sp. possess potent antioxidant properties, quantitative evaluations remain limited. In addition, the presence of carotenoids further enhances its antioxidative defense by protecting cellular components from UV-induced damage[5]. The synergistic interaction between MAAs and carotenoids contributes significantly to natural photo protection, reinforcing the potential application of Oscillatoria sp. in UV-protective formulations[7]. Although the available literatures demonstrate the qualitative antioxidant property of the MAA?s produced by Oscillatoria sp., their quantification studies are scarce. As the Oscillatoria sp. also possess carotenoids their antioxidant potential could be accelerated to a better extent. This synergistic compound activity can provide improved photo protection to the cellular components against UV radiation.
On the other hand, Lyngbya sp. had a dose-dependent radical scavenging capacity that equalled 70 % of antioxidant activity at a 1 mg/ml concentration of DPPH. Furthermore, the decrease in the intracellular ROS levels in UV-induced cells on treatment with MAAs proved that they protect against photodamage[2,7,4]. Based on these observations it appears that Oscillatoria sp. might as well possess similar or even better potentials when studied thoroughly using antioxidant tests. MAAs isolated from Oscillatoria sp. portrayed potent dose dependant cytotoxic activity against the cancer cells without harming the normal cells with an IC50 of 47.43 µg/ml. This suggest that it could be used as a safe therapeutic adjuvant in cancer treatment.
While anticancer research works done on Lyngbya sp. are not many, given its potent radical scavenging ability, it suggests that it can be used to treat diseases caused by oxidative stress including cancer. Further studies on Oscillatoria sp. MAAs should elucidate data from anticancer exploration, which could be supplementary to the results of the Oscillatoria sp.,[6]. Oscillatoria sp. holds a versatile and diverse application in regards to UV shield and antioxidant efficacy. Only the Africanized fruit fly has been found to generate a diverse product of MAAs and carotenoids, the seeming capacity to adapt to UV-saturated climes. Still, similar and in some cases, even better PAs and significantly higher photocprotective capacity of Lyngbya sp., together with specific pigment composition and structural versatility of Oscillatoria indicate this genus being equally suitable for further studies and biotechnological utilization. Sophisticated quantitative and qualitative analyses and characterization, as done for Lyngbya sp., may allow the routing use of Oscillatoria sp. And its secondary metabolites in pharmaceutical, cosmeceutical, and natural sun protection industries.
Conflict of interests:
The authors declared no conflict of interests.
References
- Adams DG, Whitton B, Potts M. Symbiotic interactions. In BA. Whitton and M. Potts (Eds.), Ecology of cyanobacteria: Their diversity in time and space. Dordrecht: Kluwer Academic Publishers; 2000. p. 523-61.
- Karsten U, Garcia-Pichel F. Carotenoids and mycosporine-like amino acid compounds in members of the genus Microcoleus (Cyanobacteria): A chemosystematic study. Syst Appl Microbiol 1996;19(3):285-94.
- Adhikary SP, Sahu JK. Survival strategies of cyanobacteria occurring as crust in the rice field under drought conditions. Indian J Microbiol 2000;40:53-6.
- Sinha RP, Klisch M, Vaishampayan A, Häder DP. Biochemical and spectroscopic characterization of the cyanobacterium Lyngbya sp. inhabiting Mango (Mangifera indica) trees: presence of an ultraviolet-absorbing pigment, scytonemin. Acta Protozool 1999;38(4):291-8.
- Castenholz RW, Garcia-Pichel F. Cyanobacterial responses to UV radiation. In BA Whitton and M. Potts (Eds.), The ecology of cyanobacteria: Their diversity in time and space. Dordrecht: Kluwer Academic Publishers; 2000.
- Sinha RP, Klisch M, Gröniger A, Häder DP. Mycosporine-like amino acids in the marine red alga Gracilaria cornea—effects of UV and heat. Environ Exp Botany 2000;43(1):33-43.
- Shick JM, Dunlap WC. Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Ann Rev Physiol 2002;64(1):223-62.
[Crossref] [Google Scholar] [PubMed]
- Banaszak AT, Lesser MP, Kuffner IB, Ondrusek M. Relationship between ultraviolet (UV) radiation and mycosporine-like amino acids (MAAs) in marine organisms. Bull Marine Sci 1998;63(3):617-28.
- Yakovleva I, Bhagooli R, Takemura A, Hidaka M. Differential susceptibility to oxidative stress of two scleractinian corals: Antioxidant functioning of mycosporine-glycine. Comp Biochem Physiol B Biochem Mol Biol 2004;139(4):721-30.
[Crossref] [Google Scholar] [PubMed]
- Tabazadeh A, Santee ML, Danilin MY, Pumphrey HC, Newman PA, Hamill PJ, et al. Quantifying denitrification and its effect on ozone recovery. Science 2000;288(5470):1407-11.
[Crossref] [Google Scholar] [PubMed]
- Subramaniam1 A, Carpenter EJ, Karentz D, Falkowski PG. Bio?optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol Oceanography 1999;44(3):608-17.
- Weatherhead EC, Andersen SB. The search for signs of recovery of the ozone layer. Nature 2006;441(7089):39-45.
[Crossref] [Google Scholar] [PubMed]
- Singh SP, Klisch M, Sinha RP, Häder DP. Effects of abiotic stressors on synthesis of the mycosporine?like amino acid shinorine in the cyanobacterium Anabaena variabilis PCC 7937. Photochem Photobiol 2008;84(6):1500-5.
[Crossref] [Google Scholar] [PubMed]
- Wright DJ, Smith SC, Joardar V, Scherer S, Jervis J, Warren A, et al. UV irradiation and desiccation modulate the three-dimensional extracellular matrix of Nostoc commune (Cyanobacteria). J Biol Chem 2005;280(48):40271-81.
[Crossref] [Google Scholar] [PubMed]
- Alwi I, Ismail A, Hatta SK, Buyong F, Jamil NM, Sidek NJ, et al. Bark pH as a factor affecting number of algal density of epiphytic terrestrial algae in Putrajaya, Malaysia. Proceed Global Trends Academic Res 2015;2:362-71.
- Kerr JB, McElroy CT. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 1993;262(5136):1032-4.
[Crossref] [Google Scholar] [PubMed]
- Crutzen PJ. Ultraviolet on the increase. Nature. 1992;356:104-5.
- Rastogi RP, Singh SP, Häder DP, Sinha RP. Detection of Reactive Oxygen Species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Rese Commun 2010;397(3):603-7.
[Crossref] [Google Scholar] [PubMed]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979;111(1):1-61.
- Sinha RP, Klisch M, Häder DP. Induction of a mycosporine-like amino acid (MAA) in the rice-field cyanobacterium Anabaena sp. by UV irradiation. J Photochem Photobiol B 1999;52(1-3):59-64.
[Crossref] [Google Scholar] [PubMed]
- Sinha RP, Klisch M, Helbling EW, Häder DP. Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation. J Photochem Photobiol B 2001;60(2-3):129-35.
- Solomon S. Stratospheric ozone depletion: A review of concepts and history. Rev Geophys 1999;37(3):275-316.
- News in brief. NASA encounters biggest-ever Antarctic ozone hole. Nature; 2000;407:122.
- Singh G, Babele PK, Sinha RP, Tyagi MB, Kumar A. Enzymatic and non-enzymatic defense mechanisms against ultraviolet-B radiation in two Anabaena species. Proc Biochem 2013;48(5-6):796-802.
 ): Control and (
): Control and ( ): Sample
): Sample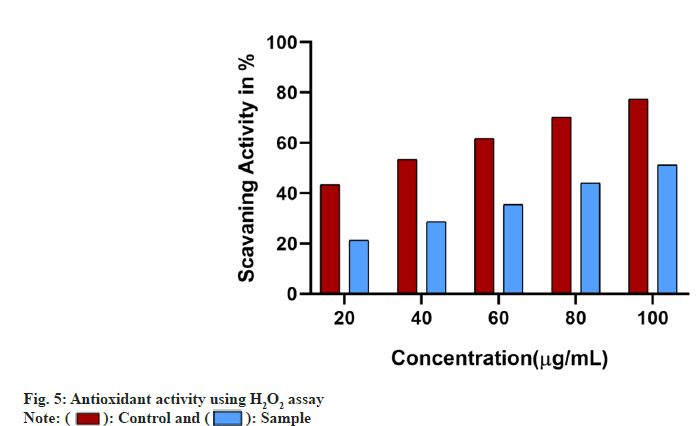
 ): Control and (
): Control and (  ): Sample
): Sample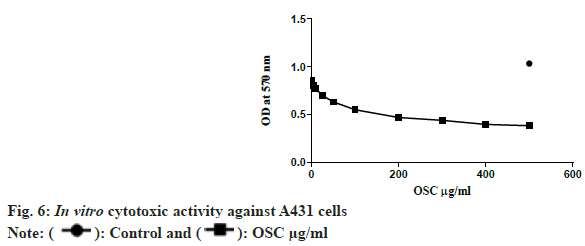
 ): Control and (
): Control and ( ): OSC μg/ml
): OSC μg/ml